Abstract
The present work investigates the applicability of a widespread bio-geochemical model (Biome BGC) to simulate monthly net primary productivity (NPP) and leaf area index (LAI) of Indian tropical deciduous forests. We simulated the monthly NPP and LAI of three plant functional types (PFTs) [dry mixed (DM), sal mixed (SM) and teak plantation (TP)] having distinct tree species compositions, canopy structure, different carbon assimilation rates and microclimate within a broad tropical deciduous forest during 2011–2012. The parameterization of 11 major eco-physiological parameters of Biome BGC was performed from in-situ physiological measurements gathered from 9 long-term ecological research plots in above three PFTs and PFT specific indices were developed. Bimodal trends, with highest peak in September during autumn and second peak in January during winter were observed for simulated monthly NPP in all three PFTs. Simulated NPP (gC/m2/year) values were 408.8 and 414.6; 376.8 and 392.9; and 327.5 and 338.2 during 2011 and 2012 in DM, SM and TP PFTs respectively. Observed NPP (gC/m2/year) values ranged between 463.4 and 493.1; 498.0 and 529.5; and 542.1 and 677.9 in 2012 in DM, SM and TP PFTs respectively. Biome BGC simulated NPP were in positive agreement with observed NPP in all PFTs (R2 = 0.92, 0.83 and 0.72 in DM, SM and TP respectively). In all PFTs Biome BGC led to an underestimation of LAI. The current investigation evaluated the operational application of Biome BGC in Indian tropical deciduous forest and opens scope for further improvement for LAI algorithms for better in-situ LAI simulation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Terrestrial net primary production (NPP), defined as the sum of photosynthetic carbon uptake by green canopy, plays a significant role in the global carbon dynamics and enhance our understanding for carbon flux studies in relation to changing climates (Bao et al. 2016; Sannigrahi 2017). Quantifying and monitoring the carbon flux in forests is essential in account of national carbon inventory and meeting the targets of Kyoto Protocol. Ground based assessment of carbon dynamics in forest ecosystems is both labour intensive and time consuming, therefore needs additional modeling approach to make the assessment easier and handy. Modeling approaches also examine and lower the uncertainty in estimating ecosystem productivity in complex terrestrial biomes for different time periods at ecosystem scale. During the last few decades, several models were introduced for biome specific modeling of terrestrial productivity ranging from meteorological parametric models to different light use efficiency (LUE) models to complex process-based models (Jiang et al. 2013; Li et al. 2014; Soudani et al. 2014; Melaku Canu et al. 2015). For understanding and predicting the potential impacts of climatic change on forest dynamics requires biogeochemical models (Haxeltine and Prentice 1997). Different models are available to estimate the NPP of forest ecosystems. Ecosystem models combine representations of plant biology, ecosystem dynamics and functioning, photosynthesis, stem and leaf, carbon and nutrient dynamics (Jiang et al. 1999). Process-based simulators (PBS) simulate eco-physiological processes to predict state variables such as LAI, keeping account of carbon, nutrient and water stocks (Running and Gower 1991). With a parameterized PBS, one could predict ecosystem NPP by simulating different physiological plant responses to climatic conditions, atmospheric properties and plant community structures (Running and Hunt 1993). Implementation of PBS for specific sites has been difficult due to the incomplete knowledge of site specific input parameters for the occurring multiple associated tree species.
Data on forest gross and net primary production (GPP and NPP) are crucial for measurement of vegetation dynamics, as they determine carbon storage and biomass. Knowledge of these carbon fluxes is indispensable for understanding the ecology and energetics of forests. Predicting the potential impacts of climatic change on natural vegetation dynamics particularly carbon fluxes requires ecosystem-scale biogeographical and biogeochemical models (Overpeck et al. 1991; Smith et al. 1992a, b; Melillo et al. 1993; Haxeltine and Prentice 1996, 1997; Foley et al. 1996; Goudriaan et al. 1999; Liu et al. 2014; Li et al. 2014; Pretzsch et al. 2015; Wang et al. 2015; Tao et al. 2015; Bao et al. 2016; Sannigrahi 2017). Biome BGC is a widely employed PBS to simulate carbon, water and nitrogen fluxes, requiring about 39 ecophysiological parameters (Thornton 1998; Thornton et al. 2002). NPP is a combined function of temperature, vapour pressure deficit, soil water content, photosynthetically active radiation, atmospheric CO2 concentration, LAI and leaf nitrogen (Churkina and Running 1998).
Advancement in ecology and systems biology progressed from simple traditional taxonomic description of individual species to non-phylogenetic group cluster of species having similar response to set of environmental and climatic variables, termed as plant functional types (PFTs). Tropical deciduous forest due to higher species richness, multiple species association, varied phenophases, irregular stem densities and basal cover, multistoried canopy architecture and different microclimate, offers dynamism to the understanding of the monthly and annual dynamics in NPP and LAI within different PFTs (Blackburn and Gaston 1996; Behera et al. 2012). There is an urgent need to understand how changing climate, as well as natural disturbances, will affect vegetation carbon dynamics in Indian tropical deciduous forests.
Biome BGC simulates carbon, water and nitrogen fluxes within the vegetation, litter and soil compartments of terrestrial ecosystem with a daily time step. Biome BGC estimates NPP as a function of temperature, vapour pressure deficit, soil water content, solar radiation, atmospheric CO2 concentration, LAI and leaf nitrogen concentration (Churkina and Running 1998). The model operates at a daily time step with prescribed meteorological (maximum and minimum temperature, precipitation, humidity, and shortwave radiation) and site description information. Biome BGC has been used extensively to simulate fluxes of temperate species: e.g. boreal black spruce (Bond-Lamberty et al. 2005), Norway spruce, Scots pine, common beech and oak (Tatarinov and Cienciala 2006), but no detail study exist reporting tropical deciduous species.
In present investigation, we performed Biome BGC simulation for 2 years (2011–2012) for three PFTs [dry mixed (DM), sal mixed (SM) and teak plantation (TP)] having distinct tree species compositions, different carbon assimilation rates and microclimate, using daily meteorological data at Katerniaghat Wildlife Sanctuary (KWLS), India. Attempt has been made to (i) to simulate the monthly NPP and LAI during 2011–2012 for 3 PFTs using widespread biogeochemical Biome BGC model (ii) to validate the model-derived NPP and LAI with observed NPP and LAI data.
Methodology
Study area
The present study was undertaken in Katerniaghat Wildlife Sanctuary (KWLS), a representative tropical deciduous forest in the upper Gangetic plains adjoining Himalayan foothills in Uttar Pradesh state, India (Fig. 1). The KWLS is situated between 27°41′ and 27°56′N latitude; and 81°48′ and 81°56′E longitude, with elevation ranges from 116 to 165 m along the southern border of Nepal. It is a dense patch of 40 km long and 10 km wide with an area of 440 km2. The site experiences climatic variation, typical of northern India with extremes of heat and cold, and winter nights are very cold and foggy and heavy dew falls regularly. Heavy monsoon rain is experienced from June end to September, and along with the winter rainfall an average annual rainfall of about 1300–1400 mm is experienced.
Long term ecological data generation
Vegetation of the whole sanctuary area was studied through random sampling of quadrates of 20 m × 20 m each in year 2008. The importance value (IVI) of each tree species was calculated based on frequency, density and abundance value. Further, cluster analysis of the trees with their IVI values were performed for identifying the plant functional type (PFT) (Behera 2017). The criteria followed to identify the 3-PFTs for further investigation in the study i.e., (i) distinct species associations and similar ecological structure, (ii) distinct canopy architecture and vertical stratification, (iii) similar microclimatic requirement (iv) similar ecological role and performance among species clusters, (v) similar phenological calendar among major species compositions, and (vi) distinct understorey growth and regeneration patterns. Three associations (dry mixed (DM) for being the most heterogeneous diverse community; sal mixed (SM) for being moderately heterogeneous with dominance of sal and being a pre-climax community; teak plantation (TP) for being a introduced species in the sanctuary and naturalized gregarious form representing complete homogeneous community were selected and further considered as three PFTs for long term ecological monitoring under broad Indian deciduous forests. Nine 1-ha long term ecological research (LTER) permanent plots (100 m × 100 m) were established (in triplicate) in three selected PFTs i.e. DM, SM and TP (Fig. 2) under Council of Scientific and Industrial Research (CSIR), New Delhi network project (NWP-020) in year 2008 and further PFT specific annual ecological inventory were conducted.
Forests of KWLS falls under tropical deciduous forest, which are two- to three storied having a distinct understory and top storey canopy, which experiences about 3–5 dry months in a year, harbour largely deciduous species with a few semi-evergreen and evergreen species and also a considerable diversity and density of lianas. SM is easily recognized by their large tall trees which constitute the upper canopy of the forest with height of 25-35 m. Although, in SM, Shorea robusta and Terminalia elliptica are the two dominant top story associates, however, Diospyros exculpta, Haldina cordifolia, Mitragyna parvifolia, Syzygium cumini, Hymenodictyon orixense are also frequently found in middle story canopy. TP has now become naturalized by their continuous regeneration, but understory layer is very poorly developed. Herb and shrub are also very poorly developed. Only few climbers like Tiliacora acuminata and some scattered trees of Mallotus philippensis and Ficus hispida are found in TP. DM with the largest stretch of the sanctuary area contains species like Syzygium cumini, Haldina cordifolia, Madhuca longifolia, Schleichera oleosa, Ficus hispida, Mitragyna parvifolia, Aegle marmelos, Cassia fistula, Miliusa tomentosa, Bridelia retusa, Ehretia laevis, Diospyros exculpta, etc. (Behera et al. 2017). Mallotus philippensis demonstrated the highest IVI in DM and SM with values of 71.88 and 104.56 respectively, completely dominating the forest understory. Shorea robusta and Tectona grandis were the 2nd and 3rd dominating tree species of DM with 40.94 and 35.16 IVI values, whereas Terminalia elliptica occupied the 3rd position in SM next to Mallotus and sal. Teak species completely dominates the TP PFT with highest IVI (256).
Leaf area index measurements
The LAI of all the LTER plots were measured with the LAI-2000 (LICOR Inc., Lincoln, NE, USA) plant canopy analyzer (PCA). All LAI measurements were conducted during periods of overcast sky between April 2010 and May 2012 (Behera et al. 2015). LAI-2000 PCA observed LAI were used to validate the monthly Biome BGC simulated LAI. Post monsoon LAI-2000 PCA LAI data were taken for all correlation and statistical analysis.
Eco-physiological observation
Individual PFT microclimatic parameters such as photosynthetically active radiation (PAR), air temperature, soil surface temperature, ambient CO2 and absolute humidity were measured using Li-Cor 840 CO2/H2O analyzer and Li-Cor line quantum sensors (Behera et al. 2012). We also measured the diurnal gas exchange and water relations parameters with LICOR-6400XT portable photosynthesis system (LICOR Inc. USA) and plant water status console (Soil Moisture Inc., USA) in 10 randomly selected representative Mallotus (Mallotus philippensis), Sal (Shorea robusta) and teak (Tectona grandis) trees along with some major understory trees in abovementioned PFTs. Finally the average value for measured eco-physiological parameter for each PFT were considered for PFT specific parameterization (Tables 1, 2).
Biomass and net primary productivity
Annual DBH measurements of each tree were subsequently converted into estimates of aboveground biomass (AGB) using allometric equations for moist deciduous forest stands that relate tree diameter to biomass (Chave et al. 2005). Two equations were explored in order to determine the biomass for dry and moist PFT independently. For DM and SM PFTs the moist allometric equations was used and for TP the dry allometric equation was used.
where Y is biomass per tree in kg, D = DBH in cm (Brown et al. 1989).
Per tree biomass of all tress within the LTER was summed for the respective tree species and total individual species biomass were calculated for each PFT in each LTER. Finally, we calculated AGB from 2010 to 2012. Stand total AGB was calculated by summing all individual tree AGB for each inventory years in each 1-ha LTER plots for each PFT. Annual net primary productivity (NPP) for 2011 and 2012 were calculated by subtracting the AGB of current year from its previous year in each PFT (Behera et al. 2017).
Model parameterization
To parameterize the BIOME-BGC variable for Indian deciduous forest PFTs, locations with homogeneous vegetation and minimal topographical variation has been identified within the long term ecological research plots (LTER) in each PFT and PFT specific physiological data were acquired during 2010–2011.
The BIOME-BGC version 4.1.2 was used for the present NPP and LAI simulation of Indian topical deciduous forests (Table 1) [Numerical Terradynamic Simulation Group (NTSG) at the University of Montana] (available online at http://www.ntsg.umt.edu.).
To generate meteorological inputs such as precipitation, daily minimum and maximum temperature, shortwave radiation and vapour pressure deficit required for BIOME-BGC, we used the automatic weather station (AWS) (Costal Inc. USA) data installed in KWLS. The installed AWS station is situated within 6–10 km of areal distance from all 3 PFTs. The meteorological data collected from the AWS for the year 2010 and 2011 were used for the current simulations.
Parameter groups include the following: (1) turnover and mortality; (2) allocation of carbon; (3) carbon to nitrogen ratios (C:N); (4) the percent of plant material in labile, cellulose and lignin pools; (5) leaf morphology; (6) leaf conductance rates and limitations; (7) canopy water interception and light extinction; and (8) the percent of leaf nitrogen in Rubisco (PLNR) (Wang et al. 2009). The nearest suitable forest biome i.e. deciduous broad-leaved forest (DBF) parameter settings were considered for present simulation. We used the default parameters for DBF to simulate both water and carbon exchanges in a Indian deciduous forest ecosystem. Out of 39 eco-physiological parameters, 11 major eco-physiological parameters were parameterized specific to PFT (Table 2). Static description of the ecophysiological characteristics of the above three PFTs in the present study were given as input variables (Table 2). DBF group was selected for the current simulation. The general eco-physiological parameterization defined by White et al. (2000) was used, except the dates of leaf onset and offset, litter fall as fraction of growing season, annual fire mortality fraction, C:N of leaves, C:N of leaf litter after retranslocations, canopy light extinction coefficient, leaf area index, maximum stomatal conductance, vapour pressure deficit (VPDf and VPDi) which were parameterized by in-situ ecophysiological measurements independently in three PFTs during 2010–2012 (Table 2). The allocation of current growth proportion, C:N of fine roots, C:N of live wood, leaf litter labile proportion, leaf litter cellulose proportion, leaf litter lignin proportion were taken from values for dry tropical deciduous forests of Vindhyan Highlands of Uttar Pradesh (Kumar and Raghubanshi 2011).
Parameterization and calibration of a process-based-simulator is the major challenge in simulating GPP and NPP. The large number of input parameters makes the calibration difficult and complicated as they are inter-dependent (Raj et al. 2014). There are several input parameters for which field values are hard to measure directly, also referred to as unknown model parameters leading to uncertainty of model output (Kennedy and O’Hagan 2001). Nutrient requirements for new growth, plant respiration rates and photosynthetic efficiency are majorly dependent on leaf carbon to nitrogen ratio (C:N). Some parameters control the allocation of photosynthetically accumulated carbon to leaf, stems and root pools among all variables (White et al. 2000). Considering the complexity of Biome-BGC model, it could not be possible to measure all (or even some specific) internal model parameters, therefore we measured only 11 input variables for all three PFTs (Table 2).
The use of Biome BGC required a preliminary calibration phase, aimed at setting its eco-physiological parameters to properly simulate the NPP. We applied the calibrated versions of Biome BGC in independent forest sites having field measurements of observed LAI and NPP within the same PFT class for testing the model output. For testing the output of the calibrated Biome BGC, eight independent plots of 50 m × 50 m within the same PFT class outside the LTER sites were considered and ground NPP and LAI data were generated. Scatter plots were analyzed to understand the agreement/fitness of model output with observed data for year 2012. Pearson correlation coefficient (R2) was calculated for each pair of modeled vs observed data and linear equations for positive agreement were derived.
Results and discussion
Simulated NPP and comparison with estimated NPP
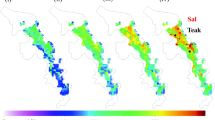
Biome BGC model was run to estimate the daily and monthly NPP in three distinct PFTs during 2011–2012 in KWLS, India (Fig. 3). Observed NPP (gC/m2/year) ranged between 463.4 and 493.1; 498.0 and 529.5; and 542.1 and 677.9 during 2012 in DM, SM and TP PFTs respectively. Simulated NPP (gC/m2/year) were found to be 408.8 and 414.6; 376.8 and 392.9; and 327.5 and 338.2 during 2011 and 2012 in DM, SM and TP respectively (Fig. 4). The simulated and observed NPP showed positive correlation (R2 = 0.92, 0.83 and 0.72 for DM, SM and TP respectively) (Fig. 5). Biome BGC confirmed a negative daily NPP in dry season for all three PFTs, indicating that plant respiration was greater than carbon uptake by plants during summer season when vegetation remain under water stress condition. The model revealed that NPP attained peak during the autumn season (particularly in September) for all 3 PFTs, while plants have maximum canopy cover leading to optimum photosynthetic gain. During autumn, soil moisture remain high after monsoon, when the duration of daylight becomes noticeably shorter and the temperature starts cooling down considerably with higher solar radiation than monsoon months. These microclimatic conditions seem to favour peak photosynthesis with optimal temperatures leading to peak in the NPP during autumn in maximum trees. Autumn ambient temperature observed positive association with simulated NPP for temperate oak-maple forests in New England forests in northeastern US (Duveneck and Thompson 2017). Precipitation and autumn temperature may had the largest control for peak NPP in September in all three PFTs, as observed in temperate forests in New England forests in US (Duveneck and Thompson 2017). Peak NPP during September month for all three PFTs at KWLS may be attributed by optimum temperature, lowest soil water potential and low atmospheric vapor pressure deficit leading to highest photosynthesis rate leading to higher carbon partitioning (Behera et al. 2012). Current simulations of Indian tropical deciduous forest physiological processes can improve our understanding of the phenological trade-offs associated with growing seasons and ecosystem respiration costs to gross primary productivity (GPP). The model simulated 84.66, 74.29 and 49.85% of the estimated NPP for DM, SM and TP respectively for 2012. These simulated NPP values for 2012 are in good agreement with estimated NPP for DM and SM, while very low as compared to estimated NPP for TP. Low NPP simulation for TP may be due to high C:N ratio, lower LAI and maximum range of vapour pressure deficit (Table 2). Deciduous species observed more seasonal variations in NPP as compared to evergreen species as observed for teak tree in TP in comparison to sal tree in SM. The simulated NPPs in teak plantation at Mae Moh, Thailand for 2004, 2005, 2006 and 2007 years of evaluation were 776.1, 740.4, 605.6 and 687.4 gC/m2/year respectively (Imvitthaya and Honda 2008), which is much higher than the simulated NPP in TP in KWLS (327.5 and 338.2 gC/m2/year during 2011 and 2012 respectively), may be lower soil N and high atmospheric vapor pressure deficit in Teak PFT lead to lower photosynthetic rate and lower foliar nitrogen content. Low total N in soil and microbial biomass carbon (MBC) attributed for lower nutrient use efficiency (NUE) and low carboxylation efficiency in TP PFT (Behera 2017; Behera et al. 2017). Simulated NPP of the undisturbed tropical rainforest was 419 gC/m2/year (Lim et al. 2009), which is similar with current simulation for DM (414.6 gC/m2/year in 2012) and higher than SM and TP (392.9 and 338.2 gC/m2/year in 2012 for SM and TP respectively).
Teak PFT sequestered maximum observed NPP in field among three PFTs due to optimum physiological performance in teak trees, (a) in absence of any other competing tree associate, (b) systematic well spread canopy due to plantation, leading to optimum utilization of lights and nutrients in absence of resource competitor, (c) fast growing in homogenous monoculture condition, (d) longer deciduous conditions (November to March–April) leading to decrease in net annual maintenance respiration, (e) large and broad leaf size for effective gas exchange. Biome-BGC model simulated lowest NPP in TP PFT among three PFTs due to less sensitiveness of model to the above listed ecological variables. Biome BGC can be further improved by incorporation of additional input variables addressing (a) tree density per area, (b) canopy architecture, (c) species associations and competition, (d) linkage between the different phenophases to its physiological vigor for different tree associates; which may be playing crucial role in net ecosystem productivity in tropical deciduous forests.
Comparison of simulated and estimated LAI
Biome BGC model was run to estimate the daily and monthly LAI in three PFTs during 2011–2012 in KWLS, India (Fig. 3). Observed LAI ranged between 4.03 to 6.44, 4.19 to 5.88 and 3.61 to 4.16 in DM, SM and TP during post monsoon season in 2012 respectively. Simulated maximum LAI were 2.38 and 2.24; 2.21 and 2.12; and 1.93 and 1.87 during 2011 and 2012 in DM, SM and TP respectively. All three PFTs simulated ‘0’ LAI in March, April and May months in both years 2011 and 2012, whereas the observed LAI ranged between 1.06–2.19, 1.17–2.41, and 0.67–1.63 in DM, SM and TP during summer season in 2012 respectively. DM accounted to the highest simulated LAI among three PFTs (2.38 and 2.24 in 2011 and 2012 respectively) in post-monsoon season in both years. This may be attributed due to higher species richness and high tree density in DM among three PFTs leading to dense canopy (Behera et al. 2012). Simulated and observed LAI measured (Fig. 5) showed good agreement with each other with significant positive correlation (R2 = 0.85, 0.79 and 0.69 in DM, SM and TP respectively). Monthly LAI pattern exhibited the highest value for the post rainy season (during October and November) in all PFTs that coincides with ground measurement (Behera et al. 2015). Maximum gap fraction in the top storey canopy in TP resulted in lower LAI among all.
Maximum LAI simulated by the model is much lower than the observed LAI in all 3 PFTs. Simulated LAI were zero during summer season in all PFTs may be due to water stress condition. The Biome BGC modeled LAI for 2012 were 44.09 , 40.92, and 41.55% of the ground measured LAI in DM, SM and TP respectively. DM is the structurally complex with distinct three storied canopy with intermingled woody lianas, observed maximum average LAI (> 5 m2 m−2) in majority of forest stands (Behera et al. 2015), which is also in agreement with the maximum simulated LAI in DM by Biome BGC. Lim et al. (2009) also obtained pessimistic result for simulated LAI (2.26) than the observed (6.2) for the tropical rainforests of Malaysia. Simulated maximum LAI for DM and SM are found to be in agreement with the LAI simulation for tropical rainforests of Malaysia. LAI has major influence on Biome BGC model since it controls canopy radiation absorption, water interception, photosynthesis, and litter inputs to detritus pools (White et al. 2000). Reducing the allocation of carbon to new leaf, fine root and stem in effect increases the LAI values.
Input data
Underestimation of NPP and LAI in all three PFTs under present simulations could be attributed to current eco-physiological input variables taken from an Indian dry deciduous forest site having lower SLA (30.0 m2/kg C), C/N ration in fine root (48.2 kg C/kg N) and live woods (78.3 kg C/kg N) (Chaturvedi and Raghubanshi 2011). Field observations for above input variables for all three PFTs with further parameterization may improve the accuracy of current simulation, as SLA and C/N ration decide the partitioning of the sequestered carbon and further NPP. Stomatal conductance (gs,max) showed negative correlation to NPP in TP PFT. Increase in gs,max reduced NPP by increasing water stress in TP PFT (Tatarinov and Cienciala 2006; White et al. 2000). VPDi and VPDf showed low negative effect on NPP in all PFTs. This negative relation with NPP was due to increased VPD which causes closure of stomata resulting in inhibition of photosynthesis (Kumar and Raghubanshi 2011).The current simulation can be further improved by additional parameterization of labile and non-labile component of litter, leaf water potential (complete conductance reduction and start of conductance reduction), canopy water interception coefficient, fraction of leaf N in Rubisco and ratio of shaded SLA: sunlit SLA to have prediction close to real time processes for above three PFTs (Jarvis 1995). Therefore, these parameters need more precision, and can be further estimated by more field observations at PFT level.
Biome BGC model based simulation
Biome BGC underestimated LAI in all PFTs in current simulations in Indian tropical deciduous forest. The underestimation may be attributed to (i) less sensitiveness of model to multi-tier/ multi-storied vertical stratification of canopy with discontinuous top story and (ii) highly dense regenerating understory and ground vegetation layers intermingled with large liana population. Moreover, the Biome BGC model misses a tree density component as input variable, which has direct influence on stand productivity; as higher tree density over unit ground cover leads to higher carbon sequestration and NPP in comparison to low density PFT stand.
In most of the cases Biome BGC modeled NPP has been validated with eddy flux covariance foot prints (Trusilova et al. 2009), but in present study outputs were validated by comparing with observed NPP of independent sites. Chiesi et al. (2007) investigated the applicability of Biome BGC by setting ecophysiological parameters to simulate the monthly GPP of three Mediterranean species (Quercus ilex, Quercus cerris and Pinus pinaster) and validated with GPP estimates of a simplified parametric C-Fix model. Majority of Biome BGC simulations were studied for temperate trees, however not much attempt has been made to understand the NPP patterns either in individual tropical tree species or PFT as a whole. DM PFT observed highest positive agreement (R2 = 0.92 for NPP and R2 = 0.85 for LAI) between simulated NPP and LAI with observed data among three PFTs. TP PFT observed lowest agreement between simulated NPP and LAI with observed data (R2 = 0.72 for NPP and R2 = 0.69 for LAI), due to open canopy architecture and least clumping of leaves in branches in Teak leading to lower LAI (Behera et al. 2015).
Conclusion
This study validated the applicability of Biome BGC to simulate LAI and NPP for tropical deciduous forest in Indian sub-continent. Bimodal trends, with highest peak in September during autumn and second peak in January during winter were observed for simulated monthly NPP in all three PFTs. NPP was simulated very well as compared to observed data, however, LAI was underestimated in current Biome BGC simulation. Biome BGC model can be suitably applied for simulating the LAI and NPP in tropical deciduous and evergreen ecosystems in other regions of India and other tropics. Current simulations of Indian tropical deciduous forest physiological processes can improve our understanding of the phenological associated trade-offs with NPP, very crucial for deciduous scenarios. We further feel for possible incorporation of tree density and species richness (SR) as additional model input variables for more precise simulation of NPP in reference to tropical species rich communities.
References
Bao G, Bao Y, Qin Z, Xin X, Bao Y, Bayarsaikan S, Chuntai B (2016) Modeling net primary productivity of terrestrial ecosystems in the semi-arid climate of the Mongolian plateau using LSWI-based casa ecosystem model. Int J Appl Earth Obs Geoinf 46:84–93. https://doi.org/10.1016/j.jag.2015.12.001
Behera SK (2017) Biomass, net primary productivity and community analysis in an Indian tropical deciduous forest. Indian Institute of Technology Kharagpur, India, PhD Thesis
Behera SK, Mishra AK, Sahu N, Kumar A, Singh N, Kumar A, Bajpai O, Chaudhary LB, Khare PB, Tuli R (2012) The study of microclimate in response to different plant community association in tropical moist deciduous forest from northern India. Biodivers Conserv 21:1159–1176
Behera SK, Behera MD, Tuli R (2015) An indirect method of estimating leaf area index in a tropical deciduous forest of India. Ecol Indic 58:356–364
Behera SK, Sahu N, Mishra AK, Bargali SS, Behera MD, Tuli R (2017) Aboveground biomass and carbon stock assessment in Indian tropical deciduous forest and relationship with stand structural attributes. Ecol Eng 99:513–524
Blackburn TM, Gaston KJ (1996) A sideways look at patterns in species richness, or why there are so few species outside the tropics. Biodivers Lett 3:44–53
Bond-Lamberty B, Gower ST, Ahl DE, Thornton PE (2005) Reimplementation of the BIOME-BGC model to stimulate successional change. Tree Physiol 25:413–424
Brown S, Gillespie AJR, Lugo AE (1989) Biomass estimation methods for tropical forest with applications to forest inventory data. For Sci 35:881–902
Chaturvedi RK, Raghubanshi AS (2011) Plant functional traits in a tropical deciduous forest. An analysis. Lambert Academic Publishing Gmbh & Co. KG, Saarbrücken
Chave J, Andalo C, Brown S, Cairns MA, Chambers JQ, Eamus D, Folster H, Fromard F, Higuchi N, Kira T, Lescure JP, Nelson BW, Ogawa H, Puig H, Riera B, Yamakura T (2005) Tree allometry and improved estimation of carbon stocks and balance in tropical forests. Oecologia 145:87–99
Chiesi M, Maselli F, Moriondo M, Fibbi L, Bindi M, Running SW (2007) Application of BIOME-BGC to simulate Mediterranean forest processes. Ecol Modell 206:179–190
Churkina G, Running SW (1998) Contrasting climate control on the estimated productivity of global terrestrial biomes. Ecosystems 1:206–215
Duveneck MJ, Thompson JR (2017) Climate change imposes phenological trade-offs on forest net primary productivity. J Geophys Res Biogeosci 122:2298–2313. https://doi.org/10.1002/2017jg004025
Foley JA, Prentice IC, Ramankutty N, Levis S, Pollard D, Sitch S, Haxeltine A (1996) An integrated biosphere model of land surface processes, terrestrial carbon balance, and vegetation dynamics. Glob Biogeochem Cycles 10:693–709
Goudriaan J, Shugart HH, Bugmann H, Cramer W, Bondeau A, Gardner RH, Hunt LA, Lauwenroth WK, Landberg JJ, Linder S, Noble IR, Parton WJ, Pitelka LF, Staford Smith M, Sutherst RW, Valentin C, Woodward FI (1999) Use of models in Global Climate studies. In: Walker B, Steffen W, Canadell J, Ingram J (eds) The terrestrial biosphere and global change: implications for natural and managed ecosystems. Cambridge University Press, Cambridge, pp 106–140
Haxeltine A, Prentice IC (1996) BIOME3: an equilibrium terrestrial biosphere model based on ecophysiological constraints, resource availability, and competition among plant function types. Glob Biogeochem Cycles 10:693–709
Haxeltine A, Prentice IC (1997) A general model for the light- use efficiency of primary prediction. Funct Ecol 10:551–561
Imvitthaya C, Honda K (2008) Evaluation of BIOME-BGC Model for Estimating NPP and LAI of Teak Plantation of Thailand Using SPOT-Data As Reference (TS 21.7). 29th Asian Conference on Remote Sensing (ACRS 2008), Colombo, Sri Lanka, 10–14 November 2008, Volume 1 of 3
Jarvis PG (1995) Scaling processes and problems. Plant, Cell Environ 18:1079–1089
Jiang H, Apps MJ, Zhang Y, Peng C, Woodard PM (1999) Modelling the spatial pattern of net primary productivity in Chinese forests. Ecol Model 122:275–288
Jiang Y, Zhang J, Xu X, Dong Z (2013) A GPP assimilation model for the Southeastern Tibetan Plateau based on CO2 Eddy Covariance flux tower and remote sensing data. Int J Appl Earth Obs Geoinf 23:213–225. https://doi.org/10.1016/j.jag.2012.08.015
Kennedy MC, O’Hagan A (2001) Bayesian calibration of computer models. J R Stat Soc B 63:425–464
Kumar M, Raghubanshi JS (2011) Sensitivity analysis of BIOME-BGC model for dry tropical forests of Vindhyan highlands, India. ISPRS archives XXXVIII-8/W20; workshop proceedings: Earth Observation for Terrestrial Ecosystem, At MPCT, Bhopal, India, Volume: XXXVIII
Li Z, Liu S, Tan Z, Bliss NB, Young CJ, West TO, Ogle SM (2014) Comparing cropland net primary production estimates from inventory, a satellite-based model, and a process-based model in the Midwest of the United States. Ecol Modell 277:1–12. https://doi.org/10.1016/j.ecolmodel.2014.01.012
Lim ACF, Suzuki M, Ohte N, Hotta N, Kume T (2009) Evapotranspiration patterns for tropical rain forests in Southeast Asia: a model performance examination of the BIOME-BGC model. Bull Tokyo Univ For 120:29–44
Liu Z, Wang L, Wang S (2014) Comparison of different GPP models in China using MODIS image and Chinaflux data. Remote Sens 6(10):10215–10231. https://doi.org/10.3390/rs61010215
Melaku Canu D, Ghermandi A, Nunes PALD, Lazzari P, Cossarini G, Solidoro C (2015) Estimating the value of carbon sequestration ecosystem services in the Mediterranean sea: an ecological economics approach. Glob Environ Chang 32:87–95. https://doi.org/10.1016/j.gloenvcha.2015.02.008
Melillo JM, McGuire AD, Kicklighter DW, Moore IIIB, Vorosmarty CJ, Schloss AL (1993) Global climate change and terrestrial net primary production. Nature 363:234–240
Overpeck JT, Bartlein PJ, Webb IIIT (1991) Potential magnitude of future vegetation change in eastern North America: comparisons with the past. Science 254:692–695
Pretzsch H, Forrester DI, Rötzer T (2015) Representation of species mixing in forest growth models. A review and perspective. Ecol Modell 313:276–292. https://doi.org/10.1016/j.ecolmodel.2015.06.044
Raghubanshi AS (2008) Nitrogen cycling in Indian terrestrial natural ecosystems. Curr Sci 94:1404–1412
Raj R, Hamm NAS, Tol CV, Stein A (2014) Variance-based sensitivity analysis of BIOME-BGC for gross and net primary production. Ecol Model 292:26–36
Running SW, Gower ST (1991) FOREST-BGC: a general model of forest ecosystem processes for regional applications. II. Dynamic carbon allocation and nitrogen budgets. Tree Physiol 9:147–160
Running SW, Hunt ER (1993) Generalization of a forest ecosystem process model for other biomes, Biome-BGC, and an application for global scale models. In: Ehleringer JR, Field CB (eds) Scaling physiological processes: leaf to globe. Academic Press. Inc., New York, pp 141–158
Sannigrahi S (2017) Modeling terrestrial ecosystem productivity of an estuarine ecosystem in the Sundarban Biosphere Region, India using seven ecosystem models. Ecol Modell 356:73–90
Singh L (1990) Biomass production and nutrient dynamics in a dry tropical forest. Ph.D. Thesis, Banaras Hindu University, India
Singh KP, Kushwaha CP (2005) Paradox of leaf phenology: Shorea robusta is a semi evergreen species in tropical deciduous forests in India. Curr Sci 88:1820–1824
Singh L, Singh JS (1991) Storage and flux of nutrient in a dry tropical forests in India. Ann Bot 68:275–284
Smith TM, Leemans R, Shugart HH (1992a) Sensitivity of terrestrial carbon storage to CO2 induced climate change: comparison of four scenarios based on general circulation models. Clim Chang 21:367–384
Smith TM, Shugart HH, Bonan GB, Smith JB (1992b) Modelling the potential response of vegetation to global climate change. Adv Ecol Res 22:93–116
Soudani K, Hmimina G, Dufrêne E, Berveiller D, Delpierre N, Ourcival J-M, Joffre R (2014) Relationships between photochemical reflectance index and light-use efficiency in deciduous and evergreen broadleaf forests. Remote Sens Environ 144:73–84. https://doi.org/10.1016/j.rse.2014.01.017
Tao X, Liang S, Wang D (2015) Assessment of five global satellite products of fraction of absorbed photosynthetically active radiation: intercomparison and direct validation against ground-based data. Remote Sens Environ 163:270–285. https://doi.org/10.1016/j.rse.2015.03.025
Tatarinov FA, Cienciala E (2006) Application of BIOME-BGC model to managed forests: 1 Sensitivity analysis. For Ecol Manag 237:267–279
Thornton PE (1998) Description of numerical simulation model for predicting the dynamics of energy, water, carbon and nitrogen in a terrestrial ecosystem. Ph.D. Thesis, University of Montana, Missoula
Thornton PE, Law BE, Gholz HL, Clark KL, Falge E, Ellsworth DS, Goldstein AH, Monson RK, Hollinger D, Falk M, Chen J, Sparks JP (2002) Modelling and measuring the effects of disturbance history and climate on carbon and water budgets in evergreen needleleaf forests. Agric For Meteorol 113:185–222
Tripathi SK, Singh KP (1992) Nutrient immobilization and release patterns during plant decomposition in a dry tropical bamboo Savanna, India. Biol Fertil Soils 14:191–199
Trusilova K, Trembath J, Churkina G (2009) Parameter estimation and validation of the terrestrial ecosystem model Biome-BGC using eddy-covariance flux measurements. Max Planck Institute for Biogeochemistry, Germany, Technical Reports 16, pp 1–60
Wang W, Ichii K, Hashimoto H, Michaelis AR, Thornton PE, Law BE, Nemani RR (2009) A hierarchical analysis of terrestrial ecosystem model Biome-BGC: equilibrium analysis and model calibration. Ecol Model 220:2009–2023
Wang S, Huang K, Yan H, Yan H, Zhou L, Wang H, Sun L (2015) Improving the light use efficiency model for simulating terrestrial vegetation gross primary production by the inclusion of diffuse radiation across ecosystems in China. Ecol Complex 23:1–13. https://doi.org/10.1016/j.ecocom.2015.04.004
White MA, Thornton PE, Running SW, Nemani R (2000) Parameterization and sensitivity analysis of the BIOME-BGC terrestrial ecosystem model: net primary production controls. Earth Interact 4:1–85
Acknowledgements
The authors are grateful to Director, CSIR-National Botanical Research Institute, Lucknow, India for providing necessary facilities and encouragement. Mr. Nayan Sahu (CSIR-SRF) and Mr. Ashish K. Mishra (PA-II, NWP-20 Project) are also acknowledged for their assistance in the field measurements. Thanks are also due to PCCF (Wildlife), Government of Uttar Pradesh, Lucknow and CCF cum Field Director (Dudhwa National Park), Bahraich for granting permission to carry out the research and facilities to visit the area. The funds to carry out this work were received from CSIR, New Delhi under NWP-020 and BSC-0109. The valuable comments made by the two anonymous reviewers and Editor is highly appreciated.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by David Hawksworth.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Behera, S.K., Tripathi, P., Behera, M.D. et al. Modeling net primary productivity of tropical deciduous forests in North India using bio-geochemical model. Biodivers Conserv 28, 2105–2121 (2019). https://doi.org/10.1007/s10531-019-01743-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10531-019-01743-6