Abstract
Investigating the tolerance of plant reproductive systems to environmental changes has become a research priority under current climate change scenarios. Successful plant conservation requires knowledge of plant reproductive biology, particularly the meiotic characteristics of planted species. Meiosis, as part of microsporogenesis, is a critical plant developmental stage controlling future pollen quality. Meiosis in a Siberian fir (Abies sibirica) plantation, established in the Forest Arboretum of the Sukachev Institute, Russia, was studied from 2002 to 2004. The microsporogenesis pattern found for the Siberian fir appeared to be largely similar to that exhibited by other conifer species. Meiosis in the Siberian fir has the following characteristics: asynchrony, rapid progression of telophases I and II, and parallel and linear spindle arrangements at different meiosis II stages. General and specific meiosis irregularities were recorded at each stage. Some specific features of meiosis and the specific development of some irregularities were revealed. Pollen development analysis showed that irregular pollen grains made up less than 1% of all grains. The specific features of meiosis identified in fir trees growing in the Arboretum indicated low resistance of male reproductive structures to climatic changes and might account for high fir pollen sterility in this new environment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Current weather observations, as well as physical and biological events, such as the retreat of glaciers, rise in sea levels, and extended growing seasons, indicate that global temperatures have increased over the past 200 years (Hulme et al. 1999). Records of global surface temperatures going as far back as 1850 revealed 1995–2006 to be the warmest period (Core et al. 2007). Boreal forests and mountain regions are particularly vulnerable in terms of climate change. Hadley Centre-derived GCM projections for Siberia predict a 4°C to 6°C temperature increase by 2100 (Gordon et al. 2000). Rapidly changing temperatures and its high absolute values are expected to have profound effects on woody species diversity (Iverson and Prasad 1998; Box et al. 1999).
The Siberian fir is an environmentally important Siberian woody species that can grow at altitudes of up to 2000–2100 m a.s.l. In mountains of southern Siberia, Siberian fir stands have been heavily disturbed by environmental changes over the past few decades (Tretyakova and Bazhina 2000). As a result, the establishment of a seed gene bank of this species became, along with the conservation of this species in its natural environment, a scientific priority.
Plants have to adapt to new environmental conditions due to global warming as well as to novel site conditions when planted. Observations conducted during artificial breeding of animals and plants have shown that new environments often have stress-like effects on them, with different species responding differently to these new conditions (Nekrasov 1971). For example, Siberian fir trees growing in the Forest Arboretum at the Sukachev Institute are characterized by high (68–92%) pollen sterility (unpublished data), which may be due to irregularities in chromosome behaviour during meiosis. The results of studies of several coniferous species planted in arboreta suggest that, on the one hand, meiosis tends to be regular in certain species, whereas it exhibits a high degree of disturbance in other species (Muraya et al. 1988; Gavrilov and Butorina 2005).
The aim of the present study was to investigate meiosis in Siberian fir (Abies sibirica) cultures at the Forest Arboretum of the Sukachev Institute, Russia. Fir responses to the changed environment, i.e. to conditions at the Forest Arboretum, can be considered as a model for the adaptation of species to changing climatic conditions. Climatic changes influence plant physiological and genetic characteristics, and their distribution. Monitoring ontogenesis of the fir plants in the non-native climatic environment of the Arboretum will provide insights into climatic effects on individual and population-scale characteristics of plants and enable us to identify mechanisms by which plants respond to environmental changes.
Materials and methods
Study site
This 3-year (2002–2004) study addressed meiosis in Siberian fir plantations located in the Forest Arboretum at the Sukachev Institute. The arboretum is located on a terrace (275 m a.s.l.) at the wildland/Krasnoyarsk interface (Akademgorodok), on the left bank of the river Yenisei. This is essentially an islet of mountain steppe supported by sod-carbonate, weakly alkaline (pH is close to neutral) soil containing little humus and mobile nitrogen (Mamaev et al. 1993). The Krasnoyarsk region is under extremely continental climate and the arboretum is considered to be an East-Siberian version of the southern taiga subzone (average annual temperature = +0.8°C). Weather information provided by the Weather Service of the town Krasnoyarsk showed that April and May air temperatures in 2002 and 2003 were similar to their average multi-year values, whereas spring in 2004 arrived 7–12 days later than usual (Varpholomeev and Maltsev 2006). Notwithstanding its proximity to Krasnoyarsk, the arboretum is not affected by pollution, as it is situated outside the zone of prevalent industrial emission transfer.
Dataset
Siberian fir saplings were transferred to the arboretum from the nursery of the Siberian Research Institute of Fruit Growing (Barnaul, Altai) in 1977 (Loskutov 1993). They were grown from seeds collected from natural stands of Siberian fir forests of the Altai region situated in a moderately continental climate (average annual temperature = 4.0°C).
Male generative buds (microstrobiles) were used for this study. Buds were collected daily, from April 23 to May 13, during meiosis and pollen development from the central parts of crowns of six trees, brought to the laboratory, and fixed. From 15 to 20 buds were collected per tree per day. The buds were kept in alcohol mixed with acetic acid at a ratio of 3:1 for one or two days, then transferred to 70% alcohol, and stained by acetohematoxylin.
Data analysis
A total of 13 507 developing pollen grains and 16 695 pollen mother cells (PMC) at different stages of meiosis were analyzed. PMC chromosomes were studied using a MBI-6 microscope and DCM130 Digital microscope camera. Normal PMC and those having irregular chromosome behaviour were counted at each stage of meiosis. STATISTICA 7.0 (StatSoft, Inc. STATISTICA 2001) was used to calculate descriptive statistics including standard deviations and probability levels. The mean percentage of irregular PMC at each stage of meiosis was compared using the Student t-test.
Results
Pollen-cone development phenology
Siberian fir was largely similar to other conifer species in terms of its microsporogenesis pattern (Mergen and Lester 1961; Rozhdestvenskii 1974; Owens and Molder 1985). Male strobili occurred on the lower side of the current year’s shoot base, and became visible in late June–early July.
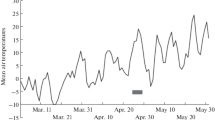
Sporogenous tissue developed in the microsporangia during August. The strobili grew in size and the cells within the microsporangia were at the primitive archesporial stage by late August–early September (Fig. 1a, b). The strobili were dormant at this stage and their development was resumed in April. The rate of development of the post-dormancy pollen cone appeared to be strongly dependent on air temperature; it increased in warm weather and decreased when it became cooler. The first meiotic divisions were observed on the 23rd of April, at an accumulated temperature of 12.9–13.7°C in 2002 and 2003, while this process only started on the 5th of May in the cold spring of 2004, at an accumulated temperature of 57.5°C (Fig. 2). Meiotic division lasted for 13–17 days (from diakinesis through the tetrad stage). The duration of meiosis differed by no more than one day among the sampled trees. Meiosis occurred at a generally higher rate in microsporangia situated in the lower part of the strobile as compared to those found in the upper part. At the end of meiosis, microspore tetrads separated quickly. Pollen grains formed in about two weeks following meiosis. At the onset of pollen development, accumulated temperatures were 152°C and 170°C in 2002 and 2004, respectively, and pollen was dispersed between 25 and 31 May.
Meiosis cytology
Archesporial cells differentiated into PMC in the buds collected on the 23rd of April. These typically rounded cells with easily distinguishable nuclei were surrounded by a transparent zone of cytoplasm. Their diameter (57.3 ± 1.1 μm) appeared to be 1.5–2.0 times that of the surrounding tapetum cells. All stages of prophase I (leptotene, zygotene, pachytene, diplotene and diakinesis) were found in fir trees sampled from the arboretum (Fig. 1c–h). The PMC contained up to five nucleoli, which disappeared during the diplotene prophase, although they were earlier believed to be present until the end of the diakinesis prophase (Mergen and Lester 1961; Nekrasova and Ryabinkov 1978). In general, one of the nucleoli was larger and better stained.
Siberian fir exhibited classical meiosis that includes the formation of 12 bivalents, which moved in the opposite direction. Bivalent morphology was determined by the position and amount of chiasmata (Fig. 1h). PMC of most of the trees sampled had up to four chiasmata per bivalent. Average chiasmata frequency of occurrence was 2.6 ± 0.1 (±SE) per bivalent and 31.2 ± 0.9 (±SE) per metaphase plate. A number of PMC of one of the trees sampled contained up to five chiasmata per bivalent.
The fir trees sampled showed all of the species-specific characteristics of meiosis described above including asynchrony, rapid PMC telophase I and II progression, and a distinct prophase II (Bazhina et al. 2007). While PMC developed synchronously up to the diakinesis stage, the time of occurrence of the following developmental stages varied among the cells. PMC prophase I and immature pollen grains were observed from the same microsporangium (Fig. 3a). Along with their normal orientation, spindles showed parallel and linear arrangements at different meiosis II stages (Fig. 3b–d). PMC with the above features accounted for 0.1–0.7% of all PMC in the trees sampled. Parallel spindle orientation might have resulted in chromosome group fusion at the poles and in the formation of pollen grains with diploid chromosome sets (Fig. 3c).
Meiosis irregularities
Although meiosis proceeded quite regularly in most cells, irregularities were found at nearly all meiotic stages in a number of cells (Fig. 4). Our analysis of irregular PMC revealed the presence of irregularities of both general and specific types. The general irregularities included chromosomes present outside spindle divisions, chaotic disjunction of chromosomes to the poles, lagging chromosomes, elongated chromosomes, and bridges consisting of multiple and tripolar configurations at anaphase II.
Irregularities of meiosis: a residual nucleolus (diakinesis), b decontraction of bivalents (diakinesis), c polyvalent associations, d complete chromosome agglutination into a ring (metaphase I), e–f chromosome agglutination (anaphase I), g elongated chromosomes (anaphase I), h elongated chromosomes (metaphase II), i chaotic chromosome arrangement (metaphase II)
Specific irregularities found in only a few cells were represented by polyvalent associations in diakinesis, fragmentation, chromosome agglutination into a ring at metaphase II, pentapolar configurations at anaphase II, and micronuclei in dyad and tetrad. Numerous irregularities were also recorded at anaphases I and II in 0.5–3.6% of all PMC, with bridges, ejections, lagging chromosomes, and chaotic disjunction of chromosomes in different combinations being the most common. We found an interesting and very rare anomaly type: asynchronous division within the same PMC, for example, prophase II and metaphase or anaphase II at the poles. The following combinations were also identified: anaphase I–metaphase II, metaphase II–anaphase II, dyad–anaphase II, and anaphase II–tetrad.
The frequency of meiotic irregularities was significantly higher than in natural population near Krasnoyarsk (Table 1). While certain irregularities, such as chromatin agglutination, residual nucleolus at prometaphase I and complete chromosome agglutination into a ring at metaphase I, tripolar configuration and chromosomes outside of spindle divisions at ana–telophase I, nuclear union in dyad, chromosome elongation, and their chaotic arrangement at metaphase II were recorded in the arboretum trees (Fig. 5), they have never been observed in trees growing in natural forest stands near Krasnoyarsk.
Stage-specific frequency of meiotic irregularities varied among observation year and trees sampled (Table 2). Meiotic irregularities were usually eliminated at the stage of interkinesis (in dyads, or tetrads). Environmental conditions were found to control the amount of irregular cells. Irregularities increased in frequency at different meiotic stages for all sample in 2004 as compared to the previous years. They were particularly numerous (15.6 to 31.2%) in meiosis I. Also, PMC ceased to develop at prophase I in all trees sampled. Chromatin was agglutinated and the nucleolus was often ejected to the cytoplasm in such cells (Fig. 5a–b) and degraded completely with time (Fig. 5c–d). The amount of PMC with this irregularity ranged from 0.20 to 2.21%. A specific developmental irregularity was found in 2004. For example, lagging chromosomes at the ana–telophase I stage stood apart in the micronucleus at the dyad stage (Fig. 5e). A number of bridges that occurred at anaphase I were also present in meiosis II (Fig. 5h–i), which may lead to partial chromosome group fusions (Fig. 5f–g).
Microspore development
Initially, microspores were angular, however, they became round with time. They grew in size for ten days. After wing formation, the haploid microspore cell divided unequally twice, producing a small lens-shaped prothallial cell on the proximal side (opposite the wings) each time (Fig. 6). The remaining large cell (“antheridial initial”) then divided unequally into a small generative and a large tube cell. Most mature Siberian fir pollen grains had four cells: two degenerated prothallial cells, a generative and a tube cell. Pollen development was slightly asynchronous: there were 2-and 4-celled pollen grains in the same microsporangia. The study showed that irregular pollen grains made up less than 1% of all pollen produced by each tree. Differences of air sacs, in terms of quantity and form, appeared to be common (Fig. 6g–i). Regular Siberian fir pollen grains have, as a rule, two large symmetrical air sacs. However, our study identified pollen grains having no air sacs, as well as grains having one, three, and four air sacs.
Discussion
The process of meiosis is determined by interactions between genotypes, chromosome structure, and the environment (Sosnikhina et al. 1994). Chromosome behaviour, as a function of environmental conditions, is also genetically determined during meiosis (Shkutina 1975). Classical genetic studies and modern molecular evolutionary approaches suggest that inbreeding depression and heterosis are predominantly caused by the presence of recessive deleterious mutations in populations (Charlesworth and Willis 2009). Some disturbances and irregularities during meiosis, observed in this study, might be due to inbreeding depression as well as heterosis, or their genetic features, for example, mutations, as meiosis is under genetic control (Golubovskaya 1975; Khvostova and Yachevskaya 1975; Sosnikhina et al. 1994; Bogdanov 2003). Chromosome lagging and agglutination, for example, are attributed to mono-or di-genic recessive mutations (Mehra and Rai 1970; Sosnikhina et al. 1994). The presence of a parallel-spindle (ps) mutagen can also be indicated by parallel spindle orientation at different stages of meiosis II. A number of authors (Mok and Peloquin 1975; Veileux et al. 1982; Butorina et al. 1985) believe that the spindles are normally at an angle of 60° to each other in meiosis II. Mutations of Siberian fir chromosomes, including ring chromosomes, have been reported by Muratova and Matveeva (1996).
Responses of ontogenetic development of fir male reproductive structures to environmental conditions at the Forest Arboretum can be considered as a model of adaptation of the species to a changing climate. In Siberian firs from natural stands near Krasnoyarsk, which are adapted to an extreme continental climate, meiosis started later (on May 7–13, at an accumulated temperature of 40.8–47.8°C), proceeded very rapidly: within 2–3 days, and are more regular (Table 1, Bazhina et al. 2007). Trees planted in the arboretum were brought from the Altai region and adapted to a different (moderately continental) climate. Drastic environmental and climatic changes resulting from extracting trees from natural stands and planting them in botanical gardens and arboreta can cause tree-growth irregularities, increased intraspecific differentiation, and reduced seed production (Nekrasov 1971; Mamaev and Andreev 1996). High sensitivity to these changes is characteristic of the juvenile ontogeny stage and the beginning of the reproductive phase, as sporo-and gameto-genesis adapt to temperature and light regime changes (Gavrilov and Butorina 2005). The climatic changes were assumed to cause an early start of meiosis and an increase in the duration of meiosis (up to 17 days for trees in the arboretum). In the Siberian fir, meiosis occurs immediately after winter dormancy. Yet only a sufficiently long period of dormancy can protect meiotic processes from damage from cold weather (Luomajoki 1977). For trees in the arboretum, dormancy terminates too early and late frosts might result in increasing the number of irregular cells, like in 2004.
Termination of the development of certain PMC, as well as irregularities were presumably caused by extreme air temperatures. In different parts of the Siberian fir’s range, local populations show differences in growth rate and in timing of growth. When plants that are native to warm western regions are transferred to areas of severe environmental conditions, they show higher sensitivities to air temperatures when compared to local plant species (Mamaev et al. 1993). Southern and western climotypes start to grow at lower air temperatures and their seasonal developmental stages start earlier at lower accumulated temperature, as compared to northern and eastern climotypes. Fir trees transferred to the more climatically severe arboretum locality start to grow earlier (i.e., at lower air temperatures) than local fir trees. As such, cell disturbances and an increasing number of meiotic irregularities occur in these trees compared to local trees. The sensitivity of meiosis to extreme air temperatures is known to vary among the different stages of meiosis (Eriksson 1968; Andersson 1980). The stages from diakinesis to anaphase I and from metaphase II to telophase II appeared to be the most sensitive. Most PMC in the arboretum were in interphase–prophase II during extreme temperature spells in 2002–2003. In 2004, PMC were at the premeiotic phase during the extreme temperature period and meiotic divisions began later, at higher accumulated temperatures. However temperature-caused PMC damage occurring at the premeiotic phase takes a long time to become visible (Luomajoki 1986). Extreme temperatures occurring during pollen formation, the stage at which meiosis is at its most temperature-sensitive, appear to have a negative impact on meiotic cell division patterns, manifested in the occurrence of irregularities or pollen sterility (Christiansen 1960; Chira 1964; Chandler and Mavrodineanu 1965; Eriksson 1968; Johnsson 1974; Luomajoki 1977; 1986; Muratova 1995; Hak and Russell 2004). These meiotic irregularities, apparently, resulted in decreasing pollen fertility in fir trees growing in the arboretum.
Chromosome behaviour irregularities during meiosis are, as a rule, eliminated prior to the tetrad stage and, in fact, do not impact developing pollen grain quality (Gavrilov and Butorina 2005). However, some defects, such as chromatin agglutination, may be reflected in pollen heterogeneity, for example, variation in the sizes and shapes of pollen grains and reduced pollen fertility (Pozhidaeva et al. 1985). We believe that irregularities at the tetrad stage became more frequent and, hence, abnormal pollen occurred due to these disturbances. The regular development of air sacs is important for maintaining population genetic diversity. Even with regular cell development, defective air sacs can reduce pollen dispersal and cross-pollination probability (Mahneva et al. 2003).
Investigating plants that have been moved to a different environment also enables us to identify their adaptive responses to a changing climate. Even with a small sample of trees, the results obtained showed a statistically significant effect on male reproductive parts of fir trees. Our results indicate that the response of the Siberian fir in the arboretum can be used to investigate the response of this species to climate change.
Conclusion
One of the major tasks of botanical gardens is the ex situ conservation of plants, primarily major woody species. Our study showed that climatic changes induced plant physiological disturbances mainly regarding meiosis and pollen development. Fir pollen development irregularities might lead to increased pollen sterility and, as a result, fertilization and seed formation failures. Results from this study allowed us to speculate that global climate change may cause Siberian fir regeneration disturbances. Our study also showed that plant material meant for ex situ conservation should be collected from locally well-adapted populations and be tested for quality using cytological and genetics techniques prior to collecting.
References
Andersson E (1980) Temperature-conditioned irregularities in pollen mother cells of Picea abies (L.) Karst. Hereditas 92:27–35
Bazhina EV, Kvitko OV, Muratova EN (2007) Meiosis at microsporogenesis and pollen viability in Siberian fir (Abies sibirica Ledeb.) in the Middle Eastern Sayan mountains. Russ For Sci (Lesovedenie) 1:57–64
Bogdanov YuF (2003) Variability and evolution of meiosis. Russ J of Genet (Genetika) 39(4):363–381
Box EO, Crumpacker DW, Hardin PS (1999) Predicted effects of climatic change on distribution of ecologically important native tree and shrub species in Florida. Climatic change 41:213–248
Butorina AK, Muraya LS, Mashkina OS et al (1985) A new type of meiotic mutation in the pine. Russ J Genet (Genetika) 21(1):103–111
Chandler C, Mavrodineanu S (1965) Meiosis in Larix laricina Koch. Contrib Boyce Thompson Inst 23(4):67–76
Charlesworth D, Willis JH (2009) The genetics of inbreeding depression. Nat Rev Genet 10:783–796
Chira E (1964) Vplyv teploty na pribeh meiozy pelovych materskych buniek Taxus bassata L (The temperature influence on Taxus bassata L. meiosis). Biologia, Bratislava 11(4):235–243
Christiansen H (1960) On the effect of low temperature on meiosis and pollen fertility in Larix decidua Mill. Silvae Genet 9(3):72–78
Core WT, Pachauri RK, Reisinger A (eds.) (2007) Climate change 2007: synthesis report. IPCC, Geneva. http://www.ipcc.ch/publications_and_data/publications_and_data_reports.htm
Eriksson G (1968) Temperature response of pollen mother cells in Larix and its importance for pollen formation. Stud For Suec 63:1–131
Gavrilov IA, Butorina AK (2005) Cytogenetics of the Canadian Hemlock upon introduction in the Voronezh district. Russ For Sci (Lesovedenie) 3:60–65
Golubovskaya IN (1975) Genetic control of chromosome behavior during meiosis. In: Khvostova VV, Bogdanov YuF (eds) Tsitologiya i genetika meioza (Cytology and genetics of meiosis). Nauka, Moscow, pp 312–322
Gordon C, Cooper C, Senior C et al (2000) The simulation of SST, sea-ice extent and ocean heat transport in a version of the Hadley Centre coupled model without flux adjustments. Climatic Dyn 16:147–168
Hak O, Russell JH (2004) Environmental effects on Yellow-cedar pollen quality. Forest Genetic Council Extension note 05:1–9
Hulme M, Mitchell JFB, Ingram W et al (1999) Climate change scenarios for global impacts studies. Glob Environ Change 9:3–19
Iverson LR, Prasad AM (1998) Predicting abundance of 80 tree species following climate change in the Eastern United States. Ecol Monographs 68(4):465–485
Johnsson A (1974) A study on the temperature response of pollen mother cells in Norway spruce. Stud For Suec 116:1–32
Khvostova VV, Yachevskaya GL (1975) Chromosome rearrangements in meiosis. In: Khvostova VV, Bogdanov YuF (eds) Tsitologiya i genetika meioza (Cytology and genetics of meiosis). Nauka, Moscow, pp 232–262
Loskutov RI (1993) Dekorativnye drevesnye rasteniya dlya ozeleneniya gorodov i poselkov (Decorative tree plants for greenery of towns and settlements). Izd-vo Krasnoyarskogo instituta lesa, Krasnoyarsk
Luomajoki A (1977) Effects of temperature on Spermatophyte Male Meiosis. Hereditas 85:33–48
Luomajoki A (1986) Timing of microsporogenesis in trees with reference to climatic adaptation. Acta For Fenn 196:1–33
Mahneva SG, Babushkina LG, Zueva GV (2003) Sostoyanie muzhskoy generativnoy sphery Pinus sylvestris pri technogennom zagryznenii sredy (The state of Pinus sylvestris male generative sphere at technogenic pollution of environment). Ural university publisher, Ekaterinburg
Mamaev SA, Andreev LN (1996) Role of botanical gardens of Russia in conservation of floristic diversity. Russ J of Ecol (Ekologia) 6:432–437
Mamaev SA, Dorofeeva LM, Alexandrova MS et al (1993) Adaptacia i izmenchivost dreveshych rastenii lesnoy zone Evrasii (Adaptation and variability of woody plant in forest zone of Eurasia). Nauka, Ekaterinburg
Mehra RC, Rai KS (1970) Cytogenetic studies of meiotic abnormalities in Collinsa tinctoria. I. Chromosomal stikness. Can J Genet Cytol 12(4):560–569
Mergen F, Lester DT (1961) Microsporogenesis in Abies. Silvae Genet 10(5):146–156
Mok DWS, Peloquin SJ (1975) Three mechanisms of 2n pollen formation in diploid potatoes. Can J Genet Cytol 17(2):217–225
Muratova EN (1995) Specific features of meiosis in Scots pine near the northern boundary of its range. Russ J Dev Biol (Ontogenez) 26(2):128–139
Muratova EN, Matveeva MV (1996) Caryological features of the Siberian Fir under different conditions of growing. Russ J of Ecol (Ekologia) 2:96–102
Muraya LS, Butorina AK, Dudetskaya EM (1988) Meiosis during microsporogenesis and development of male gametophyte in the Chinese Douglas fir upon introduction. Russ For Sci (Lesovedenie) 5:37–44
Nekrasov VI (1971) Some theoretical problems of the formation of introduced populations of forest trees. Russ For Sci (Lesovedenie) 5:26–30
Nekrasova TP, Ryabinkov AP (1978) Plodonoshenie pychty sibirscoi (Abies sibirica seed production). Nauka, Novosibirsk
Owens JN, Molder M (1985) The reproductive cycles of true firs. Information Services Branch Ministry of Forests, Victoria, British Columbia, 35 pp
Pozhidaeva IM, Butorina AK, YuN Isakov (1985) Defects of meiosis during microsporogenesis and level of fertility in the Scots pine. In: Nekrasova TP (ed) Polovoe razmnozhenie khvoinykh rastenii (Sexual reproduction of coniferous plants). Nauka, Novosibirsk, p 31
Rozhdestvenskii YuF (1974) Specific features of microsporogenesis in the Scots pine in the Urals and its dependence on ecological factors. Russ J of Ecol (Ekologia) 1:49–53
Shkutina FM (1975) Meiosis in remote hybrids and amphiploids. In: Khvostova VV, Bogdanov YuF (eds) Tsitologiya i genetika meioza (Cytology and Genetics of Meiosis). Nauka, Moscow, pp 263–283
Sosnikhina SP, Fedotova YuS, Smirnov VG et al (1994) A study of genetic control of meiosis in the rye. Russ J Genet (Genet) 30(8):1043–1056
StatSoft, Inc. STATISTICA (2001) Data analysis software system, version 6. http://www.statsoft.com
Tretyakova IN, Bazhina EV (2000) Structure of crown as well as pollen and seed viability of fir (Abies sibirica Ledeb.) in disturbed forest ecosystems of the Khamar-Daban Mts. Ecology Bratislava 19(3):280–294
Varpholomeev IV, Maltsev YuM (eds) (2006) Gosudarstvennii doklad O sostoyanii i ohrane okruzhajuscheii sredii v Krasnoyarskom kraye v 2006 (State report about environmental status and protection in Krasnoyarsk region in 2006). Priroda, Krasnoyarsk
Veileux RE, McHale NA, Lauer FI (1982) 2n gametes in diploid solanum: frequency and types of spindle abnormalities. Can J Genet Cytol 24(3):301–314
Acknowledgments
This research was supported by the Russian Foundation of Basic Research-Krasnoyarsk Region Foundation of Science Support (grants No 09-04-98000, r_sibir_a).
The authors are grateful to the editor and two anonymous reviewers who provided valuable comments on the manuscript as well as the organisers of the EUROGARD V conference for linguistic improvements.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bazhina, E.V., Kvitko, O.V. & Muratova, E.N. Specific features of meiosis in the Siberian Fir (Abies sibirica) in the forest Arboretum of the V. N. Sukachev Institute, Russia. Biodivers Conserv 20, 415–428 (2011). https://doi.org/10.1007/s10531-010-9958-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10531-010-9958-y