Abstract
The present study deals with the cytogenetics of Aponogeton bruggenii S.R.Yadav and Govekar (Aponogetonaceae) sporting asexual propagation. Mitotic metaphase in the root-tips revealed that the species is a polyploid with 2n = 56 chromosomes. Male meiosis was mostly abnormal. A total of 2023 pollen mother cells were observed, of which 448 showed normal and the remaining 1575 abnormal meiosis. Mostly, the stickiness and precocious separation was recorded at diakinesis and metaphase (I and II), respectively. Chromatin bridges, laggards, disorientation, ring formation were detected at various stages of meiosis ranging from diakinesis to telophase. Consequent to chromatin transfer, heterogeneously sized pollen grains, i.e. small, normal, large and fused were observed, hampering gametic efficiency and fruit set. However, the species resorts to vegetative propagation by means of underground tubers, possibly as a survival strategy to sustain dispersion and speciation.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aponogeton L.f. (Aponogetonaceae) is a genus of aquatic plants which is distributed in the tropical and subtropical regions of the Old World [19]. Plants usually bear tubers except A. rigidifolius H.Bruggen which is rhizomatous [12]. The genus consists of 60 species, of which ten species occur in India [2]. Most of the species are bisexual but there are five dioecious species (A. decaryi Jum. ex Humbert, A. dioecus Bosser, A. nudiflorus Peter, A. satarensis Sundararagh., A.R.Kulk. & S.R.Yadav and A. troupinii J.Raynal) [19].
Karyological studies on the family are far from complete. So far chromosome numbers are known for only 16 species [15]. It has been observed that the small size of the chromosomes (0.5–2.5 μm long) and their strong tendency to stick together makes it very difficult to analyse even the best metaphases [20]. The chromosome number ranges from 2n = 16 (A. distachyos and A. madagascariensis) to 2n = 126 (A. junceus Lehm.) (modified after Chougule et al. [3]). A base number of x = 8 has been suggested for the genus [20]. Aneuploidy and infraspecific polyploidy are commonly observed in the genus [20]. The polyploidy phenomenon is important in plant evolution and also it is considered as an important biodiversity engine that promotes speciation, adaptation and expansion of range. The species exhibiting polyploidy are ecologically more tolerant than their progenitor species [18].
Aponogeton bruggenii S.R.Yadav & Govekar is an endemic species of the Western Ghats, India that grows in the paddy fields along the Tarkarli river at Nerurpar in Sindhudurg district, Maharashtra [23]. As a part of our ongoing studies on Aponogeton [2,3,4] we observed that although A. bruggenii exhibited abundant flowering in nature, the fruit set was quite low. Nevertheless, there was no decline in the population of this species and plants propagated vegetatively. Therefore, we studied the cytogenetics of this species in order to analyse the meiotic course, microsporogenesis and pollen viability. In the present study the absence of fruit/seed set correlated to irregular synapsis leading to abnormal meiosis and heteromorphic pollen grains and low genetic diversity on account of small population size.
Materials and methods
Plant collection: Aponogeton bruggenii was collected from its type locality (Nerurpar in Sindhudurg district, Maharashtra) (16°01′ 08.0″ N, 73° 37′ 32.4″ E, 32 m msl) and maintained in the Botanical Garden of the Department of Botany, Shivaji University, Kolhapur, Maharashtra, India. Voucher specimens (RNC 232, RNC 245, RNC 257) were deposited in the Herbarium of the Department of Botany (SUK), Shivaji University, Kolhapur. Observations on morphology and ecology were made in the field.
Fruit set: For determining fruit set, plants (N = 20) growing in the collection locality were randomly selected. The values were presented as mean ± standard error. Morphological features such as inflorescence per plant and number of flowers per inflorescence were recorded by observing live plants. The percentage fruit set was calculated by using the following formula:
Cytogenetics and pollen viability: The methodology for preparation of meiotic and mitotic chromosomes is provided elsewhere [2]. Freshly prepared slides were examined to determine the chromosome number and meiotic abnormalities at different stages using the LEICA DM750 microscope. A total of 2023 Pollen mother cells (PMCs) were observed. Pollen viability was estimated through stainability using glyceroaceto-carmine (1:1) mixture. A total of 1110 pollen grains were observed. Stained pollens were taken as fertile while shrivelled and unstained pollens sterile. For calculating the diameter, pollen grains (N = 15) were measured and the values presented as mean ± standard error. Suitable mitotic cells and PMCs at different stages were photographed from freshly prepared slides with LEICA DM 750 microscope with attached camera at 1000 × and photo plates were prepared by using Adobe Photoshop 7.0 software.
Results
Plant morphology and ecology
Aponogeton bruggenii is a tuberous plant and grows as a weed in rice fields (Fig. 1a). Each individual produces a single spike with dark pink flowers (Fig. 1b–d). The species starts growing with the onset of monsoon and as the monsoon recedes, the above-ground leaves wither and the plant undergo dormancy till the next monsoon. However, by the end of each growing season, a new tuber is added to the previous one. Consequently, a chain of tubers is formed (Fig. 1g).
Vegetative propagation in Aponogeton bruggenii by means of tubers. a, b habit, c, d inflorescence showing anther dehiscence (arrowheads), e abortive inflorescence, f infructescence showing some follicles (arrowheads), g formation of new plantlet on tuber (arrow), note chain of tubers. Scale bars = 1 cm
Fruit set
The average number of inflorescences per plant was 1.1 ± 0.07 whereas the average flowers per inflorescence 47.95 ± 2.56. Fruit set was observed in only six individuals and hence the fruit set 4.37% (Fig. 1e, f).
Cytogenetics
The root-tip mitosis revealed the presence of 2n = 56 chromosomes. Karyotype characterization does not fall under the scope of the present work and hence not dealt in herewith.
The meiotic course of A. bruggenii was observed and diakinesis revealed the presence of n = 28 bivalents. Most of the PMCs exhibited abnormal meiosis. Observation of 2023 PMCs revealed that only 448 (22.15%) underwent normal meiosis whereas the remaining 1575 (77.85%) exhibited anomalies. Anomalies, namely chromosome bridges (single/multiple), disorientation, laggards, ring formation, stickiness and precocious separation were recorded. Stickiness contributed to 89.72% of the anomalies at diakinesis whereas precocious separation was responsible for 67.05%, 68.52% and 56.67% of the anomalies at metaphase I, metaphase II and meta-anaphase II, respectively. Laggards dominated anaphase I (38.55%), anaphase II (38.85%), telophase I (45%) and telophase II (52.83%). At anaphase II, single bridge and laggards were the most common and observed in 43.88% of PMCs. The phase-wise occurrence of various meiotic anomalies is listed in Table 1 and depicted in Figs. 2 and 3. Despite considerable meiotic anomalies, high pollen viability (88.83%) was observed. Pollen grains of heterogeneous size (diameter) were observed. Based on diameter, pollen grains were categorized as small (14.48 ± 0.76 µm), normal (23.13 ± 0.51 µm), large (30.47 ± 0.72 µm) and fused (35.43 ± 3.86 µm) (Figs. 3 z-bb). Normal pollen grains were the most common (77.30%) followed by the small ones (11.08%). Large and fused pollen grains were represented by 8.74% and 2.88%, respectively. Small pollen grains were completely sterile whereas normal, large and fused pollen grains were fertile.
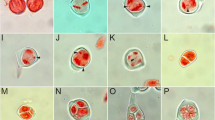
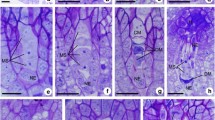
Somatic chromosomes and meiotic abnormalities in Aponogeton bruggenii. a Mitotic metaphase chromosomes (2n = 56), b-e PMCs in prophase I: b PMC at leptotene, c PMC at zygotene, d PMC at pachytene, e PMC at diplotene, f PMC showing n = 28 bivalents at diakinesis, g PMC showing chromatin material arranged in S shape at diakinesis, h PMC showing stickiness and typical ring formation (arrow) at diakinesis, i PMC showing ring formation (arrowhead) and extra chromatin material (arrow) at diakinesis, j PMC showing inter bivalent connections (arrow) at diakinesis, k PMC showing stickiness (arrow) at diakinesis, l PMC at normal metaphase-I, m PMC showing stickiness at metaphase-I, n, o, p PMCs showing precocious separations (arrow) at metaphase-I, q PMC showing ring formation at metaphase-I, r, s PMCs showing disorientation of chromosomes at anaphase-I, t PMC at normal anaphase-I, u PMC showing stickiness and precocious separation (arrow) at anaphase-I, v PMC showing laggards (arrowhead) and precocious separation (arrow) at anaphase-I, w PMC showing laggards (arrowhead) at anaphase-I, x PMC showing single bridge (arrow) at anaphase-I, y PMC showing single bridge (arrow), laggards (arrowhead) and precocious separations (encircled) at anaphase-I, z PMC showing multiple bridge (arrow) at anaphase-I, aa PMC showing multiple bridges (arrow), laggards (arrowhead) and precocious separations (encircled) at anaphase-I, bb PMC showing 14:14 chromosomes at anaphase-I. Scale bars = 5 μm
Meiotic abnormalities in A. bruggenii. a PMC at normal telophase-I, b PMC showing laggards (arrowhead) at telophase-I, c PMC showing single bridge (arrow) at telophase-I, d PMC showing single bridge (arrow) and laggard (arrowhead) at telophase-I, e PMC at normal metaphase-II, f PMC showing precocious separations (arrow) at metaphase-II, g PMC showing bridge (arrow) at metaphase-II, h PMC showing bridge (arrowhead) and precocious separations (arrow) at metaphase-II, i PMC showing stickiness at metaphase-II, j PMC showing ring (encircled) at metaphase-II, k PMC at meta-anaphase-II, l PMC showing bridge (arrowhead) and precocious separations (arrow) at meta-anaphase-II, m PMC at normal anaphase-II, n PMC showing bridge (arrow), laggards (arrowhead) and precocious separations (encircled) at anaphase-II, o, p, q PMCs showing laggards (arrowhead) and bridge (arrow) at anaphase-II, r PMC at normal telophase-II, s, t PMC showing laggard (arrowhead) at telophase-II, u PMC showing single bridge (arrow) at telophase-II, v PMC showing single bridge (arrow) and laggards (arrowhead), w PMC showing multiple bridges (arrow) and laggards (arrowhead) at telophase-II, x triad, y tetrad, z heterogeneous sized pollen grains, aa normal, large sized fertile pollen and small sized sterile pollen, bb fused fertile pollen grains (arrowhead). Scale bars = 5 μm
Discussion
Plant morphology and ecology
Aponogeton bruggenii is an endemic species of Maharashtra state [14]. It has been placed under the Vulnerable category of IUCN red list [21]. It is so far known from only two localities in Sindhudurg district, i.e. Nerurpar, Kudal taluka (type locality) and Satose village, Sawantwadi taluka [6]. Both the localities have been considered as one location [21]. Also, the estimated area of occupancy (AOO) and the estimated extent of occurrence (EOO) for the species are 3 and 25 km2, respectively [21] which indicates small population of the species.
The species grows in the rice fields and since rice cultivation and growing season of the species coincide, the underground tubers get split (during field preparation/ploughing and rice plantation) and start serving as a new plant.
Fruit set
In general, Aponogeton species have features that indicate pollination by tiny insects [19]. Also, the presence of spinules on the pollen suggest aerial pollination by insects [19]. Aponogeton bruggenii bears a pink-coloured spike. The exine of the pollen is microreticulate and microechinate [16]. Although no pollination study is on record, aforementioned features indicate insect pollination syndrome. Meiotic anomalies leading to failure of sexual reproduction account for the low fruit set. Also, small population and multiplication by means of vegetative propagation (through tubers) seem to be responsible for the low genetic diversity that severely impact subsequent fruit set.
Cytogenetics and pollen viability
Chromosomal pairing during meiosis is crucial to the understanding genome homology, particularly in polyploids and interspecific hybrids (homoploid and alloploid) as it provides information on the reproductive success of these species. In the present study, the meiotic course in the meiocytes of A. bruggenii was examined in detail for the first time. The meiotic course was found to be abnormal. Anomalous behaviour observed in the meiocytes varied across the stages of meiosis. In general chromosome stickiness dominated the prophase of meiosis I (diplotene and diakinesis) and continued to later stages such as metaphase II, anaphase II. Kaur and Singhal [7] observed frequently chromosome stickiness in metaphase I of many dicot plants from Indian cold deserts. The precocious separation, bridges and laggards were more prominent in the later stages such as I and II metaphase, anaphase and telophase. Similar observations were made by Kumar et al. [9] in Clematis flammula L. Kumar et al. [9] also reported chromatin bridges in anaphase and telophase. In the present study we also observed the single or multiple chromatin bridges in metaphase and anaphase I/II. Chromosome stickiness was responsible for the disorientation of chromosomes, delayed separation of bivalents at anaphase I and II (laggards) and chromosomal bridges. Both genetic and environmental factors are responsible for chromosome stickiness, see Mendes-Bonato et al. [13]. It is difficult to ascertain the cause of chromosome stickiness in A. bruggenii at this point of time. At diakinesis bivalents (synapsed homologues) exhibited fuzzier appearance and some were seen falling apart indicating irregular synapsis. This kind of chromosomal behaviour at diakinesis is characteristic of synaptic mutants [8]. Synaptic mutants have not been reported so far in Aponogetonaceae, see Koduru and Rao [8], but the occurrence of this phenomenon cannot be ruled out in the family. Synaptic mutations leading to asynapsis or desynapsis are common in apomicts [8]. Aponogeton decaryi, a Madagascan species is a well-known apomict [22]. Megaspore mother cell (MMC) exhibited asynapsis during first meiotic division [22]. Yadav [22] studied the megasporogenesis in this species and reported the species as gametophytic apomict exhibiting diplospory. However, male meiosis has not been studied in this species so far as male plants are very few in numbers as compared to females [19].
Nonsynchronous early disjunction of bivalents or precocious separation was mainly observed at metaphase I, II and meta-anaphase II. Precocious separation at metaphase can be accounted for by precocious terminalization of chiasma at diakinesis [17]. Also, structural differences in homologous chromosomes can lead to precocious movement of chromosomes [1]. Structural differences in the homologous chromosomes may arise as a result of some mutation or in case of interspecific hybridization which brings two different genomes together. The late disjunction of bivalents on account of stickiness was responsible for lagging chromosomes and chromatin bridges. Chromatin bridges (1–3) were responsible for chromatin transfer which resulted in heterogeneous sized pollen grains. The large pollen grains (30.47 ± 0.72 µm) were double the size of the smaller ones (14.48 ± 0.76 µm). The size of the normal pollen grains (23.13 ± 0.51 µm) was intermediate between large and small grains whereas fused pollen grains (35.43 ± 3.86 µm) were even bigger than the large ones. Smaller pollen grains might be the product of hypoploid PMCs and larger ones hyperploid PMCs. The double-sized large pollen grains are in fact unreduced (2n) gametes and can act to produce a polyploid population. Fusion of two pollen grains by the formation of cytoplasmic channels was also observed, however the percentage was quite low (2.88%). Pollen grains of heterogeneous size have also been observed in Clematis flammula [9], Echinodorus palaefolius (Nees & Mart.) J.F.Macbr. [11], Clematis graveolens Hook. [7], etc. Stress conditions like low temperature may lead to fusion of PMCs and pollen grains [7].
Anomalous meiotic course in A. bruggenii did not have much effect on the pollen viability. High pollen viability in the presence of erratic meiosis has also been observed in Ledebouria revoluta (L.f.) Jessop population collected from Ratnagiri district, Maharashtra, India (pers. obs.). These bulbous plants exhibited an odd chromosome count in the root-tip cells, i.e. 2n = 45 but the pollen viability (based on staining by 1% acetocarmine) was very high (78.14%). Fruit set is almost absent and the leaf-tips produce bulbils which later on get detached and become an individual plant. The glyceroaceto-carmine (1:1) pollen staining is not very specific and hence cannot be the absolute measure of pollen fertility. Pollen germination test (in vivo and in vitro) should be performed to assess the actual fertility. Also, it is possible that bulbous/tuberous or geophytic monocots have resorted to vegetative propagation to circumvent the ill-effects of abnormal meiosis. Moreover, maintaining high pollen viability enables them to participate in hybridization events (wherever and whenever different populations of a species overlap) in nature and produce a gamut of cytotypes/species adapted to varied ecological niches. Hence, high pollen viability despite anomalous meiosis could be advantageous to generate genetic diversity during the course of evolution. Contrastingly, vegetative propagation in A. bruggenii reduced the genetic diversity of the small population by ensuring multiplication. Consequently, the fruit set is adversely affected.
Conclusion
Habitat divergence and environmental stress may impose meiotic and mitotic errors in germline and somatic tissues, enabling the establishment of new genomic states facilitating fixation of reproductively isolated lineages [10]. This is consistent with the evolutionary events of genome duplication encountered at the end of the Cretaceous as a surviving escape strategy [5]. Accordingly, an escape route to asexual reproduction seems to be in order to overcome the limitation of sexual inefficiency in the endemic zone where species is thought to be in evolutionary flux.
A. bruggenii is a polyploid species with 2n = 56 chromosomes. Male meiosis is abnormal on account of irregular synapsis and consequently many anomalies (chromosome bridges, disorientation, laggards, ring formation, stickiness and precocious separation of chromosomes, etc.) and pollen grains of heterogeneous size are observed. Erratic meiosis along with low genetic diversity hinders fruit set. Vegetative propagation in this species seems to be a survival escape route to overcome sexual inefficiency.
Data availability
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.
References
Anis M, Wani AA. Caffeine induced morpho-cytological variability in Fenugreek, Trigonella foenum-graecum L. Cytologia. 1997;62(4):343–9.
Chougule RN, Surveswaran S, George A, Yadav SR, Lekhak MM. Roxburgh was right: Aponogeton microphyllus and Aponogeton undulatus are distinct species. J Biosci. 2023;48:53.
Chougule RN, Surveswaran S, Yadav SR, Lekhak MM. Medicinal potential of the Cape-pondweed family (Aponogetonaceae): a review. S Afr J Bot. 2022;149:994–1007.
Chougule RN, Yadav SR, Lekhak MM. Leaf anatomical studies in Indian Aponogetonaceae. Phytomorphology. 2022;72(3&4):41–54.
Fawcett JA, Maere S, Van de Peer Y. Plants with double genomes might have had a better chance to survive the Cretaceous–Tertiary extinction event. Proc Natl Acad Sci USA. 2009;106(14):5737–42.
Gaikwad SP, Sardesai MM, Yadav SR. Aquatic flowering plants diversity of coastal plains of Konkan, Goa and Karnataka. In: Pullaiah T, editor. Biodiversity in India, vol. 4. New Delhi: Regency Publications; 2002. pp. 89–200.
Kaur D, Singhal VK. Meiotic abnormalities affect genetic constitution and pollen viability in dicots from Indian cold deserts. BMC Plant Biol. 2019;19(1):10.
Koduru PRK, Rao MK. Cytogenetics of synaptic mutants in higher plants. Theor Appl Genet. 1981;59(4):197–214.
Kumar P, Singhal VK, Kaur J. Cytomixis induced meiotic abnormalities in Pollen mother cells of Clematis flammula L. (Ranunculaceae). Cytologia. 2008;73(4):381–5.
Lavania UC. Plant speciation and polyploidy: in habitat divergence and environmental perspective. Nucleus. 2020;63(1):1–5.
Lekhak MM, Yadav SR. Anomalous male meiosis in an aquarium plant, Echinodorus palaefolius (Alismataceae). Nucleus. 2016;59(3):207–10.
Manawaduge CG, Yakandawala D. Morphometric and taxonomic update to the Sri Lankan Aponogetonaceae. Phytotaxa. 2018;365(3):201–24.
Mendes-Bonato AB, Pagliarini MS, do Valle CB, Penteado MIO. A severe case of chromosome stickiness in pollen mother cells of Brachiaria brizantha (Hochst) Stapf (Gramineae). Cytologia. 2001;66(3):287–91.
Nayar TS, Rasiya Beegam A, Sibi M. Flowering plants of the Western Ghats, India. Vol. 2 Monocots. Thiruvananthapuram: Jawaharlal Nehru Tropical Botanic Garden and Research Institute;2014. p. 944.
Rice A, Glick L, Abadi S, Einhorn M, Kopelman NM, Salman-Minkov A, Mayzel J, Chay O, Mayrose I. The Chromosome Counts Database (CCDB)—a community resource of plant chromosome numbers. New Phytol. 2015;206(1):19–26.
Severova EE, Yadav SR, Sokoloff DD. Pollen morphology of Indian Aponogeton (Aponogetonaceae, Alismatales) and the problem of recognizing palynotypes in a taxonomically diverse and ancient genus. Phytotaxa. 2020;475(3):187–200.
Srivastava A, Kapoor K. Seed yield is not impaired by chromosome stickiness in sodium azide treated Trigonella foenum-graecum. Cytologia. 2008;73(2):115–21.
Tantray YR, Singhal VK, Gupta RC. Deciphering the meiotic behaviour in species of genus Artemisia from cold deserts of Ladakh (Jammu and Kashmir). Flora. 2020;262: 151520.
van Bruggen HWE. Monograph of the genus Aponogeton (Aponogetonaceae). Bibl Bot. 1985;33:1–76.
van Bruggen HWE. Aponogetonaceae. In: Kubitzki K, editor. The families and genera of vascular plants, vol. 4. Berlin: Springer-Verlag; 1998. pp. 21–5.
Watve A. Aponogeton bruggenii. The IUCN Red List of Threatened Species 2011: e.T177177A7384396. https://doi.org/10.2305/IUCN.UK.2011-1.RLTS.T177177A7384396.en. Accessed 20 Jan 2024.
Yadav SR. Mechanism of apomixis in Aponogeton decaryi jumelle. Phytomorphology. 1993;43(3&4):201–7.
Yadav SR, Govekar RS. Aponogeton bruggenii (Aponogetonaceae), a new species from India. Rheedea. 1994;4(1):34–6.
Acknowledgements
The authors are thankful to the Head, Department of Botany, Shivaji University, Kolhapur (SUK) for providing the necessary research facilities. RNC thanks DBT, Govt. of India, New Delhi for financial support. SRY is thankful to University Grants Commission (UGC), New Delhi and Indian National Science Academy (INSA), New Delhi for the award of fellowship.
Author information
Authors and Affiliations
Contributions
RNC: Chromosome preparations, writing original draft and preparation of figures. SRY: Conceptualization, review and editing. MML: Conceptualization, writing original draft, review and editing.
Corresponding author
Ethics declarations
Conflict of interest
Authors have no conflict of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Corresponding Editor: Umesh C. Lavania: Reviewers: Ahmet L. Tek, Geeta Sharma.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chougule, R.N., Yadav, S.R. & Lekhak, M.M. Asexual propagation could be an escape to sustain the constraints of inefficient gametic system in the evolving endemic species: evidence from meiotic anomalies encountered in Aponogeton bruggenii (Aponogetonaceae). Nucleus (2024). https://doi.org/10.1007/s13237-024-00469-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13237-024-00469-3