Abstract
Arthropods have been regarded as good indicators of habitat quality due to their sensitivity to changes in habitat state. However, there are many constraints to working with arthropods that make them inaccessible to land managers and most volunteer-driven initiatives. Our study examined a novel approach for detecting changes in web-building spider communities by focussing on the types of webs that spiders build rather than the spider itself. This method was cost-effective, easy-to-use, and importantly, we found a strong congruency between the diversity of web architecture and the diversity of web-building spider genera. The metrics derived from this method could distinguish differences in web-building communities among habitat types that represented a successional gradient, and thus we concluded that the method was useful for monitoring the progress of restoration. Many other applications for the method are possible such as environmental impact assessment and agricultural pest management, and we encourage development in these areas.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Assemblages of terrestrial arthropods have been reported as useful indicators of ecological change and habitat quality (Rosenberg et al. 1986; Kremen et al. 1993). However, there are many difficulties involved in their practical use in environmental monitoring and assessment programmes (Curtis 1981; Hodkinson and Jackson 2005). Indeed, the use of arthropods as indicators has been referred to as more a topic of ‘scientific discourse than a part of land management practice’ (Andersen et al. 2002). In particular, problems stem from two interrelated factors. The first is the reality that the process of arthropod identification is time consuming and therefore expensive. Typically, arthropod surveys require lengthy laboratory sorting using high-powered microscopes, pinning or mounting of specimens, and identification and curation. The second factor is the decline of taxonomy as a science. There is limited specialist expertise available for the identification of most taxa (Hopkins and Freckleton 2002) and most groups contain significant proportions of species for which there are no means of identification (e.g. identification keys, Gotelli 2004). The sheer volume of material that can be collected intensifies problems due to both of these issues.
Various strategies have been employed to minimise the problems associated with arthropod diversity. These have included the use of: bioindicators—where one or a few taxa are used to represent the status of others (e.g. Cardoso et al. 2004a); environmental attributes as surrogates; morphospecies sorting; functional groups (e.g. Oliver and Beattie 1996; Takada et al. 2008; Gollan et al. 2009), and higher taxa (e.g. Mandelik et al. 2007).
Higher taxa as surrogates can be particularly useful for groups that are difficult to identify to species level. For instance, in a typical field survey, the majority of spiders collected cannot be identified to species because they are juveniles (Brennan et al. 2004). While higher-taxon surrogates of spiders using genus or family level have shown congruency with species (Cardoso et al. 2004b), identification by trained personnel, lengthy laboratory, time and technical equipment (e.g. microscopes) are needed. Furthermore, this type of surrogacy does not necessarily reduce sampling effort and employs lethal indiscriminate sampling methods that are considered counterproductive to the goal of conserving and indeed, restoring biodiversity (Bowie and Frampton 2004).
In this study, we developed a method for assessing web-building spider communities based solely on characteristics of web architecture. As a substitute for collecting spider specimens, the aim was to minimise the need for laboratory time, expert involvement and technical equipment. We then used the substitute to examine the response of web-building spiders to the changes in habitats caused by native revegetation. We sought to determine if native revegetation has been successful for re-establishing web-building spider communities by comparing web assemblages in three types of habitat: (1) unplanted grassland (acting as procedural controls), (2) revegetated habitat, and (3) uncleared remnant forests (acting as references).
Methods
Study area
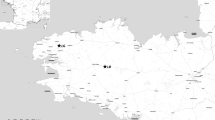
Our study was conducted within the Hunter Catchment of New South Wales, Australia, which covers an area of approximately 22,000 km2 (Fig. 1 inset).
The catchment can be divided into 8 botanical landscapes, each of which supports broadly similar vegetation formations within their boundaries (Peake 2003). We included only 3 types in our study, namely ‘Floodplain open woodland and grassland’, ‘Forests and woodlands of the valley lowlands’, and ‘Forests of dissected sandstone plateaux and sub-coastal sandstone hills’ (sensu Peake 2003). We restricted our study to these three botanical landscapes because they represent the majority of the total catchment (~80%) and have been the target for planting programs over the past two decades. Plant species of the former two landscapes are primarily Eucalyptus spp., Casuarina spp., while E. moluccana, E. albens, E. dawsonii, Corymbia maculata, and Allocasuarina luehmannii dominate the latter.
Web-types as a substitute for web-building spider communities
The substitute for measuring web-building spider communities was developed over 3 years (between 2004 and 2007) prior to the main survey of this study (see below). The aim was to produce a methodology for assessing web diversity of aerial web-building spiders. While web forms are rarely species specific over a large geographic area, many are characteristic for a genus or species in a regional context. We considered that focussing on these differences in web architecture should produce a useable, if slightly approximate, method for estimating spider diversity.
In the initial stages of developing the web key, diagrams and general information on different web-forms were collated from Main (1976), and a preliminary version was produced. Following some field testing at 12 sites within the study region (Fig. 1), further web-types were added and refinements were made to existing definitions of types. Spiders were also collected from all webs found within 10 strip transects (~10 × 2 × 1.8 m high) at each of the 12 test-sites to assess the correlation between spider richness and web richness. An author (HMS) identified specimens to species level if possible. Many specimens could not be identified to species since a large proportion were juveniles (58%), but even spiderlings could be identified to genus by a combination of comparison with adult and intermediate instars and collecting and hatching egg sacs.
Terminology was checked and improvements on the general set-out of the web identification guide were made following advice from other arachnologists (see Acknowledgments), and the usability of the key for non specialists was improved by incorporating feedback from trials by people who were not familiar with spider biology or using scientific keys. Generally, these people were volunteers from local conservation groups, as well as private landholders. The final version of the web diversity guide, called Web2Spider, can be found on the Australian Museum website (Smith et al. 2008) and as supplementary material (S1).
Survey design and analyses for examining the response of web-building spiders to native revegetation
To test the response of web-building spiders to native revegetation, two of the authors (JRG and MB) used the web identification guide to collect data in three types of habitat. All revegetated plots that we used exceeded 7 years of age with canopy species between 5 and 10 m in height. The study region also encompassed land set-aside for conservation purposes in the form of national parks, state forests and wildlife refuges, and these areas were used as reference communities. So that any changes observed in web-building spider communities could be attributed to the plantings themselves, we also included previously cleared, but unplanted grassland areas. These are considered low in terms of conservation values and are typical of areas that are targeted in restoration attempts (pers. obs.).
Five locations (100 × 100 m), which were different from those used for the field-testing of the web identification guide, were chosen for sampling throughout the catchment. Locations were chosen so that treatments were not clustered (Fig. 1), thus avoiding a spatially confounded experimental design (Hulbert 1984). Within each location, we searched for webs within five haphazardly positioned strip transects (~10 × 2 × 1.8 m high). A pressurised hand-held water mister was used to aid location of webs. When a web was exposed, the web was identified using the web identification guide (as described above) and assigned to one of 32 web-types. We note that 33 web-types are included in the final Web2Spider guide (Smith et al. 2008) because one web type, not found in early trials, was added after this work was carried out. Surveys were carried out during daylight hours, over a 6 day period (between 2 and 7 May 2008), and the order in which locations and habitat types were surveyed was random. No rain fell during the 6 days.
Web richness (the number of different web types), abundance and web composition were analysed using a fully nested analysis of variance, and the assumption of homogeneity of variances was tested using Cochran’s Test (Winer et al. 1991). Web composition was analysed using a permutational analysis of variance using the software PERMANOVA V1.6. Analyses were performed on Bray-Curtis dissimilarity distances calculated from the raw data. A two-dimensional ordination using non-metric multidimensional scaling and the average of the five locations of each habitat type was used to illustrate differences among habitat types and locations.
Characteristic web-types were identified for each habitat type using the Indicator value (Indval) method and a random reallocation procedure was used to test the significance of the Indval measures for each web-type (Dufrêne and Legendre 1997). Web-types with significant Indvals >70%, a subjective benchmark following McGeoch et al. (2002), were regarded as characteristic for the habitat type in question. The randomisation testing procedure was conducted using the freeware programme, INDVAL V.2, using 999 permutations. Frequency histograms of web types within each location were used to display the distribution of webs among locations and within each habitat type.
Results
Web-types as a substitute for measuring web-building spider communities
Spider generic richness (the number of different genera) correlated strongly with web richness (R2 = 0.83; Fig. 2). Cross-checking spider specimens and web-types confirmed that this was not a perfect correlation. Some genera produced more than one web-type, which led to a tendency to overestimate generic richness. This was often the result of juveniles building a different web-type than adults, or distinctive web characteristics that were usually but not always constructed by a species (e.g. web decorations). In other cases it was found that a single web-type could be produced by more than one genus, which led to a tendency to underestimate generic richness (see Table 1).
Response of web-building spiders to native revegetation
A total of 2,373 webs were counted over the 6 day period. Mean (±SE) web abundance ranged from a low of 4.0 (±0.8) in unplanted grassland to a high of 59.0 (±3.2) in reference locations. Mean web abundance was intermediate in revegetated habitat (31.4 ± 2.7). While there was significant variability in mean web abundance among locations of the same habitat type (F12,60 = 2.1, P < 0.05), there were also significant differences among the three habitat types (F2,12 = 68.7, P < 0.0001). Post hoc tests showed that mean web abundance was significantly higher in reference locations compared to revegetated habitat, and mean web abundance was significantly higher in revegetated habitat than in unplanted grassland (Fig. 3 top).
Twenty-nine different web-types were found. Mean (±SE) web richness ranged from a low of 2.2 (±0.4) in unplanted grassland to a high of 11.8 (±0.4) in reference locations. Mean web richness was intermediate in revegetated habitat (7.2 ± 0.4). While there was significant variability in mean web richness among locations (F12,60 = 2.4, P < 0.05), there was also a significant difference among the three habitat types (F2,12 = 82.5, P < 0.0001). Post hoc tests showed that mean web richness was significantly higher in reference locations compared to revegetated habitat, and mean web richness was significantly higher in revegetated habitat than in unplanted grassland (Fig. 3 bottom).
For web composition, there was significant variability among locations of the same habitat type (F12,60 = 3.5, P < 0.01), and significant differences among the three habitat types (F2,12 = 5.8, P < 0.01). Pair-wise post hoc comparisons of web composition showed significant differences among all three habitat types. The ordination plot displayed the patterns in web composition, which showed good discrimination of reference locations. Web composition in revegetated locations formed a distinct cluster between unplanted and reference locations. This pattern indicated that web composition in revegetated habitat was intermediate of unplanted grassland and reference habitat (Fig. 4).
Non-metric multidimensional plot of web-type composition. Non-transformed abundances and Bray-Curtis dissimilarity values using the mean (n = 5) of each of five locations in unplanted, revegetated and reference habitat were used. Ellipses have been added to highlight locations of the same habitat type
Indicator value (Indval) analysis (Fig. 5) and frequency histograms (Fig. 6) revealed that eight web-types (W10, W14, W17, W25, W26, W27, W28, W29) were characteristic of reference habitat i.e. these types were found in high abundance and mostly in reference habitat. One of those web-types (W29) had a perfect (100) and significant Indval in reference habitat. Frequency histograms across all locations and habitat types also showed that three web-types (W18, W19, W32) were common to most locations and were not characteristic of any particular habitat type. This was reflected by low and non-significant Indvals for these three web-types (46, 53 and 52, respectively). Twenty-two of the 29 web-types reached maximum Indvals in reference habitat, while only five and one, reached maximums in revegetated and unplanted grassland habitat, respectively.
Discussion
A common practice in vertebrate monitoring and research is the use of animal signs—such as tracks or scats—for the census of unseen fauna (Moller et al. 1997; Chame 2003; Glen and Dickman 2003; Birks et al. 2005). In contrast, arthropod signs have rarely been exploited. The issue of scale, of course, makes it impossible or rather impractical to identify scats or footprints of arthropods. However, as we, and others have proposed (e.g. Barros 2001; Chame 2003; Schlick-Steiner et al. 2006), arthropods do produce other signs that are measurable and are useful for detecting trends.
The architecture of webs is fairly well conserved across web-building taxa (Blackledge 1998; Herberstein et al. 2000). This is reflected in our findings that the relationship between generic richness and web richness was strong and positive. Therefore, we consider this approach to be a good substitute for assessing web-building spider communities at a generic level at least. We must stress that the web identification guide may need to be modified for other habitat types or other regions because the relationship we found may not translate to all ecosystems. In areas of high generic richness for instance, the relationship with web richness is likely to weaken because species rich areas can reduce the resolution of generic surrogates (Lovell et al. 2007). Nonetheless, we encourage others to investigate the applicability of this type of substitute to their region of interest.
In further development of this substitute the use of night surveys could also be considered. Night surveys have the benefit of finding the webs of nocturnal species that might be removed during the day, but may not find all day-active species. We chose not to conduct night surveys for reasons that included consideration of safety (especially when volunteers were involved) and some logistical difficulties involved in working at night in remote areas. As a consequence, our method has been tailored to pick up day-time web diversity. Night surveys would find orb webs belonging to genera such as Deliochus, which is strictly nocturnal, and more webs of Tetragnatha and araneid genera such as Eriophora and Araneus. During the day these webs may be left in place but they often degrade quickly. In an exposed, windy situation therefore, night surveys would be of benefit. Night surveys would also find web types such as the “net” of Deinopis, the net-casting spider. Countering this however, many of the diurnal orb-web species take down their webs in the evening before rebuilding pre-dawn. Thus many webs, decorated orbs especially, may be missing for all or part of the night. Part webs of some of these spiders (the remaining tangle), and other web builders with semi-permanent webs might be found both by day and by night. If both day and night surveys were to be carried out some method would need to be devised to avoid including such webs twice.
The objectives of a study will determine whether such rigor is required. Since the application of the method in our study was for comparative purposes among different stages of restoration and we always conducted surveys during the day, the oversight of web types set at night would not have affected results or the interpretation of results. Also noteworthy is that weather may also influence the web count. In particular, windy weather or heavy rain is likely to destroy webs or deter spiders from building them. Suitable weather conditions need to be considered in any survey work using our method. During our survey among restoration sites, the weather was always calm and there was no rain during the surveys and so comparisons are valid.
When generic richness was plotted against web richness, the variability around the regression line suggested web richness sometimes overestimated or underestimated generic richness. This relationship could be improved by the addition of web-types that use more subtle features to distinguish those webs that are made by different genera. The taxonomic resolution of the web identification guide would also be improved by incorporating resources that allow the identification of the actual spider. Indeed, the use of webs only is an ‘extreme’ approach, especially since some web-building spiders have easily identifiable attributes e.g. it is easy to overlook the decorations in an Austracantha web (web-type 8), but the spiders themselves are distinctive, with yellow/black coloration and a spiny abdomen. Since producing the web guide, an additional guide has been produced that gives examples of spiders that make each web type and information about particular spiders’ ecology and behaviour. This additional guide is included as supplementary material (S2) and can be found on the Australian Museum website (Smith 2008). This supplement is specific to our study region, but could be easily adapted and modified for other regions.
While there are many good reasons to include terrestrial arthropods in monitoring programmes, there are also many disadvantages. Most disadvantages are due to the fact that it is time consuming to collect and identify them; translating to high costs. Once refined and tested, our substitute offers several advantages over ‘traditional’ collecting techniques. Firstly, collecting data on web-types is non-lethal. Most traditional arthropod monitoring approaches require animals to be collected, a process considered counterproductive to the philosophy of ‘conservation’ (Bowie and Frampton 2004). Second, costs are lowered because there is no collecting involved. Furthermore, time associated with the processing of unidentifiable specimens is avoided. For spiders, this cost can be high because large proportions of collected material are juvenile, damaged, or undescribed. Unidentifiable specimens have been estimated to make up to 70% of collected material (Brennan et al. 2004). The lower proportion of juveniles in our study (58%) was mainly due to seasonal factors (many spiders were adult at the time) and generic identifications were only possible for all specimens due to the relatively well known, but fairly limited fauna of the area. It should be noted however (as indicated in Table 1) that generic definitions are constantly under revision and it will become ever more difficult to identify juvenile spiders to genus. Since the work we describe was carried out an ongoing revision of Australian orb-web spiders has resulted in several name changes (Framenau and Scharff 2008; Framenau et al. 2010). Similarly, some of our common theridiids (comb-footed spiders) have also been moved to other genera (Yoshida 2008). The upside of these and other future changes is that there will be a tighter relationship between our web identification and generic richness than we have recorded here (see Fig. 2 and Table 1). For instance, at present, spiders of the genus Araneus make five web types in our identification guide. However, at least three of these web types are made by species that belong in what will be new genera when Australian Araneus is revised (V. Framenau, pers. comm.).
People who are not specialised in spider identification can also use our method. In particular, our substitute offers a method that enables community involvement. Participation of community volunteers in environmental and ecological monitoring projects has been described as a global phenomenon (Danielson et al. 2003). The most obvious advantage of using volunteers in environmental projects is that volunteers provide an inexpensive and potentially large labour force. However, there are many challenges faced by volunteer organisations in monitoring outcomes to ensure their efforts are not being wasted. Apart from disadvantages such as the complexity of some scientific methods rendering them unsuitable for wide-scale volunteer use (e.g. DNA analysis), data collected by volunteers have been considered unreliable (Underwood and Chapman 2002; see references in Newman et al. 2003). Despite such scepticism, there has been much research showing that given even limited amounts of training, volunteers can collect data that are just as credible as those of professional scientists (Greenwood 1994; Fore et al. 2001; Brandon et al. 2003; Newman et al. 2003; Gofreddo et al. 2004). Our experience with community groups during the refinement of our web guide is that given supervision and training, community volunteers can collect data on web-types that are comparable to ours (unpublished data).
Our web identification guide also provides a way in which non-specialists and community groups can work with invertebrates in a meaningful way, and limits the need for specialist help. So far, community monitoring initiatives have been restricted to a few invertebrate groups such as aquatic invertebrates and butterflies (for examples see Firehock and West 1995; Kuehn et al. 2008). We are not suggesting that substitutes like the one we developed will replace invertebrate scientists. Indeed, specialists in spider taxonomy, behaviour and ecology were (and are) required to develop the web identification guide, but we do encourage invertebrate researchers to develop methods that allow non-specialist involvement in invertebrate conservation. This will go towards overcoming some of the ‘perception’ problems that invertebrates suffer where many sectors of the community only recognize ‘the dirty cockroach’ and the ‘nuisance fly’ (Samways 2007).
Lastly, the use of webs as a substitute for monitoring the biodiversity of web-building spiders is cost-effective. Any monitoring program—for whatever purpose—must be cost-effective, otherwise the programme will run into financial difficulties and is likely to fail (Caughlan and Oakley 2001). Indeed, monitoring programmes will have a much greater chance of success if cost considerations are explicitly incorporated into the program framework. Monitoring strategies also have costs that are not easily measured in financial terms and these should also be considered. These non-market values refer to goods and services that cannot be bought or sold, but still have economic value (Green and Tunstall 1991). Examples are the costs borne from not accounting for Type II statistical errors (failing to detect a change that has occurred). To deal with such a cost, our rapid and cost-effective substitute allows repeated sampling in both space and time, and so will increase the power of tests and reduce the probability of making statistical errors. In contrast, there are also non-market costs that are positive and add value to a monitoring program. These include valuing the social aspects such as learning that citizens might gain from being involved in sampling programs.
The trends we observed among the three habitat types suggested that native revegetation has had a positive impact on web-building spider communities. Web abundance, richness and composition in revegetated habitat were intermediate between unplanted and reference habitats. While we did not measure habitat attributes, it is likely that the trends we observed are a response to increasing the availability of web construction sites brought about by introducing woody native tree species. Spider richness, abundance and even spider assemblages have been shown to vary with variation in habitat structure (Greenstone 1984; Riechert and Gillespie 1986; Uetz 1991; Bell et al. 2001; Langellotto and Denno 2004). For web-builders in particular, an increase in structural complexity increases the availability of potential refuges and structural supports suitable for web construction. Experiments that have altered existing features, or added artificial substrates, to a habitat, have demonstrated positive or negative recruitment depending on whether structures are added or removed (Rypstra 1983; McNett and Rypstra 2000). Also important is the presence of dead twigs, branches and large trees with rough bark, which comes with maturing vegetation. Examples of genera that require this substrate are Paramatachia, which live in holes in dead twigs (web-type 22), and stiphidiid sheet web weavers (web-type 28), which need bark crevices or rotting logs.
Our method also detected trends in individual web-types. Indval analyses in our study revealed that eight web-types were characteristic of reference habitat with one of those eight being exclusive to reference habitat type. Others have also found unique assemblages of web-builders associated with successional vegetative states (Gibson et al. 1992; Baines et al. 1998; Wheater et al. 2000; Brennan et al. 2006). The different responses we observed for individual web-types highlight the importance of examining the response of individuals, and not relying on simple metrics such as abundance and richness that can mask trends in certain groups. Indeed, these eight characteristic web-types could be used as ecological indicators of success in our study area.
Conclusion
The web substitute showed strong congruency with spider genera across our study sites. The substitute is a cost effective and easy approach for surveying the diversity of web-building spiders and it appears to be sensitive enough to detect differences in habitat types. Furthermore, due to the structural constraints and the functional role of capture webs they are also likely to provide information regarding functional potential and spatial complexity within study sites. We found this method useful for assessing the effects of revegetation. Many other applications for the web substitute are possible, such as environmental impact assessments and agricultural pest management, and we encourage its development.
References
Andersen AN, Hoffmann BD, Muller WJ, Griffiths AD (2002) Using ants as bioindicators in land management: simplifying assessment of ant community responses. J Appl Ecol 39:8–17
Baines M, Hambler C, Johnson PJ et al (1998) The effects of arable field margin management on the abundance and species richness of Araneae (spiders). Ecography 21:74–86
Barros F (2001) Ghost crabs as a tool for rapid assessment of human impacts on exposed sandy beaches. Biol Conserv 97:399–404
Bell JR, Wheater CP, Cullen WR (2001) The implications of grassland and heathland management for the conservation of spider communities: a review. J Zool (Lond) 255:377–387
Birks J, Messenger J, Braithwaite T (2005) Chapter 12: are scats surveys a reliable method for assessing distribution and population status of pine Martens? In: Harrison DJ, Fuller AK, Proulx G et al (eds) Martens and Fishers (Martes) in human-altered environments an international perspective. Springer, US, pp 235–252
Blackledge TA (1998) Signal conflict in spider webs driven by predators and prey. Proc R Soc Lond B Biol 265:1991–1996
Bowie MH, Frampton CM (2004) A new technique for non-destructive monitoring of soil surface invertebrates for ecological restoration programmes. Ecol Restor Manage 5(1):34–42
Brandon A, Spyreas G, Molano-Flores B et al (2003) Can volunteers provide reliable data for forest vegetation surveys? Nat Area J 23:254–262
Brennan KEC, Moir ML, Majer JD (2004) Exhaustive sampling in a Southern Hemisphere global biodiversity hotspot: inventorying species richness and assessing endemicity of the little known jarrah forest spiders. Pac Conserv Biol 10:241–260
Brennan KEC, Ashby L, Majer JD et al (2006) Simplifying assessment of forest management practices for invertebrates: how effective are higher taxon and habitat surrogates for spiders following prescribed burning? For Ecol Manage 231:138–154
Cardoso P, Silva I, de Oliveira HG, Serrano ARM (2004a) Indicator taxa of spider (Araneae) diversity and their efficiency in conservation. Biol Conserv 120:517–524
Cardoso P, Silva I, de Oliveira NG, Serrano ARM (2004b) Higher taxa surrogates of spider (Araneae) diversity and their efficiency in conservation. Biol Conserv 117:453–459
Caughlan L, Oakley KL (2001) Cost considerations for long-term ecological monitoring. Ecol Indic 1:123–134
Chame M (2003) Terrestrial mammal feces: a morphometric summary and description. Memorias do Instituto Oswaldo Cruz 98:71–94
Curtis DJ (1981) Describing communities of land invertebrates—attempting the impossible? Scott Field Stud 1981:18–27
Danielson F, Mendoza MM, Alviola P et al (2003) Biodiversity monitoring in developing countries: what are we trying to achieve? Oryx 37:407–409
Dufrêne M, Legendre P (1997) Species assemblages and indicator species: the need for a flexible asymmetrical approach. Ecol Monogr 67:345–366
Firehock K, West J (1995) A brief history of volunteer biological water monitoring using macroinvertebrates. J North Am Benthol Soc 14:197–202
Fore LS, Paulsen K, O’Laughlin K (2001) Assessing the performance of volunteers in monitoring streams. Freshw Biol 46:109–123
Framenau VF, Scharff N (2008) The orb-weaving spider genus Larinia in Australia (Araneae: Araneidae). Arthropod Syst Phylogeny 66:227–250
Framenau VF, Dupérré N, Blackledge TA, Vink CJ (2010) Systematics of the new Australasian orb-weaving spider genus Backobourkia (Araneae: Araneidae: Araneinae). Arthropod Syst Phylogeny 68:79–111
Gibson CWD, Hambler C, Brown VK (1992) Changes in spider (Araneae) assemblages in relation to succession and grazing management. J Appl Ecol 29:132–142
Glen AS, Dickman C (2003) Monitoring bait removal in vertebrate pest control: a comparison using track identification and remote photography. Wildl Res Manage Conserv 30:29–33
Gofreddo S, Piccinetti C, Zaccanti F (2004) Volunteers in marine conservation monitoring: a study of the distribution of seahorses carried out in collaboration with recreational scuba divers. Conserv Biol 18:1492–1503
Gollan JR, Ashcroft MB, Cassis G et al (2009) Testing common habitat based surrogates in a semi arid rangeland. Biodivers Conserv 18:1147–1159
Gotelli NJ (2004) A taxonomic wish-list for community ecology. Phil Trans R Soc Biol 359:585–597
Green CH, Tunstall SM (1991) Is the economic evaluation of environmental resources possible? J Environ Manage 33:123–141
Greenstone MH (1984) Determinants of web spider species diversity: vegetation structural diversity vs. prey availability. Oecologia (Berlin) 62:299–304
Greenwood JJD (1994) Trust the wildlife volunteers. Nature 368:490
Herberstein ME, Craig CL, Coddington JA (2000) The functional significance of silk decorations of orb-web spiders: a critical review of the empirical evidence. Biol Rev 75:649–669
Hodkinson ID, Jackson JK (2005) Terrestrial and aquatic invertebrates as bioindicators for environmental monitoring, with particular reference to mountain ecosystems. Environ Manage 35:649–666
Hopkins GW, Freckleton RP (2002) Declines in the number of amateur and professional taxonomists: implications for conservation. Anim Conserv 5:245–249
Hulbert SH (1984) Pseudoreplication and the design of ecological field experiments. Ecol Monogr 54:187–211
Kremen C, Colwell RK, Erwin TL et al (1993) Terrestrial arthropod assemblages: their use in conservation planning. Conserv Biol 7:796–808
Kuehn E, Feldmann R, Harpke A (2008) Getting the public involved in butterfly conservation: lessons learned from a new monitoring scheme in Germany. Israel J Ecol Evol 54:89–103
Langellotto GA, Denno RF (2004) Responses of invertebrate natural enemies to complex-structured habitats: a meta-analytical synthesis. Oecologia (Berlin) 139:1–10
Lovell S, Hamer M, Slotow R, Herbert D (2007) Assessment of congruency across invertebrate taxa and taxonomic levels to identify potential surrogates. Biol Conserv 139:113–125
Main BY (1976) Spiders. The Australian Naturalist Library. William Collins Publishers Pty Ltd, Sydney, Australia
Mandelik Y, Tamar D, Chikatunov V, Kravchenko V (2007) Reliability of a higher-taxon approach to richness, rarity, and composition assessments at the local scale. Conserv Biol 21:1506–1515
McGeoch MA, Van Rensburg BJ, Botes A (2002) The verification and application of bioindicators: a case study of dung beetles in a savanna ecosystem. J Appl Ecol 39:661–672
McNett BJ, Rypstra AL (2000) Habitat selection in a large orb-weaving spider: vegetational complexity determines site selection and distribution. Ecol Entomol 25:423–432
Moller H, Clapperton BK, Fletcher DJ (1997) Density of rabbits (Oryctolagus cuniculus L.) in the Mackenzie Basin, South Island, New Zealand. N Z J Ecol 21:161–167
Newman C, Buesching CD, Macdonald DW (2003) Validating mammal monitoring methods and assessing the performance of volunteers in wildlife conservation—“Sed quis custodiet ipsos custodies ?”. Biol Conserv 113:189–197
Oliver I, Beattie AJ (1996) Designing a cost-effective invertebrate survey: a test of methods for rapid assessment of biodiversity. Ecol Appl 6:594–607
Peake T (2003) Hunter bushland resources kit. Hunter Catchment Management Trust, Tocal, NSW
Riechert SE, Gillespie RG (1986) Habitat choice and utilization in web-building spiders. In: Shear WA (ed) Spiders: webs, behavior and evolution. Standford University Press, Standford, pp 22–48
Rosenberg DM, Danks HV, Lehmkuhl DM (1986) Importance of insects in environmental impact assessment. Environ Manage 10:773–783
Rypstra AL (1983) The importance of food and space in limiting web-spider densities; a test using field enclosures. Oecologia 59:312–316
Samways MJ (2007) Insect conservation: a synthetic management approach. Annu Rev Entomol 52:465–487
Schlick-Steiner BC, Steiner FM, Moder K et al (2006) Assessing ant assemblages: pitfall trapping versus nest counting (Hymenoptera, Formicidae). Insect Soc 53:274–281
Smith H (2008) BugWise Web2Spider Supplement. Available at http://publications.australianmuseum.net.au/pdf/1561_complete.pdf. Accessed 29 June 2010
Smith H, Gollan RJ, Bulbert M (2008) BugWise Web2Spider a tool for monitoring the diversity of web-building spiders. Available at http://publications.australianmuseum.net.au/pdf/1560_complete.pdf. Accessed 29 June 2010
Takada M, Baba YG, Yanagi Y et al (2008) Contrasting responses of web-building spiders to deer browsing among habitats and feeding guilds. Environ Entomol 37:938–946
Uetz GW (1991) Chapter 18: Habitat structure and spider foraging. In: Bell SS, McCoy ED, Mushinsky HR (eds) Habitat structure, the physical arrangement of objects in space. Chapman and Hall, New York, pp 325–348
Underwood AJ, Chapman MG (2002) Conservation of coastal organisms depends on scientific realism, not community ‘monitoring’. In: Lunney D, Dickman C, Burgin S (eds) A clash of paradigms: community and research-based conservation. Royal Zoological Society of New South Wales, Mosman, pp 20–37
Wheater CP, Cullen WR, Bell JR (2000) Spider communities as tools in monitoring reclaimed limestone quarry landforms. Landsc Ecol 15:401–406
Winer BJ, Brown DR, Michels KM (1991) Statistical principles in experimental design. McGraw-Hill, New York
Yoshida H (2008) A revision of the genus Achaearanea (Araneae: Theridiidae). Acta Arachn Tokyo 57:37–40
Acknowledgments
We thank Dr. Mike Gray, Dr. Barbara York Main, Dr. Volker Framenau and Graham Milledge for their knowledge and expertise in spider taxonomy, behaviour and ecology. We also thank the Coal & Allied Community Trust and the NSW Environmental Trust for financial support (Project Ref. # 2003/RD/G0001 and 2006/EG/0005). Our project could not have been possible for the many volunteers and landholders (too many to list personally!) who were involved in refining the guide and who permitted access to survey sites. In particular we thank Conservation Volunteers Australia, National Parks Association NSW, Upper Hunter River Rehabilitation Initiative, and the Hunter-Central Rivers, Murray, Murrumbidgee, Southern Rivers and Hawkesbury-Nepean CMAs for their assistance. An anonymous reviewer improved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gollan, J.R., Smith, H.M., Bulbert, M. et al. Using spider web types as a substitute for assessing web-building spider biodiversity and the success of habitat restoration. Biodivers Conserv 19, 3141–3155 (2010). https://doi.org/10.1007/s10531-010-9882-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10531-010-9882-1