Abstract
Habitat-based surrogates are a low cost alternative to intensive biodiversity surveys, though they have been poorly investigated in semi-arid ecosystem compared to others such as temperate woodlands. In this study we tested potential habitat-based surrogates of invertebrate richness in a semi-arid rangeland in northwest Australia. Potential surrogates were: distance from artificial watering-point; soil hardness; habitat complexity; and individual complexity components. Generalised additive models (GAMs) were used to relate abundance and richness of selected invertebrates with environmental factors and cluster analysis was used to examine similarity in species composition. The most frequently selected factor was soil hardness, but taxa varied as to whether biodiversity was higher in soft or hard soils. Where distance from watering-point was an important predictor, there were generally higher abundances and richness closer to watering-points than further away. Abundance and species richness could be partially explained using individual complexity components, but relationships were weak and there were no consistent trends among taxa. Therefore, although habitat complexity has been correlated with species richness under some circumstances, our results cast doubt on the generality of this relationship. There are also dangers in assuming that all taxa respond in a manner similar to indicator taxa, as we observed that different taxa had higher richness at opposite extremes of some environmental gradients. Grazing may have a negative impact on biodiversity in some environments, but in regions where water is limiting, the net effect may be positive due to the creation of waterholes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Developing conservation strategies for every component of biodiversity are far beyond our reach because of the mammoth task of collecting all the relevant data on every component (Stoms et al. 2005). Using surrogates to predict biodiversity (typically expressed in terms of species richness) in areas for which biodiversity information is not available is one way of addressing this problem (Gaston and Blackburn 1995).
Habitat-based surrogates are environmental variables that act as indirect measures of diversity (Hughes et al. 2000) and are a cheaper alternative to intensive biodiversity surveys. Vegetation condition scores, individual vegetation features (e.g. amounts of dead wood or tree basal area), habitat classification (e.g. aspen, meadow, and spruce) and climatic variables have all been used to predict faunal diversity (Catling and Burt 1995; Hughes et al. 2000; Ferrier and Guisan 2006; Grove 2002; Gillison et al. 2003; Fraser et al. 2007). Some habitat-based surrogates are commonly used, and justification for their use stems from long-standing ecological theory. For example, habitat complexity is positively correlated with faunal species richness (Hansen 2000; Lassau and Hochuli 2005; Lassau et al. 2005; Jiménez-Valverde and Lobo 2007), which supports the hypothesis that structurally diverse habitats support more species (MacArthur and MacArthur 1961).
Arthropods, particularly ground-active ones, may respond more directly to soil properties such as hardness and texture (Crawford 1988; Stapp 1997; Whitford et al. 1999; Bestelmeyer and Wiens 2001) than those based on amounts of vegetation cover or leaf litter. The links between the soil and invertebrate organisms are appreciated for conservation (Lal 1991), and the impacts of soil management on invertebrates are well studied (e.g. Sharley et al. 2008). It would be expected then that soil parameters would make useful habitat-based surrogates for invertebrate diversity.
Surrogates have also been used to represent environmental gradients and subsequently used in environmental impact assessment. For example, assessment of grazing impacts on biodiversity in rangelands used ‘distance from artificial watering-point’ as a surrogate for grazing intensity (e.g. Ludwig et al. 1999; Hoffman 2000). Based on the piosphere effect of radial grazing (Osborn et al. 1932), negative effects of grazing are deduced when a positive trend between distance from watering-point and measures of biodiversity are found. Whether positive or negative, distance from watering sources could also prove to be a useful habitat-based surrogate.
In this study, we sought to identify habitat-based surrogates of invertebrate biodiversity by testing the strength of a number of environmental correlates in a semi-arid rangeland in Northwest Australia. Many such rangelands throughout the world have been substantially altered as a consequence of human activities, and almost all are degraded to a greater or lesser degree (Perrings and Walker 1995). Efficient monitoring strategies are required. Potential surrogates, namely distance from artificial watering-point, soil hardness and habitat complexity have been shown to be important for explaining invertebrate patterns elsewhere, however, their utility to act as habitat-based surrogates in this environment is unclear. Total abundance and species richness (as a measure of diversity) were used to describe invertebrate biodiversity. Although there are problems with using species richness in conservation management (Fleishman et al. 2005), modelling spatial variation in species richness is the most common strategy when there is insufficient knowledge on the distribution of individual species (Ferrier et al. 2007). Furthermore, species richness can contribute to biodiversity conservation planning provided it is not used in isolation and other metrics are also used (Fleishman et al. 2005). Therefore, we also examined how these environmental factors were related to species composition.
Methods
Study region
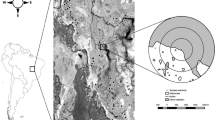
This study was conducted on the Hamersley pastoral lease in the Pilbara region of Western Australia (Fig. 1, inset) in April 2005. The area is part of a zone that is characterised by a hot and semi-arid climate, and extensive hummock grassland (Fisher et al. 2004). The Pilbara is an important pastoral region and grazing is the dominant (~60%) land use (Fisher et al. 2004). Permanent artificial watering-points are scattered across the Pilbara’s grazing region.
Distance from artificial watering-points
We chose five artificial watering-points (Ridge bore, Pindering well, Two-mile bore, Balbina bore and Kangeenarina well) spaced 5–20 km apart (Fig. 1). A 1-km transect was established from each artificial watering-point. Four trapping lines (perpendicular to each transects) of five pitfall traps were established at 100, 200, 500 and 1,000 m intervals. Traps were positioned 5 m apart along each trapping line.
Invertebrate sampling
Pitfall traps for invertebrate sampling were 9 cm in diameter and 14 cm in depth. All traps were: one-third filled with ethylene glycol as preservative; buried and placed flush to the ground surface; covered by an upturned pot-plant base held above the opening with clothes pegs; and, collected after 9 days.
All traps and specimens were processed using the laboratory sorting protocols of Wilkie et al. (2003), which is a quality control procedure for laboratory sorting and identification of invertebrate specimens. In brief, the quality control procedure involves a series of feedback loops and checks that are implemented throughout the sorting stages so that errors are corrected as they occur, and errors that cannot be controlled, are minimised. Ants (Formicidae), wasps (Hymenoptera but excluding Formicidae), beetles (Coleoptera) and flies (Diptera) were identified to morphologically recognisable units (morphospecies). Morphospecies are hereafter referred to as species for simplicity, and species richness is used to refer to the number of morphospecies. All other taxa were identified to ordinal level only and counted.
Habitat-based surrogates and complexity
We used a modification of the methods of Lassau and Hochuli (2005) to measure seven habitat-based surrogates associated with habitat complexity. This involved using an ordinal scoring system of habitat variables (tree canopy cover; shrub canopy cover; ground herb cover; amount of leaf litter; amount of logs and debris; substrate rockiness; soil moisture) at each trapping line (i.e. within 1-m either side of the trapping line; Table 1). Soil moisture was excluded from analyses because it was found that soil was very dry and there was little difference between sites. Habitat complexity was then determined as a sum of six variables at each trapping line.
Soil hardness
Soil hardness was measured as the pressure (kg/cm2) required for the end of a hand-held soil penetrometer (Humboldt, 200 mm Pocket Penetrometer) to penetrate the soil to a depth of 6.5 mm. Soil hardness was measured at three random points around each pitfall trap (but within 1-m of each trap). The average of the 15 values recorded along each trapping line was used in the analyses.
Data analyses
The samples from all five traps at each trapping line were pooled prior to analyses. Several traps at 100, 200 m (Ridge bore) and 200 m (Pindering well) were lost due to disturbance by cattle. As a consequence, for species richness (the total number of species present on each trapping line), these three trapping lines were discarded. For abundance (the absolute number of individuals on a trapping line), we discarded only traps that were disturbed and standardised values as the number of individuals per trap. Abundance and species richness were log10 (x + 1) transformed. Distance from watering-point was also log10 (x + 1) transformed.
Relationships between environmental variables and: (1) ant, wasp, beetle, and fly species richness; (2) species richness of the four groups combined (herein ‘overall species richness’); (3) total abundance of each of the 11 most abundant invertebrate groups; and (4) total abundance of all invertebrates, were investigated using Generalised Additive Models (GAMs) (Hastie and Tibshirani 1990). Distance from artificial watering-point, tree canopy cover, shrub canopy cover, ground herb cover, habitat complexity and soil hardness were considered as splines with two degrees of freedom. The remaining habitat variables were included as linear terms since splines could not be used (< 4 unique values).
Environmental variables were assessed using a variation of the D 2 (deviance explained) method. We calculated D 2 by disregarding each observation in turn and determined the minimum deviance explained by the remaining observations. This method was designed to penalise models that over-fitted to outliers (similar to Cook’s distance in linear regression; Cook 1977).
Models were created by testing each combination of one and two predictor models. The best models were selected by maximising the D 2, and P values for the models were determined by simulating the modelling process using random response variables. In short, we simulated the modelling process 1,000 times using the real predictor observations, but with random response variables. The P-value (0.05) was determined as the D 2 threshold that only 50 of the 1,000 models exceeded. This method for determining P-values ensures that the entire model building process is considered including how well the gradients were sampled, the correlation between predictor variables, the sample size, the data type (categorical/continuous), the degrees of freedom and our modification to the D 2 statistic. While we used a P-value of 0.05 to determine significant relationships, we also examined near-significant relationships (0.05 < P < 0.20) to determine if different taxa displayed similar trends to each potential surrogate.
The similarity in species composition was analysed for all 17 trapping lines using cluster analysis (Bray–Curtis similarity using presence/absence data) for all four target taxa individually, and in combination (hereafter ‘overall composition’). The resulting dendogram was used to determine if patterns in species composition were due to differences between artificial watering-points (a potential spatial bias), distance from artificial watering-point, soil hardness or habitat complexity.
Results
In total, 12,661 individuals were trapped. Ants and flies were the two most abundant groups’ trapped (58 and 15% of total abundance, respectively). Of the four taxa that were identified to species level, wasps were the most diverse, followed by ants, beetles and flies (72, 60, 47 and 30, respectively).
Soil hardness was the habitat variable that was best able to explain multiple components of invertebrate biodiversity. Hardness was selected in 19 of the 34 models produced (56%), which was more than distance from water (12 of 34 or 35%), habitat complexity (4 from 34 or 12%), and the individual components of habitat complexity (up to five models each). Soil hardness was also selected in six of the eight models that were significant (P < 0.05), while no other factor was in more than two of the significant models. Soil hardness could not be used as a surrogate for multiple components of biodiversity, however, because taxa varied in their responses to the gradient. For example, ant richness peaked on hard soils (Fig. 2b, P < 0.05), Orthopteran abundance peaked in soft soils (Fig. 2j, P < 0.05), while Acarina abundance peaked at intermediate values (Fig. 2l, P < 0.05).
Distance from water was significant (P < 0.05) in the two-parameter models for Orthopteran abundance (Fig. 2i) and Araneae abundance (Fig. 2h), and near significant (P < 0.20) in the two parameter model for ant abundance (Fig. 2c), the one parameter model for overall abundance, and the one parameter model for wasp richness. In all five cases, the responses peaked at low distances, indicating higher abundance and richness of these taxa in close proximity to waterholes. The correlation between distance from water and hardness was low (r 2 < 0.03), so the two most commonly selected predictors each explained different aspects of the distribution of biodiversity.
Neither complexity, nor the individual components of complexity were selected consistently across the 34 models. Trends in the significant and near significant models were also variable, with different taxa peaking at either high or low complexity. Tree canopy was significant (P < 0.05) in the models for ant and wasp richness, and ground herbage was significant in the model for Acarina abundance. In all three of these models the responses peaked at the more complex end of the gradient (Fig. 2a, e, k). However, the abundance of ants (Fig. 2d, P < 0.20) and Araneae (Fig. 2g, P < 0.05) was higher at the lower complexity end of the shrub canopy gradient, and the richness of wasps peaked at low overall complexity (Fig. 2f, P < 0.05). Therefore, there was no consistent trend that biodiversity was higher in either low or high complexity environments.
Cluster analysis revealed that soil hardness was the most important factor in explaining overall species composition. Three distinct site clusters: all soft (Cluster 1); a mix of soft, medium and hard (Cluster 2); and all hard (Cluster 3) could be delimited from the dendrogram (Fig. 3a). Soil hardness was also important for clustering sites with respect to ants, but instead two distinct clusters: soft (Cluster 1), and a mix of medium and hard (Cluster 2) could be delineated. However, two sites (Pw 1000 and Kw 500) were exceptions to this pattern (Fig. 3b). Soil hardness did not appear important for determining clusters of any other single taxa. There was no evidence to indicate that location, distance from artificial watering-point and habitat complexity were important for determining site clusters of overall species composition, or the species composition of any single target taxon.
Dendrograms from cluster analysis showing the similarity in overall a and ant b species composition between sites. Abbreviations for sites at each artificial watering-point are: Rb, Ridge bore; Pw, Pindering well; Tb, Two-mile bore; Bb, Balbina bore and Kw, Kangeenarina well. Following site abbreviations are: distance from artificial watering-point (100, 200, 500 or 1,000 m), hardness of soil (kg/cm2) as indicated by penetrometer (S)oft (<0.5), (M)edium (0.5–2.0) and (H)ard (>0.5), and habitat complexity scores, respectively
Discussion
Habitat-based surrogates of biodiversity are potential cost-cutting tools and the first step in identifying them is to determine the correlation between the potential surrogate and the entity in which it is supposed to be a substitute for (McGeoch 1998). In this study, we investigated strengths of correlations between the abundance and species richness of different invertebrate groups, and a number of environmental variables in a semi-arid rangeland. We also examined the influence of environmental factors on community composition using cluster analysis.
We found that none of the environmental factors tested were adequate surrogates for all components of invertebrate biodiversity. Soil hardness was the factor that was best able to explain the distribution of abundance and species richness, but taxa varied as to whether biodiversity peaked on hard, soft or intermediate soils. There was consistently higher abundance and richness near watering-points, but this factor was not as consistent as hardness in explaining all components of biodiversity. Habitat complexity, and the individual components of complexity, also performed poorly. None of the complexity predictors were consistently selected in the models, and taxa varied as to whether they peaked at high or low complexity. Therefore, our results support the notion that different taxa have different habitat preferences, and one should consider this when choosing surrogates. What this means is that one habitat variable should not be used as a surrogate for all taxa.
Habitat-based surrogates have been used in attempts to cut costs in biodiversity surveys, but there has also been interest in indicator taxa in recent years (Caro and O’Doherty 1999). Indicator taxa are sub-components of the total community that are correlated with the biodiversity of other groups (Noss 1990). Ants (Formicidae) in particular have been used extensively as focal taxa in studies of human impacts throughout the world (Perfecto and Snelling 1995; Bestelmeyer and Wiens 1996, 2001; Andersen 1997; Majer and Nichols 1998) because they are abundant, their taxonomy is well-known relative to other groups, and their responses may indicate environmental patterns that determine the distribution of other organisms over a wide range of scales and environments (Andersen 1997). While an evaluation of the utility of ants as bioindicators in rangelands, which includes parallel studies of the response of ants and other taxa to environmental gradients, have been called for (e.g. Bestelmeyer and Wiens 2001), we found no evidence that other invertebrate groups show the same patterns for ants. In fact, we found that orthopterans showed the opposite response to ants with respect to soil hardness. Ants aside, it is generally unclear how species richness of particular taxonomic groups is correlated with each other (Sauberer et al. 2004). The parameters that we tested have potential to act as surrogates for some groups and for some metrics, but not all. Thus, if different taxa are responding to different environmental factors then we cannot expect one to act as an indicator of the other. Poor correlation between species richness of different invertebrate groups have led authors to suggest that a ‘shopping basket’ approach that estimates or monitors a variety of taxa is required (di Castri et al. 1992). We also support a shopping basket approach with one that also considers multiple habitat-based surrogates.
Our study has narrowed down a few habitat factors such as soil hardness and habitat complexity for a few groups, but we must acknowledge that a limitation to our study is the narrow scale of space and time that we have tested them at. Testing at different spatial and temporal scales could clarify their usefulness over larger or smaller areas, over seasons or years.
How our habitat-based surrogates predict different combinations of taxa may also yield stronger relationships than we detected. Assuming that all invertebrates are equally likely to fall into a pitfall trap is a limitation of pitfall data, and integrating other collection techniques would be useful for investigation. Similarly, only including species or groups that are most likely to fall into a pitfall trap might yield stronger relationships. Although how this probability could be determined for every species is an unfeasible task—if not an impossible one.
Assessments of the impacts of grazing on biodiversity use ‘distance from artificial watering-point’ as a surrogate for grazing intensity (e.g. Ludwig et al. 1999; Hoffman 2000). In our study, where distance from watering point was an important predictor, partial response curves showed that there were more individuals and species proximal to watering-points. This trend was particularly strong for beetles and overall species richness, and abundance of Araneae, beetles, wasps, ants, Orthoptera and of all individuals. While this could be a result of grazing, a negative relationship between distance and abundance and species richness could also be an effect of the water and not grazing per se. Indeed, grazing pressure is one of the hardest to quantify (Pringle and Landsberg 2004). Thirteen important factors can modify the influence of distance from water on the distribution of livestock grazing pressure (Pringle and Landsberg 2004). Factors include: paddock and water source configuration; proximity to natural surface water; salinity of water; and, the species of livestock. Alternatively, water is a resource that is limiting in dry areas and a lack of water causes inactivity or death more quickly than other essentials such as food (James et al. 1999). To separate the potential positive effects of water from the effects of grazing, field-studies that use distance from watering-point as a surrogate of grazing should include trapping lines close to watering points that were not grazed.
We found poor relationships between habitat complexity and the species richness and abundance of nearly all arthropod groups. However, wasp species richness was negatively associated with habitat complexity in two-parameter models. Notably, this is not consistent with the habitat heterogeneity hypothesis, which predicts that habitat complexity would be positively correlated with species diversity (MacArthur and MacArthur 1961). This result is also opposed to the findings of Lassau and Hochuli (2005) who found higher species richness of wasps in highly complex woodland habitats than ones with less structural diversity. In contrast to Lassau et al. (2005), we also found no positive relationship between beetle richness and habitat complexity. Our finding of poor relationships may not be surprising. Tews et al. (2004) review of habitat heterogeneity-animal species diversity studies show variable responses between taxa and structural parameters measured. Importantly, habitat complexity, and indeed other habitat-based attributes, which are often inferred from remote sensing, may not adequately reflect the variation that is important to some animal taxa. There may be consequences for management strategies that use habitat-based surrogates developed in other areas or under different sets of conditions—particularly when ‘high’ values of complexity are used as surrogates of high diversity. For example, management strategies using surrogate measures for one group of taxa might be detrimental for others.
We found that soil hardness was a consistent predictor in two-predictor models (8/12 and 4/5 for abundance and species richness, respectively), with ant abundance and species richness and orthopteran abundance strongly related. Soil hardness was a factor in overall species composition, although unduly influenced by ants, with the other three taxa not clustered with respect to soil hardness. Bestelmeyer and Wiens (2001) also found soil hardness important for explaining community variation of ants in a grazed short-grass desert habitat in the south-west of the United States. Variations in soil characteristics affect abundance and diversity of other surface-active arthropod groups such as beetles in arid grasslands of New Mexico (Crawford 1988) and Colorado (Stapp 1997). Other soil factors, including erodibility and composition of clay and sand, are associated with structure of rangeland orthopteran communities such as grasshoppers (Quinn et al. 1991 Schell and Lockwood 1997). Soil parameters are clearly important factors influencing invertebrate communities in rangelands—and an aspect that is often neglected (Bestelmeyer and Wiens 2001).
Conclusion
Biodiversity in many parts of the world, including semi-arid regions in Australia, is poorly understood and the means of tracking change in biodiversity are not available (Fisher et al. 2004). Such limitations have prompted biologists to take shortcuts in biodiversity assessment (Kerr et al. 2000). Shortcuts include both habitat-based surrogates and indictor species. Samways (2007) noted that both surrogates and indicators of invertebrate diversity are not perfect and there are inherent risks in using them in ecological assessments. Our results support this notion and the need for examining a variety of invertebrate taxa in order to obtain a more complete picture of biodiversity and how human impacts might affect invertebrate communities. While we found that soil hardness was the best habitat-based surrogate, measures of single habitat features may not be relevant to all components of biodiversity and one cannot assume that richness of one invertebrate group correlates with richness of others. As the distribution of biodiversity is influenced by a variety of factors, we recommend that predictions are based on models that include non-linear responses to multiple environmental gradients, and not on the assumption that one gradient provides a sufficient surrogate. If regional conservation strategies are to be effective for managing biodiversity, monitoring and inventories need to be based on a set of factors reflecting important aspects for varying groups of invertebrates. Thus, management plans will need to vary accordingly.
References
Andersen AN (1997) Using ants as bioindicators, multiscale issues in ant community ecology. Conserv Ecol 1(1):8. http://www.ecologyandsociety.org/vol1/iss1/art8/. Cited 12 Dec 2007
Bestelmeyer BC, Wiens JA (1996) The effects of land use on the structure of ground-foraging ant communities in the Argentine Chaco. Ecol Appl 6:1225–1240. doi:10.2307/2269603
Bestelmeyer BC, Wiens JA (2001) Ant biodiversity in semiarid landscape mosaics: the consequences of grazing vs. natural heterogeneity. Ecol Appl 11(4):1123–1140. doi:10.1890/1051-0761(2001)011[1123:ABISLM]2.0.CO;2
Caro TM, O’Doherty G (1999) On the use of surrogate species in conservation biology. Conserv Biol 13:805–814. doi:10.1046/j.1523-1739.1999.98338.x
Catling PC, Burt RJ (1995) Studies of ground-dwelling mammals of eucalypt forests in south-eastern New South Wales: the effect of environmental variables on distribution and abundance. Wildl Res 22:669–685. doi:10.1071/WR9950669
Cook RD (1977) Detection of influential observation in linear regression. Technometrics 19:15–18. doi:10.2307/1268249
Crawford CS (1988) Surface-active arthropods in a desert landscape: influences of microclimate, vegetation, and soil texture on assemblage structure. Pedobiologia (Jena) 3:373–385
di Castri F, Robertson J, Vernhes J, Younes T (1992) Inventorying and monitoring biodiversity. Biol Int 27:1–27
Ferrier S, Guisan A (2006) Spatial modelling of biodiversity at the community level. J Appl Ecol 43:393–404. doi:10.1111/j.1365-2664.2006.01149.x
Ferrier S, Manion G, Elith J, Richardson K (2007) Using generalized dissimilarity modeling to analyse and predict patterns of beta diversity in regional biodiversity assessment. Divers Distrib 13:252–264
Fisher A, Hunt L, James C et al (2004) Review of total grazing pressure management issues and priorities for biodiversity conservation in rangelands: a resource to aid NRM planning. Desert knowledge CRC and tropical savannas management. CRC, Alice Springs
Fleishman E, Noss RF, Noon BR (2005) Utility and limitations of species richness metrics for conservation planning. Ecol Indic 6(3):543–553. doi:10.1016/j.ecolind.2005.07.005
Fraser SEM, Dytham C, Mayhew PJ (2007) Determinants of parasitoid abundance and diversity in woodland habitats. J Appl Ecol 44(2):352–361. doi:10.1111/j.1365-2664.2006.01266.x
Gaston KJ, Blackburn TM (1995) Mapping biodiversity using surrogates for species richness: macro-scales and new world birds. Proc R Soc Lond B Biol Sci 262(1365):335–341. doi:10.1098/rspb.1995.0214
Gillison AN, Jones DT, Susilo F-X et al (2003) Vegetation indicates diversity of soil macroinvertebrates: a case study with termites along a land-use intensification gradient in lowland Sumatra. Org Divers Evol 3(2):111–126. doi:10.1078/1439-6092-00072
Grove S (2002) Tree basal area and dead wood as surrogate indicators of saproxylic insect faunal integrity: a case study from the Australian lowland tropics. Ecol Indic 1:171–188. doi:10.1016/S1470-160X(01)00016-4
Hansen RA (2000) Effects of habitat complexity and composition on a diverse litter microarthropod assemblage. Ecology 81:1120–1132
Hastie T, Tibshirani R (1990) Generalized additive models. Chapman and Hall, New York
Hoffman BD (2000) Changes in ant species composition and community organization along grazing gradients in semi-arid rangelands of the northern territory. Rangeland J 22(2):171–189. doi:10.1071/RJ0000171
Hughes JB, Daily GC, Ehrlich PR (2000) Conservation of insect diversity: a habitat approach. Conserv Biol 14(6):1788–1797. doi:10.1046/j.1523-1739.2000.99187.x
James CD, Landsberg J, Morton SR (1999) Provision of watering points in the Australian arid zone: a review of effects on biota. J Arid Environ 41:87–121. doi:10.1006/jare.1998.0467
Jiménez-Valverde A, Lobo J (2007) Determinants of local spider (Araneidae and Thomisidae) species richness on a regional scale: climate and altitude vs. habitat structure. Ecol Entomol 32(2):113–122
Kerr JT, Sugar A, Packer L (2000) Indicator taxa, rapid biodiversity assessment, and nestedness in an endangered ecosystem. Conserv Biol 14(6):1726–1734. doi:10.1046/j.1523-1739.2000.99275.x
Lal R (1991) Soil conservation and biodiversity. In: Hawkesworth DL (ed) The biodiversity of microorganisms and invertebrates: its role in sustainable agriculture. CAB International, University of Arizona Press, pp 89–103
Lassau SA, Hochuli DF (2005) Wasp community responses to habitat complexity in Sydney sandstone forests. Aust Ecol 30(2):179–187. doi:10.1111/j.1442-9993.2005.01435.x
Lassau SA, Cassis G, Flemons PKJ et al (2005) Using high-resolution multi-spectral imagery to estimate habitat complexity in open-canopy forests: can we predict ant community patterns? Ecography 28(4):495–504. doi:10.1111/j.0906-7590.2005.04116.x
Ludwig JA, Eager RW, Williams RJ et al (1999) Declines in vegetation patches, plant diversity, and grasshopper diversity near cattle watering-points in the Victoria River district, Northern Australia. Rangeland J 21(1):135–149. doi:10.1071/RJ9990135
MacArthur RH, MacArthur HJ (1961) On bird species diversity. Ecology 42(3):594–598. doi:10.2307/1932254
Majer JD, Nichols OG (1998) Long-term recolonization patterns of ants in western Australian rehabilitated bauxite mines with reference to their use as indicators of restoration success. J Appl Ecol 35:161–182. doi:10.1046/j.1365-2664.1998.00286.x
McGeoch MA (1998) The selection, testing and application of terrestrial invertebrates as bioindicators. Biol Rev Camb Philos Soc 73:181–201. doi:10.1017/S000632319700515X
Noss RF (1990) Indicators for monitoring biodiversity, a hierarchical approach. Conserv Biol 4:355–364. doi:10.1111/j.1523-1739.1990.tb00309.x
Osborn TG, Wood JG, Paltidge TB (1932) On the growth and reaction to grazing of the perennial saltbush (Atriplex vesicarium). An ecological study of the biotic factor. Proc Linn Soc N S W 57:377–402
Perfecto I, Snelling R (1995) Biodiversity and the transformation of a tropical agroecosystem: ants in coffee plantations. Ecol Appl 5:1084–1097. doi:10.2307/2269356
Perrings C, Walker BW (1995) Biodiversity loss and the economics of discontinuous change in semiarid rangelands. In: Perrings C, Mäler K, Folke C, Holling CS, Jansson B (eds) Biodiversity loss economic and ecological issues. Cambridge University Press, Cambridge, pp 190–212
Pringle HJR, Landsberg J (2004) Predicting the distribution of livestock grazing pressure in rangelands. Austral Ecol 29:31–39. doi:10.1111/j.1442-9993.2004.01363.x
Quinn MA, Kepner RL, Walgenbach DD et al (1991) Habitat characteristics and grasshopper community dynamics on mixed-grass rangeland. Can Entomol 123(1):89–105
Samways MJ (2007) Insect conservation: a synthetic management approach. Annu Rev Entomol 52(1):465–487. doi:10.1146/annurev.ento.52.110405.091317
Sauberer N, Zulka KP, Abensperg-Traun M et al (2004) Surrogate taxa for biodiversity in agricultural landscapes of eastern Austria. Biol Conserv 117:181–190. doi:10.1016/S0006-3207(03)00291-X
Schell SP, Lockwood JA (1997) Spatial analysis of ecological factors related to rangeland grasshopper (Orthoptera: Acrididae) outbreaks in Wyoming. Environ Entomol 26(6):1343–1353
Sharley DJ, Hoffmann AA, Thomson LJ (2008) The effects of soil tillage on beneficial invertebrates within the vineyard. Agric For Entomol 10(3):233–243. doi:10.1111/j.1461-9563.2008.00376.x
Stapp P (1997) Microhabitat use and community structure of darkling beetles (Coleoptera: Tenebrionidae) in shortgrass prairie: effects of season, shrub cover, and soil type. Am Midl Nat 137:298–311. doi:10.2307/2426849
Stoms DM, Comer PJ, Crist PJ, Grossman DH (2005) Choosing surrogates for biodiversity conservation in complex planning environments. J Conserv Plann 1:44–63
Tews J, Brose U, Grimm V et al (2004) Animal species diversity driven by habitat heterogeneity/diversity: the importance of keystone structures. J Biogeogr 31:79–92
Whitford WG, Van Zee J, Nash MS et al (1999) Ants as indicators of exposure to environmental stressors in North American desert grasslands. Environ Monit Assess 54:143–171. doi:10.1023/A:1005858918860
Wilkie L, Cassis G, Gray M (2003) A quality control protocol for terrestrial invertebrate biodiversity assessment. Biodivers Conserv 12:99–106. doi:10.1023/A:1021242329800
Acknowledgments
This research was undertaken as part of the Australian Museum–Rio Tinto partnership and funded through the Rio Tinto WA Future Fund. We thank M. Elliott, L. Kampen and M. Bulbert for help with fieldwork. Thanks to D. Smith (ants), S. Ginn (wasps, flies) and G. Brown (beetles) for taxonomic sorting and/or identifications, and Dr C. Reid, Dr D. Bickel, Dr D. McAlpine, and Dr S. McEvey for their taxonomic advice. We also thank two anonymous reviewers for useful comments on an earlier draft.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gollan, J.R., Ashcroft, M.B., Cassis, G. et al. Testing common habitat-based surrogates of invertebrate diversity in a semi-arid rangeland. Biodivers Conserv 18, 1147–1159 (2009). https://doi.org/10.1007/s10531-008-9504-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10531-008-9504-3