Abstract
Red deer (n = 149) from eight geographical locations, including the endangered endemic populations from the Tyrrhenian islands (Sardinia and Corsica), were analysed at eight polymorphic microsatellite loci. Two questions were addressed: (1) Is there a founder effect in the Corsican population, which was reintroduced to the island using Sardinian deer after the species’ extinction on Corsica? (2) What is the origin of the Tyrrhenian or Corsican red deer (Cervus elaphus corsicanus)? Our results showed signs of a founder effect for the red deer on Corsica in that these deer showed differentiation from the Sardinian population as measured by FST values, assignment tests (with and without a priori definition of populations) and individual-based dendrograms. Genetic variability, however, did not differ significantly between the two populations. With respect to the phylogeography of C. e. corsicanus we found that both deer from North-Africa and Mesola on the Italian mainland were genetically close to the Corsican red deer, but phylogenetic trees based on genetic distances were only poorly supported statistically. Among all populations studied the Mesola red deer showed the lowest distance values from Corsican red deer and yielded allele frequencies that were more similar to those of C. e. corsicanus than were those of North-African red deer. These results are in line with recent palaeontological and archaeozoological findings which suggest that the Corsican red deer is derived from small Italian red deer introduced from the mainland to Sardinia and Corsica during the Late Neolithic and just before the beginning of Classical Antiquity, respectively. They also suggest a possible recent introduction of Tyrrhenian red deer to North-Africa (rather than the other way around), thus accounting for the close genetic relationship (especially based on mitochondrial DNA) that has repeatedly been found between C. e. corsicanus and C. e. barbarus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Corsican red deer (Cervus elaphus corsicanus Erxleben, 1777) is not only the smallest of red deer but also one of the most threatened cervid taxa of the world. It is confined to the Tyrrhenian islands of Sardinia and Corsica and listed as endangered by the IUCN (IUCN Red List 2007, Wemmer 1998). Recent population and conservation genetic studies revealed very low levels of genetic variability in the Corsican red deer, in particular at nuclear microsatellite loci (Zachos et al. 2003; Lorenzini et al. 2005; Hmwe et al. 2006a), but over the last decades the deer have recovered from a bottleneck of only 100–150 remaining animals, numbering at least 2,000–2,300 head at the end of the twentieth century (Krumbiegel 1982; Mattioli et al. 2001; Lovari et al. 2007; Banwell (1998) gives an estimate of 1,500 head). After the red deer had become extinct on Corsica around 1970 (Vigne and Marinval-Vigne 1988), the Corsican population was refounded with altogether 13 individuals (four males and nine females) introduced from Sardinia in 1985, 1987 and 1994 (Dolan 1988; Gonzalez and Kidjo 2002; Kidjo et al. 2006; Gonzalez personal communication). A fourteenth specimen, a male, died a few days after its introduction (Kidjo personal communication). These animals were kept in breeding enclosures, and since 1998 there have been repeated releases of deer into the wild resulting in a free-ranging population of about 200 head in 2005 (Kidjo et al. 2006).
The origin of C. e. corsicanus has been a matter of considerable debate (reviewed in Vigne 1988; Zachos and Hartl 2006). There are basically two unresolved problems—where exactly did it come from, and how did it reach the Tyrrhenian islands, naturally or through human introduction? The human introduction hypothesis was already put forward by Lydekker (1898), and Groves (personal communication to Geist 1998) thought that the Corsican red deer was derived from the North-African Barbary red deer (C. e. barbarus) and introduced to the Tyrrhenian islands by the Phoenicians. In accordance with this, Ludt et al. (2004), in a comprehensive phylogeographic study based on cytochrome b sequences, found close relationships between Sardinian and Barbary red deer, which together formed a clade quite distinct from the rest of the Eurasian red deer studied. Introduction from North-Africa to Sardinia has very probably occurred in other mammal species such as hares (Lepus sp., Scandura et al. 2007). It has also been hypothesized that Corsican, Barbary and Spanish (C. e. hispanicus) red deer are closely related (Flerov 1952), which was partly supported for Spanish and Corsican deer by Zachos et al. (2003) and Hmwe et al. (2006a) based on sequences of the mitochondrial control region, but other studies or markers did not confirm these results (Zachos et al. 2003; Ludt et al. 2004; Hmwe et al. 2006a). Yet another genetically based hypothesis was put forward by Hartl et al. (1995) who favoured the Near East as the place of origin of C. e. corsicanus because the Sardinian deer they studied showed mitochondrial RFLP affinities to Bulgarian red deer. This latter population might be phylogenetically similar or even identical to the eastern subspecies C. e. maral (see Groves and Grubb 1987), but none of the subsequent genetic studies corroborated this hypothesis.
Interestingly, preliminary results based on nuclear microsatellite markers showed close relationships between C. e. corsicanus and European mainland red deer (Zachos et al. 2003), in particular those from Mesola in the Po delta (Hmwe et al. 2006a). The Mesola population is special in that it is probably the only truly autochthonous red deer population in the Italian peninsula and thus, it may represent a remnant of the original Italian gene pool (Mattioli 1990; Mattioli et al. 2003). This is in line with close morphological similarities between Holocene bone series from southern Italy and nineteenth and early twentieth century samples from the now extinct original Corsican red deer population (Vigne 1988) and would suggest an introduction of Italian red deer to Sardinia and Corsica or even a natural colonization of the Tyrrhenian islands from mainland Italy, possibly during the maximum of the last (or an earlier) glacial when the sea-level was up to 130 m lower than today (Shackleton 1987). The hypothesis of a natural colonization, however, stands in clear contrast to palaeontological and archaeozoological data (Section “Discussion”). Unfortunately, Hmwe et al. (2006a) did not include Barbary deer into their analysis to directly compare the nuclear genetic affinities of the Corsican red deer to Mesola deer on the one hand and African deer on the other.
Most phylogeographic studies are carried out using mitochondrial sequences such as the control region or the cytochrome b gene. There is, however, a growing body of evidence that these markers are in many cases too conservative to unravel recent demographic events at or below the species level (cf. Cronin 1993; Taberlet et al. 1998; Funk and Omland 2003; Hmwe et al. 2006a). This means that coalescent times of the sequences studied may considerably predate the time of population sundering resulting in a systematic mismatch of gene trees and population trees (Avise 1989, 2000; Pamilo and Nei 1988). Microsatellites, with their very high mutation rates, may be well suited to close this gap between genetic and population “coalescence” and have successfully been used to solve phylogeographic questions in cervids (Goodman et al. 2001). These markers are short tandem-repetitive DNA units which have often been used in population genetic analyses before (e. g., Estonba et al. 2006; Hmwe et al. 2006b; Kalz et al. 2006; Nussey et al. 2006; Redeker et al. 2006; Zachos et al. 2006). In the present study, we analysed for the first time Corsican red deer together with their potentially phylogenetically closest populations (Mesola, representing Italy, northern Africa and Spain) by using a panel of polymorphic microsatellite loci. To make the sampling more comprehensive we also included red deer from several further, taxonomically distinct European populations. In addition, since we had samples from both Sardinia and Corsica, we used our data to address the question of a possible founder effect in the reintroduced Corsican population as compared to its population of origin on Sardinia.
Methods
Sampling
A total of 149 red deer samples was collected from eight different sites in Europe and North-Africa (Fig. 1). We collected tissue samples from culled animals where the species is hunted (Scotland, France, Spain and Romania) or obtained blood samples taken by jugular puncture from live-caught animals (Sardinia and Mesola, cf. Hmwe et al. 2006a). The Corsican samples consisted of pieces of ear tissue sampled when the animals were individually marked for a monitoring project. For details on the sampling of Tunisian Barbary deer see Hajji et al. (2007).
The taxonomic designation of the populations is given in Table 2. There have been many attempts at classifiying red deer into different subspecies, and although the subspecies concept is partly arbitrary and hence not useful as an evolutionary unit (Mayr 1963), subspecific names may serve as terms to designate geographically, morphologically or genetically different populations. In the case of the red deer, the subspecies listed in Table 2 are all morphologically differentiated although this does not necessarily imply that the deer from France and Mesola (both classified as C. e. hippelaphus) are genetically and historically closer to one another than members of different subspecies. It should be made clear that the present study cannot serve as a general systematic analysis of red deer subspecies. For such an analysis to be valid the subspecific designations would first have to be genetically substantiated, and to achieve this, a much more extensive sampling would be necessary in order to cover the whole range of “intra-subspecific” variability. The present choice of populations merely aimed at including geographically and genetically diverse specimens. The taxonomic designations follow Dolan (1988) except for the Carpathian red deer which we separated from the Central European red deer since its genetic distinctness has recently been confirmed (Feulner et al. 2004). Apart from Scandinavian populations or subspecies our sampling thus comprises the great majority of European-type red deer.
Molecular analysis
DNA extraction from tissue samples was carried out either using the Dneasy Tissue Kit (QIAGEN, Hilden, Germany) or following the standard method of proteinase-K digestion, phenol chloroform extraction and precipitation in ethanol (Sambrook and Russel 2001). DNA from faecal material (some of the Tunisian samples) was extracted by means of the QIAGEN QIAMPR DNA stool mini Kit.
Individual genotypes were established through amplification of eight polymorphic microsatellite loci: INRA 121, OarVH110, BL42, BM203, BM757, BMC1009, IDVGA55 and TGLA53 (for further information on these loci and amplification conditions see Bonnet et al. 2002). Due to the comparatively low amount of DNA extracted from the non-invasive Tunisian samples and in order to reduce the risk of overlooking null alleles and large allele dropout we carried out the PCR and allele determination for each Tunisian specimen twice for skin samples, four times for hair samples and six times for faecal samples (multiple-tubes approach, see Taberlet et al. 1996; see also Hajji et al. 2007). Length determination of the microsatellite alleles was conducted with automatic sequencers and the Genescan and Genetic profiler software.
Statistical analysis
The eight loci were tested for pairwise linkage disequilibrium (for each population and over all populations) using the GENEPOP software (Raymond and Rousset 1995). In addition to the multiple-tubes approach we tested our data for null alleles, large allele dropout and stutter bands with the MICRO-CHECKER programme (van Oosterhout et al. 2004) because these potential error sources are known to distort allele frequencies and estimates of heterozygosity (Pemberton et al. 1995; Wattier et al. 1998; Ewen et al. 2000). Especially with null alleles, however, these tests can only serve as a vague indication since null allele probabilities are based on heterozygote deficiencies which are also caused by a variety of other factors such as inbreeding or population substructuring.
To give a general population genetic characterization of the populations studied we carried out the following calculations. Observed (HO) and expected (HE) heterozygosity as well as deviations from Hardy-Weinberg-Equilibrium (HWE) were computed with Arlequin (Schneider et al. 2000). Values of allelic richness were computed with the FSTAT software (Goudet 1995) as a measure of allelic diversity corrected for differences in sample size. Private alleles (alleles present exclusively in one population) were counted and their relative proportions calculated. To gain insight into the total genetic variability of C. e. corsicanus we also calculated variability parameters for the pooled data set of Corsica and Sardinia. To examine the possible differentiation between the Corsican red deer and its population of origin from Sardinia we looked for alleles present in one but not in the other population and tested for significant differences in variability parameters (observed and expected heterozygosity, allelic richness) between Corsica and Sardinia by means of Wilcoxon matched-pairs signed-ranks tests. Apart from this and the FST-based test of population differentiation (see below) we also constructed an individual-based dendrogram with the neighbor-joining algorithm and 1000 bootstrap replicates implemented in the PHYLIP software (Felsenstein 1993) to see if the Corsican deer cluster together relative to the Sardinian ones. Pairwise genetic distances between individuals from Sardinia and Corsica were quantified by (1-Dps) distances (see below). Finally, we used the Bayesian approach of testing for population subdivision without a priori definition of populations implemented in STRUCTURE (Pritchard et al. 2000). The analysis was run under admixture conditions with a burn-in length of 100,000 and a simulation length of 1,000,000 repetitions testing for a subdivision into two different groups.
Differentiation among all populations was tested for with pairwise FST-values. Overall FST- and RST-values (the latter being a microsatellite-specific analogue of FST based on the stepwise mutation model) were calculated with Arlequin as a measure of the amount of genetic variation accounted for by differentiation among (as opposed to variability within) populations.
The question of the phylogeographic origin of the Corsican red deer was statistically addressed as follows. The proportions of C. e. corsicanus alleles present in the other populations were calculated. Assignment tests based on individual genotypes were conducted on the 149 individuals using the Bayesian and the frequency approach as implemented in GENECLASS (Piry et al. 2004). All individuals are assigned to the population of which their genotype is most typical so that incorrect assignments indicate genetic similarity between the respective populations. As in a previous analysis on the phylogeography of the Corsican red deer (Hmwe et al. 2006a) genetic differentiation was further quantified with pairwise Nei’s (1972) standard distances D, Cavalli-Sforza and Edwards’ (1967) chord distances (both computed with PHYLIP) and with the proportion-of-shared-alleles-distances Dps (Bowcock et al. 1994), calculated as (1-Dps) with the programme MICROSAT (Minch et al. 1995). Dps is based on the sums of allele frequencies shared between populations and has been shown to be especially suitable for resolving relationships of closely related taxa with microsatellite data (Cornuet et al. 1999). The distance values were used for tree reconstruction with the neighbour-joining algorithm of the PHYLIP package. Support for nodes was assessed by 1000 bootstrap replicates. The calculation of genetic distances and tree reconstruction was conducted twice–with Corsica and Sardinia as separate populations and with the two samples pooled as C. e. corsicanus.
The significance level of 0.05 was adjusted using Bonferroni corrections (Rice 1989) whenever multiple tests were carried out.
Results
There was no linkage disequilibrium between any two loci, neither in each single population nor over all populations pooled. Therefore, all loci were included in the multi-locus calculations. There was no evidence of large allele dropout in our data set. In one population (Carpathians) locus OarVH110 showed signs of allele frequency distortion through PCR stuttering. Evidence of null alleles was found for single loci in all eight populations, especially in Tunisia and the Tyrrhenian islands (Corsica and Sardinia), which are known to be inbred. Significant deviations from HWE due to an excess of homozygotes occurred in most populations for single loci (the maximum was Tunisia with five loci not at HWE), but none of the loci showed deviations in all populations. In many, but not all cases the loci with significant deviations matched those for which the MICRO-CHECKER analysis suggested the possibility of null alleles.
Values of observed and expected heterozygosity as well as allelic richness and data on private alleles are given in Table 1. We found eight alleles in Corsica that were absent from our Sardinian sample while 21 Sardinian alleles were not found in Corsica. The Wilcoxon tests for differences in variability parameters between Corsica and Sardinia did not yield significant results (observed heterozygosity: P = 0.889, expected heterozygosity: P = 0.293, allelic richness: P = 0.206). However, while for the single populations deviations from HWE were only found at two loci for Sardinia (and at none for Corsica), the pooled data set yielded significant deviations due to an excess of homozygotes at five loci.
Both overall FST (0.202) and RST (0.317) were significantly different from zero, indicating that about 20% or 32% of the genetic variance were accounted for by differentiation among populations and 80% or 68% by variability within them. Pairwise FST-values were all significantly different from zero showing that all population were genetically different from one another. The value between Sardinia and Corsica was 0.151. The assignment tests confirmed these results in that both methods correctly assigned 145 out of 149 individuals to their population of origin. The frequency-based approach incorrectly assigned two Sardinian deer to Corsica, one Scottish deer to the Carpathians and one Carpathian specimen to Scotland. The Bayesian test incorrectly assigned the same two Sardinian samples to Corsica and the same Carpathian deer to Scotland as the frequency-based test but additionally assigned one Corsican deer to Sardinia. The STRUCTURE analysis also yielded results indicating differentiation between Corsican and Sardinian red deer: all 10 Corsican specimens were assigned to group one (probability ranging from 79% to 98%), while only six out of 23 Sardinian individuals were assigned to this group (with lower probabilities ranging from 53% to 90%); the other 17 Sardinian deer were assigned to group two with probabilities between 75% and 98%. Although the two groups are not reciprocally exclusive with respect to the assignment of Corsican and Sardinian individuals, the results clearly show a non-random assignment of the red deer from the two islands indicative of differentiation between Corsica and Sardinia.
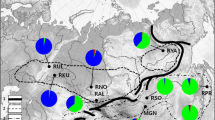
The number of C. e. corsicanus alleles present in the other populations along with their relative proportions (calculated as the percentage of all C. e. corsicanus alleles and as the percentage of alleles present in each population) are shown in Table 2. A graphic representation of allele frequencies at five loci for C. e. corsicanus (Sardinia and Corsica pooled) and the two sites probably closest to it, Tunisia and Mesola, is given in Fig. 2. From the histograms it is obvious that, in terms of allele distribution and frequencies, Mesola is more similar to the Corsican red deer than Tunisia.
Comparative allele frequencies at five microsatellite loci for the Corsican red deer, Tunisia and Mesola. Horizontal axis: allele length, vertical axis: frequency. The histograms are arranged in such a way that the same alleles are located below each other for direct comparison. The five columns of histograms refer to the loci, the three lines to populations
Pairwise genetic chord and (1–Dps) distances are shown in Table 3. Nei’s standard distances are not shown because their relative values were very similar to those of the other two distance measures (smallest value between Corsica and Sardinia; Tunisia, France and Mesola closest to Corsica and Sardinia; small values between Tunisia and Spain etc.). Distance values between the pooled C. e. corsicanus samples and the other populations are given in Table 2.
The trees yielded similar topologies for all three distance measures and irrespective of whether Sardinia and Corsica were combined or not (Fig. 3). The only well-supported node, however, was the one linking Sardinia and Corsica (72–98% bootstrap support). Tunisia and Spain on the one hand and Scotland and the Carpathians on the other were paired in all trees with low support (35–61%). In line with the distance values given in Tables 2 and 3 the population closest to C. e. corsicanus was either Mesola (Dps trees and chord distance tree with Sardinia and Corsica treated separately) or France (standard distance trees and chord distance tree with Sardinia and Corsica combined), but all of these clades yielded bootstrap supports of less than 50%.
The individual-based Dps-tree for Sardinia and Corsica (not shown) yielded two main branches, one consisting of 18 Sardinian deer and one combining all 10 Corsican individuals with five Sardinian deer. The latter contained a subcluster of seven Corsican and two Sardinian individuals. Bootstrap support, however, was low for all nodes.
Discussion
The present study encompasses, for the first time, red deer from North-Africa, Mesola, Corsica and Sardinia in one microsatellite analysis aiming at elucidating the phylogeographic origin of C. e. corsicanus, one of the world’s most threatened deer taxa (Dolan 1988). At the same time, it is also the first genetic analysis of the recently re-founded Corsican red deer population, making it possible to study the founder effect in this population by comparing its genetic characteristics to those of its population of origin on Sardinia.
Although one cannot totally rule out the possibility of the presence of null alleles in our data set, the multiple-tubes approach in our Tunisian samples and the fact that the calculated probability of null alleles was particularly high in populations known to be bottlenecked and inbred favour the conclusion that the detected heterozygote deficiencies and deviations from HWE are better explained by inbreeding than by null alleles.
Founder effect in the Corsican population
C. e. corsicanus clearly still shows the signature of its past bottleneck—as do the populations from Tunisia and Mesola. These three stocks exhibit low or very low genetic variability in a European comparison (see, apart from the other populations in this study, e.g., Zachos et al. 2003, 2007; Feulner et al. 2004; Hmwe et al. 2006a), and antler malformations possibly indicative of inbreeding depression have been observed in C. e. corsicanus (Banwell 1998; see Zachos et al. 2007 for morphological inbreeding depression in a genetically depleted red deer population). Interestingly, there is a considerable amount of genetic differentiation between Sardinia and Corsica as revealed by the significant FST-value of 15%. The fact that the pooled corsicanus sample showed significant deviations from HWE at five loci—while the Corsican population yielded none at all and the Sardinian sample only two—is further evidence of this differentiation as it is best explained by the Wahlund effect—a relative excess of homozygotes in a pooled sample due to substructuring. This is in line with the non-random pattern of Corsican deer in the individual-based tree, the presence of two almost island-specific groups in the results yielded by the STRUCTURE software and the fact that only a minor fraction of C. e. corsicanus specimens was incorrectly assigned to their population of origin. Given that our sample sizes were 23 and 10, respectively, it is not surprising that we found alleles in either Sardinia or Corsica absent from the other one. However, the fact that a 2.3-fold increase in sample size only yielded an increase in allele number of less than 50% (30 alleles from Corsica, 44 from Sardinia) suggests that we have actually detected the majority of alleles present in C. e. corsicanus at the loci studied (otherwise a more linear relationship between sample size and allele number would have been expected). Thus, mutually exclusive alleles may be an additional sign of genetic differentiation. Since the introduction of Sardinian deer on Corsica was carried out only about twenty years ago (Kidjo et al. 2006), corresponding to roughly three generations in red deer, any differentiation found must have been present from the beginning due to the random sample of alleles drawn from the Sardinian population. This founder effect has resulted in incipient genetic differentiation through drift but has not led to a reduced genetic variability in the newly-founded Corsican population as was shown by the lack of significant differences in heterozygosity and allelic richness between the two islands. This may be explained by the low genetic diversity of the source population on Sardinia: a small number of founder individuals is more likely to represent a substantial proportion of the source population’s gene pool if the latter only harbours comparatively small numbers of alleles.
Phylogeography of Cervus elaphus corsicanus
Recent studies based on mitochondrial markers (Ludt et al. 2004; Skog et al. submitted) have shed light on the general phylogeographic patterns in European red deer and identified two main glacial refugia (Iberian peninsula and the Balkans) from which Europe was re-colonized in Holocene times. Palaeontological evidence, on the other hand, suggests up to five refugia including the Italian peninsula, southern France and the Carpathians (Sommer and Nadachowski 2006). In any case, the question of the origin of the red deer on Sardinia and Corsica has been answered differently by various authors. Table 4 summarizes alternative hypotheses based on phenotypic and different kinds of genetic data. The two most probable scenarios involve either mainland Italy or North-Africa, and a direct comparison of these two candidate populations has been one main aim of the present study. Both Spain and Bulgaria/the Near East have not been corroborated as probable origins of C. e. corsicanus in extensive studies with very large data sets of several hundred European red deer (Ludt et al. 2004; Skog et al. submitted), and the present study again shows no evidence of close nuclear genetic relationships between C. e. hispanicus and C. e. corsicanus as can be seen from the genetic distance values, the resulting trees and the relatively low proportion of corsicanus alleles in the Spanish population.
While all populations showed significant genetic differences (see FST-values, private alleles and the very high proportion of correct assignments of individuals to their origin), in terms of genetic similarity to C. e. corsicanus our data clearly yielded two groups of populations: Tunisia, Mesola and France were much closer to the Corsican red deer than Spain, Scotland or the Carpathians. Genetic similarity to Corsican red deer was expected for Tunisia and Mesola but certainly not for France. Introductions of French deer into the gene pool of C. e. corsicanus cannot be excluded, but it also seems possible that French and Corsican red deer would be more distinct if the number of loci analysed were higher (which would be in line with the rather low bootstrap support in the neighbour-joining tree).
Nevertheless, our study again confirmed the close relationship between C. e. corsicanus, Mesola and North-Africa, and among the latter two, Mesola showed lower values of genetic distance from Corsican red deer than Tunisia did. Also, allelic frequencies as shown in Fig. 2 were more similar between Mesola and C. e. corsicanus than between the latter and Tunisia. The present study confirms the results of Hmwe et al. (2006a), but with eight completely different nuclear loci, which adds to the credibility of the genetic similarity between red deer from mainland Italy and the Tyrrhenian islands. This is consistent with archaeozoological analyses which yielded close morphological similarities (small size, simplified antlers, Vigne 1988, 2003) between the late Holocene and modern Corsican red deer on the one hand and the small Holocene red deer from archaeological sites in Italy on the other, indicating that the latter could have given rise to the former during Holocene times. It is highly improbable, though, that the extant Tyrrhenian red deer are direct descendants of the putative Mid-Pleistocene red deer (C. e. rossii, Pereira 2001) because, in spite of the analysis of tens of thousands of animal bones from Corsico-Sardinian palaeontological and archaeological sites, no red deer bones have so far been found for the time between the early Holocene (ca. 11,500 cal. BP) and the mid-Holocene (5000 cal. BP or later) (Vigne et al. 1997; Vigne 1999; Costa et al. 2003). Conversely, large numbers of bones from small C. elaphus specimens have frequently been found in sites from the Late Neolithic (4,500 cal BP) in Sardinia (Fonzo 1987) and from Classical Antiquity in Corsica (Vigne 1988), suggesting human introductions (Vigne 1992, 1999; Masseti 1998, 2002a).
Colonisation events such as these were rather common at the time. Deer were introduced to numerous islands for hunting as early as the 11th millennium BP (Vigne 1993; Masseti 1996, 2002a, b), and a whole wave of non-flying terrestrial mammals (large and small) colonised all the large Mediterranean islands, starting at about 10,500 cal. BP in Cyprus, and more generally speaking, at the beginning of the Neolithic on various islands (Vigne and Alcover 1985; Davis 1987; Vigne 1992, 1999; Blondel and Vigne 1993; Schüle 1993; Dobson 1998; Masseti 1998).
The historical and biogeographical context, the morphological proximity between the Corsican red deer and the early Holocene deer of mainland Italy and the genetic evidence presented and reviewed in the present paper consistently corroborate that the population of C. e. corsicanus was probably founded on the Tyrrhenian islands with Italian mainland deer introduced by humans in the second half of the Holocene. Archaeological and historical observations suggest that these deer introductions aimed at increasing the diversity of large game species on these islands, deer hunting being valued both for meat supply and for socio-symbolic reasons (Vigne 1993).
In North Africa, most of the Late Pleistocene/Early Holocene deer remains belong to Megaceroides algericus, an extinct species which could not have given rise to C. e. barbarus (Hadjouis 1990). The presence of a Late Pleistocene Cervus species in the Maghreb is still questionable (Cheylan 1991), and it is much more probable that modern Barbary red deer are descendants from late Holocene introductions by humans (Kowalski and Rzebik-Kowalska 1991). The present study, having confirmed the genetic similarity of the Corsican red deer to both Mesola and North-Africa in a direct comparison, suggests that red deer from Sardinia/Corsica were introduced to North-Africa (see also Dobson 1998), thus reversing the ancestor-descendant relationship of C. e. corsicanus and C. e. barbarus which has hitherto mostly been depicted as Barbary red deer giving rise to Corsican red deer.
With all the recorded and unrecorded translocations of red deer in Europe (Hartl et al. 2003) it will probably be impossible to uncover the species’ distribution history in all details. The present study, however, has at least reconciled genetic data based on different markers and palaeontological as well as archaeozoological data on the biogeographic history of C. e. corsicanus and its probable origin.
References
Avise JC (1989) Gene trees and organismal histories: a phylogenetic approach to population biology. Evolution 43:1192–1208
Avise JC (2000) Phylogeography. The history and formation of species. Harvard University Press, Cambridge
Banwell B (1998) Description of Cervus elaphus corsicanus. IUCN Deer Spec Group Newslett 14:9
Blondel J, Vigne J-D (1993) Space, time and man as determinants of diversity of birds and mammals in the mediterranean region. In: Ricklefs RE, Schluter D (eds) Species diversity in ecological communities. Historical and geographical perspectives. Chicago University Press, Chicago
Bonnet A, Thévenon S, Maudet F, Maillard JC (2002) Efficiency of semi-automated fluorescent multiplex PCRs with 11 microsatellite markers for genetic studies of deer populations. Anim Genet 33:343–350
Bowcock AM, Ruiz-Linares A, Tomfohrde J, Minch E, Kidd JR, Cavalli-Sforza LL (1994) High resolution human evolutionary trees with polymorphic microsatellites. Nature 368:455–457
Cavalli-Sforza LL, Edwards AWF (1967) Phylogenetic analysis: models and estimation procedures. Am J Hum Genet 19:233–257
Cheylan M (1991) Patterns of Pleistocene turnover, current distribution and speciation among Mediterranean mammals. In: Groves RH, Di Castri F (eds) Biogeography of Mediterranean invasions. Cambridge University Press, Cambridge
Cornuet JM, Piry S, Luikart G, Estoup A, Solignac M (1999) New methods employing multilocus genotypes to select or exclude populations as origins of individuals. Genetics 153:1989–2000
Costa L, Vigne J-D, Bocherens H, Desse-Berset N, Heintz C, de Lanfranchi F, Magdeleine J, Ruas M-P, Thiébault S, Tozzo C (2003) Early settlement on Tyrrhenian islands (8th millennium cal. BC): mesolithic adaptation to local resources in Corsica and Northern Sardinia. In: Larsson L, Kindgre H, Knutsson K, Loeffler D, Åkerlund A (eds) Mesolithic on the Mour. Oxbow Books, Oxford
Cronin MA (1993) Mitochondrial DNA in wildlife taxonomy and conservation biology: cautionary notes. Wildlife Soc Bull 21:339–348
Davis SJM (1987) The archaeology of animals. Batsford Ltd., London
Dobson M (1998) Mammal distributions in the western Mediterranean: the role of human intervention. Mammal Rev 28:77–88
Dolan JM (1988) A deer of many lands—a guide to the subspecies of the red deer Cervus elaphus L. Zoonooz, LXII(10):4–34
Estonba A, Solís A, Iriondo M, Sanz-Martín MJ, Pérez-Suárez G, Markov G, Palacios F (2006) The genetic distinctiveness of the three Iberian hare species: Lepus europaeus, L. granatensis, and L. castroviejoi. Mamm Biol 71:52–59
Ewen KR, Bahlo M, Treloar SA, Levinson DF, Mowry B, Barlow JW, Foote SJ (2000) Identification and analysis of error types in high-throughput genotyping. Am J Hum Genet 67:727–736
Felsenstein J (1993) PHYLIP (Phylogeny Inference Package) version 3.5c. Distributed by the author. Department of Genetics, University of Washington, Seattle
Feulner PGD, Bielfeldt W, Zachos FE, Bradvarovic J, Eckert I, Hartl GB (2004) Mitochondrial DNA and microsatellite analyses of the genetic status of the presumed subspecies Cervus elaphus montanus (Carpathian red deer). Heredity 93:299–306
Flerov CC (1952) Musk deer and deer. In: Fauna of the USSR. Mammals, vols 1, 2. Academy of Sciences, Moscow
Fonzo O (1987) Reperti faunistici in Marmilla e Campidano nell’Età del Bronzo e nella prima Età del Ferro. In: La Sardegna nel Mediterraneo tra il secondo et il primo millennio a.C. Amministrazione Provinciale, Cagliari
Funk DJ, Omland KE (2003) Species-level paraphyly and polyphyly: frequency, causes, and consequences, with insights from animal mitochondrial DNA. Ann Rev Ecol Evol Syst 34:397–423
Geist V (1998) Deer of the world. Their evolution, behavior, and ecology. Stackpole Books, Mechanicsburg
Gonzalez G, Kidjo N (2002) A research project on conservation biology of Corsican red deer (Cervus e. corsicanus). IUCN Deer Spec Group Newslett 17:1–3
Goodman SJ, Tamata HB, Wilson R, Nagata J, Tatsuzawa S, Swanson GM, Pemberton JM, McCullough DR (2001) Bottlenecks, drift and differentiation: the population structure and demographic history of sika deer (Cervus nippon) in the Japanese archipelago. Mol Ecol 10:1357–1370
Goudet J (1995) FSTAT (version 1.2): a computer program to calculate F-statistics. J Hered 86:485–486
Groves CP, Grubb P (1987) Relationships of living deer. In: Wemmer CE (ed) Biology and management of the Cervidae. Smithsonian Institution Press, Washington
Hadjouis D (1990) Megaceroides algericus (Lydekker, 1890), du gisement des Phacochères (Alger, Algérie). Etude critique de la position systématique des Mégacéroides. Quaternaire 1:247–258
Hajji GM, Zachos FE, Charfi-Cheikrouha F, Hartl GB (2007) Conservation genetics of the imperilled Barbary red deer in Tunisia. Anim Conserv 10:229–235
Hartl GB, Nadlinger K, Apollonio M, Markov G, Klein F, Lang G, Findo S, Markowski J (1995) Extensive mitochondrial-DNA differentiation among European Red deer (Cervus elaphus) populations: implications for conservation and management. Z Saeugetierkd 60:41–52
Hartl GB, Zachos F, Nadlinger K (2003) Genetic diversity in European red deer (Cervus elaphus L.): anthropogenic influences on natural populations. CR Biologies 326:S37–S42
Hmwe SS, Zachos FE, Eckert I, Lorenzini R, Fico R, Hartl GB (2006a) Conservation genetics of the endangered red deer from Sardinia and Mesola with further remarks on the phylogeography of Cervus elaphus corsicanus. Biol J Linn Soc 88:691–701
Hmwe SS, Zachos FE, Sale JB, Rose HR, Hartl GB (2006b) Genetic variability and differentiation in red deer (Cervus elaphus) from Scotland and England. J Zool 270:479–487
Kalz B, Jewgenow K, Fickel J (2006) Structure of an otter (Lutra lutra) population in Germany–results of DNA and hormone analyses from faecal samples. Mamm Biol 71:321–335
Kidjo N, Feracci G, Bideau E, Gonzalez G, Marchand B, Aulagnier S (2006) Extinction and reintroduction of the Corsican red deer in Corsica. In: Abstracts of the 1st European congress of conservation biology “Diversity for Europe”, Eger, Hungary 22–26 August 2006
Kowalski K, Rzebik-Kowalska B (1991) Mammals of Algeria. Polish Academy of Science, Wroklaw
Krumbiegel I (1982) Der Korsika-Rothirsch (Cervus elaphus corsicanus, Erxleben 1777) und sein Biotop. Saeugetierkdl Mitt 30:281–286
Lorenzini R, Fico R, Mattioli S (2005) Mitochondrial DNA evidence for a genetic distinction of the native red deer of Mesola, northern Italy, from the Alpine populations and the Sardinian subspecies. Mamm Biol 70:187–198
Lovari S, Cuccus P, Murgia A, Murgia C, Soi F, Plantamura G (2007) Space use, habitat selection and browsing effects of red deer in Sardinia. Ital J Zool 74:179–189
Ludt CJ, Schroeder W, Rottmann O, Kuehn R (2004) Mitochondrial DNA phylogeography of red deer (Cervus elaphus). Mol Phylogenet Evol 31:1064–1083
Lydekker R (1898) The deer of all lands. A history of the family Cervidae living and extinct. Rowland Ward, London
Masseti M (1996) The postglacial diffusion of the genus Dama Frisch, 1775, in the Mediterranean region. Suppl Ric Biol Selvag 25:7–29
Masseti M (1998) Holocene and anthropochorous wild mammals of the Mediterranean islands. Anthropozoologica 28:3–20
Masseti M (2002a) Uomini e (non solo) topi. Firenze University press, Florence
Masseti M (2002b) The red deer of Lampedusa (Pelagian islands, Italy): literary references and osteological evidence. Arch Nat Hist 29:51–66
Mattioli S (1990) Red deer in the Italian peninsula with particular reference to the Po delta population. Deer 8:95–98
Mattioli S, Fico R, Lorenzini R, Nobili G (2003) Mesola red deer: physical characteristics, population dynamics and conservation perspectives. Hystrix Ital J Mammal 14:87–94
Mattioli S, Meneguz PG, Brugnoli A, Nicoloso S (2001) Red deer in Italy: recent changes in range and numbers. Hystrix Ital J Mammal 12:27–35
Mayr E (1963) Animal species and evolution. The Belknap Press of Harvard University Press, Cambridge
Minch E. Ruiz-Linares A, Goldstein D, Feldman M, Cavalli-Sforza LL (1995) MICROSAT (version 1.5b): a computer program for calculating various statistics on microsatellite allele data. Stanford University Press, Stanford
Nei M (1972) Genetic distance between populations. Am Nat 106:283–292
Nussey DH, Pemberton J, Donald A, Kruuk LEB (2006) Genetic consequences of human management in an introduced island population of red deer (Cervus elaphus). Heredity 97:56–65
Pamilo P, Nei M (1988) Relationships between gene trees and species trees. Mol Biol Evol 5:568–583
Pemberton JM, Slate J, Bancroft DR, Barrett JA (1995) Nonamplifying alleles at microsatellite loci: a caution for parentage and population studies. Mol Ecol 4:249–252
Pereira E (2001) Cervus elaphus rossii (Mammalia, Artiodactyla), a new endemic sub-species from the middle Pleistocene in Corsica. Palaeovertebrata 30:189–213
Piry S, Alapetite A, Cornuet J-M, Paetkau D, Baudouin L, Estoup A (2004) GENECLASS2: a software for genetic assignment and first-generation migrant detection. J Hered 95:536–539
Pritchard JK, Stephens M, Donnelly P (2000) Inference of population structure using multilocus genotype data. Genetics 155:945–959
Raymond M, Rousset F (1995) GENEPOP (version 1.2): population genetics software for exact tests and ecumenicism. J Hered 86:248–249
Redeker S, Andersen LW, Pertoldi C, Madsen AB, Jensen TS, Jørgensen JM (2006) Genetic structure, habitat fragmentation and bottlenecks in Danish bank voles (Clethrionomys glareolus). Mamm Biol 71:144–158
Rice WR (1989) Analyzing tables of statistical tests. Evolution 43:223–225
Sambrook J, Russel DV (2001) Molecular cloning: a laboratory manual. Cold Spring Harbour Press, New York
Scandura M, Iacolina L, Ben Slimen H, Suchentrunk F, Apollonio M (2007) Mitochondrial CR-1 variation in Sardinian hares and its relationships with other old world hares (Genus Lepus). Biochem Genet 45:305–323
Schneider S, Roessli D, Excoffier L (2000) Arlequin ver 2.000: a software for population genetics data analysis. Genetics and Biometry Laboratory, University of Geneva, Switzerland
Schüle W (1993) Mammals, vegetation and the initial human settlement of the Mediterranean islands: a palaeoecological approach. J Biogeogr 20:399–412
Shackleton NJ (1987) Oxygen isotopes, ice volume and sea level. Quaternary Sci Rev 6:183–190
Skog A, Zachos FE, Rueness EK, Feulner PGD, Mysterud A, Langvatn R, Lorenzini R, Hmwe SS, Lehoczky I, Hartl GB, Stenseth NC, Jakobsen KS (submitted) Phylogeography of red deer (Cervus elaphus) in Europe
Sommer RS, Nadachowski A (2006) Glacial refugia of mammals in Europe: evidence from fossil records. Mammal Rev 36:251–265
Taberlet P, Fumagalli L, Wust-Saucy A-G, Cosson J-F (1998) Comparative phylogeography and postglacial colonization routes in Europe. Mol Ecol 7:453–464
Taberlet P, Griffin S, Goossens B, Questiau S, Manceau V, Escaravage N, Waits LP, Bouvet J (1996) Reliable genotyping of samples with very low DNA quantities using PCR. Nucleic Acids Res 24:3189–3194
van Oosterhout C, Hutchinson WF, Wills DPM, Shipley P (2004) MICRO-CHECKER (Version 2.2.3): Software for identifying and correcting genotyping errors in microsatellite data. Mol Ecol Notes 4:535–538
Vigne J-D (1988) Les Mammifères post-glaciaires de Corse, étude Archéozoologique (XXVIe suppl. Gallia Préhistoire). CNRS, Paris
Vigne J-D (1992) Zooarchaeology and the biogeographical history of the mammals of Corsica and Sardinia since the last ice age. Mammal Rev 22:87–96
Vigne J-D (1993) Domestication ou appropriation pour la chasse: histoire d’un choix socio-culturel depuis le Néolithique. L’exemple des cerfs (Cervus). In: Desse J, Audoin-Rouzeau F (eds) Exploitation des animaux sauvages à travers le temps. APDCA éd, Antibes
Vigne J-D (1999) The large „true” Mediterranean islands as a model for the Holocene human impact on the European vertebrate fauna? Recent data and new reflections. In: Benecke N (ed) The holocene history of the European vertebrate fauna. Modern aspects of research. Verlag Marie Leihdorf, Rahden
Vigne J-D (2003) L’exploitation des animaux à Torre Sabea. Nouvelles analyses sur les débuts de l’élevage en Méditerranée centrale et occidentale. In: Guilaine J, Cremonesi G (eds) Torre Sabea, un établissement du Néolithique ancien en Salento. Ecole Française, Rome (Collection de l’Ecole Française de Rome 315)
Vigne J-D, Alcover J-A (1985) Incidence des relations historiques entre l’Homme et l’Animal dans la composition actuelle du peuplement amphibien, reptilien et mammalien des îles de Méditerranée occidentale. Actes 110e Congrès Nat. Sociétés Savantes (Montpellier, 1985). Sect Sci 2:79–91
Vigne J-D, Marinval-Vigne M-C (1988) Contribution à la connaissance du Cerf de Corse (Cervus elaphus, Artiodactyla, Mammalia) et de son histoire. Bull Ecol 19:177–187
Vigne J-D, Bailon S, Cuisin J (1997) Biostratigraphy of amphibians, reptiles, birds and mammals in Corsica and the role of man in the Holocene turnover. Anthropozoologica 25–26:587–604
Wattier R, Engel CR, Saumitou-Laprade P, Valero M (1998) Short allele dominance as a source of heterozygote deficiency at microsatellite loci: experimental evidence at the dinucleotide locus Gv1CT in Gracilaria gracilis (Rhodophyta). Mol Ecol 7:1569–1573
Wemmer C (1998) Deer. Status survey and conservation action plan. IUCN/SSC Deer Specialist Group, Gland, Cambridge
Zachos FE, Althoff C, v Steynitz Y, Eckert I, Hartl GB (2007) Genetic analysis of an isolated red deer (Cervus elaphus) population showing signs of inbreeding depression. Eur J Wildlife Res 53:61–67
Zachos FE, Hartl GB (2006) Island populations, human introductions and the limitations of genetic analyses: the case of the Sardinian red deer (Cervus elaphus corsicanus). Hum Evol 21:177–183
Zachos F, Hartl GB, Apollonio M, Reutershan T (2003) On the phylogeographic origin of the Corsican red deer (Cervus elaphus corsicanus): evidence from microsatellites and mitochondrial DNA. Mamm Biol 68:284–298
Zachos FE, Hmwe SS, Hartl GB (2006) Biochemical and DNA markers yield strikingly different results regarding variability and differentiation of roe deer (Capreolus capreolus, Artiodactyla: Cervidae) populations from northern Germany. J Zool Syst Evol Res 44:167–174
Acknowledgements
F. Klein, R. Unici, J. Sale, H. Rose, S. Aulagnier, A. J. Garcia Diaz and N. Kidjo kindly assisted in obtaining samples. Their help is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hajji, G.M., Charfi-Cheikrouha, F., Lorenzini, R. et al. Phylogeography and founder effect of the endangered Corsican red deer (Cervus elaphus corsicanus). Biodivers Conserv 17, 659–673 (2008). https://doi.org/10.1007/s10531-007-9297-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10531-007-9297-9