Abstract
Invasive plants often modify the structure of the community of native plants and animals, but their potential impact on the plant–soil interface is poorly studied. In this study, we looked at the impact of invasive knotweed (Reynoutria spp.) on the taxonomic and functional structure of three trophic levels (plants, detritivores and predators). We wanted to determine if knotweed had a cascading impact from plants to predators. The plants and soil invertebrates were sampled in seven sites in northern France in three knotweed cover classes (control, mid and high). Our results showed that knotweed had a low impact on invertebrate communities despite decreasing plant richness and functional diversity. However, we observed that the functional diversity of detritivores (based on palatability traits) and predators (based on feeding traits) were highly correlated in control sites without knotweed, but that this correlation was no longer present in knotweed invaded sites. This result suggests that feeding interactions are an important feature determining community structure in control plots, but that unidentified factors are more important in the presence of knotweed. Consequently, it can be hypothesised that the presence of knotweed disrupts functional linkages within the soil food web, which may ultimately modify ecosystem stability and functioning.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
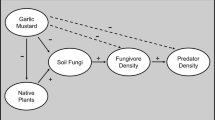
The interactions between plants and soil organisms are known drivers of many ecological processes, such as litter decomposition and nutrient cycling (Bardgett and Shine 1999; Wardle et al. 2004). In turn, they may influence many aspects of plant communities, including successional dynamics or invasibility (Bonkowski and Roy 2012). Although changes in trophic interactions are less visible and harder to detect than impacts on populations and species diversity, especially for plant–soil interactions, they could have important effects on the functioning of ecosystems (Laigle et al. 2018b; Valiente-Banuet et al. 2015). Invasive plant species specifically have been shown to drastically change plant communities and interactions within plants (Lavoie 2017; Wardle et al. 2011). But to our knowledge, few attempts have been made to depict such changes between plants and soil fauna following the ingress of an invasive species. The occurrence of interactions between pairs of species is directly related to their respective characteristics through trait matching (Bartomeus et al. 2016). For example, the biting force of consumers can be related to the toughness of their resources (Brousseau et al. 2018b; Ibanez et al. 2013), while the body size of a predator can be related to the body size of its prey (Gravel et al. 2013). It was shown that matching traits of consumers (feeding traits) and resources (palatability traits) tend to be co-distributed, but perturbations, such as plant invasion, can modify the co-distribution (Brousseau et al. 2019; Le Provost et al. 2017), potentially decreasing the connectance of the food web (Gravel et al. 2016). By reducing the species richness of plants (Hejda et al. 2009), invasive plants also reduce the diversity in palatability traits in the leaf litter (Mincheva et al. 2014), resulting in a lower diversity of niches available for detritivores (Gravel et al. 2016). Thus, we could hypothesise that a lower diversity of palatability traits in leaf litter will have a cascading effect on the functional diversity of the detritivores and the predators.
To test this hypothesis, we focused on invasive knotweed (Reynoutria spp.), a plant complex originating from East Asia that has spread in Europe and North America in a wide range of habitats (e.g. riverbanks, urban areas and roadsides). The plant possesses several characteristics that greatly increase its competitiveness towards local plant species, such as clonal growth, high density, fast growth rate and allelopathic root exudation, which make it one of the most problematic invasive plants in Europe (Rumlerová et al. 2016).
The effect of knotweed invasion on different aspects of the ecosystems in invaded countries was investigated. The invasion always simplifies plant communities (Aguilera et al. 2010; Hejda et al. 2009), but the repercussions for other organisms seem to be variable and may include higher abundance and diversity of pollinators (Davis et al. 2018), higher abundance of amoebae (Bischoff and Connington 2016) and lower abundance and diversity of microarthropods (Skubala and Mierny 2009). The observed reactions of soil macroarthropods and Gasteropoda are inconclusive and could be dependent on the feeding guild studied, with detritivorous species often being more abundant in knotweed-invaded sites (Kappes et al. 2007; Lavoie 2017; Topp et al. 2008). Recently, two meta-analyses (McCary et al. 2016; Abgrall et al. 2019) clearly underlined the role of habitat structure in determining the impact of invasive plant species, including knotweed’s impact on soil fauna. Specifically, it seems that invasion of a closed habitat (e.g. forests) may favour soil faunal abundance, contrary to invasion of an open habitat (e.g. meadows and grasslands).
Looking at the functional traits of plants and invertebrates could help to better understand the impact of knotweed on local communities. Invasive plants, such as knotweed, can modify the structure of invertebrate communities by changing soil chemistry (Dassonville et al. 2007) or microclimate as well as the quantity and quality of decomposable material. The litter of knotweed is not generally regarded as being of good quality, but the high quantity produced could favour fungal growth, which can promote an increase in detritivore abundance in an invaded open habitat (Mincheva et al. 2014). In closed habitats, the amount of litter produced by knotweed, while being the same as in open habitats, may not drastically increase the amount of pre-existing litter on the ground originating from surrounding trees. Nonetheless, in both open and closed habitats, the presence of knotweed decreases the diversity of litter (species richness) and, thus, the diversity of the available feeding niche. As a result, we could expect detritivores communities in knotweed invaded sites to be less diversified functionally than in comparable uninvaded sites. Predators could also be affected in a cascading way by the simplification of detritivore functionality (Brousseau et al. 2019), or more directly if the knotweed leaf litter modify the hiding and foraging opportunities of predators (Bultman and Uetz 1984; Wolkovich et al. 2009).
Identifying the functional response traits of invertebrates susceptible to being influenced by a knotweed invasion can be problematic, as few invertebrate traits have been associated with concrete ecological filters so far (Brousseau et al. 2018a). Furthermore, the few traits that can be generalised to a large number of taxa, such as body size, encompass too many functions to identify mechanisms implied in the modification of the functional structure of the community (Brousseau et al. 2018a; Moretti et al. 2017). Nonetheless, it can be hypothesised that changes in available resources will impact feeding traits, such as biting force (Brousseau et al. 2019), and the hunting strategies of predators (Podgaiski et al. 2013). The changes in the litter structure can also change the moving abilities of the invertebrates and, thus, favour a peculiar body shape or leg length (Kaspari and Weiser 2007).
The objective of this study was first to determine the impact of knotweed on the taxonomic and functional structure of detritivores and predators in open (meadows) and closed (forests) habitats. Then, we wanted to determine if knotweed had a cascading impact from plants to predators and if this impact was habitat-dependent. Based on previous studies on soil macro-invertebrates (Kappes et al. 2007; Lavoie 2017; Topp et al. 2008), we hypothesised that there would be a low impact on their species richness and species community structure. However, we hypothesised that the functional diversity of the resources (plants and prey) would follow the same trajectory as the functional diversity of consumers (detritivores and predators). Thus, by reducing the functional diversity of plants, knotweed will have an indirect cascading deleterious impact on the functional diversity of the detritivores and predators.
Material and methods
Study sites
We selected seven riparian sites in Normandy, France, where knotweed was present but not managed. Knotweeds were morphologically identified as Japanese knotweeds (Reynoutria japonica Houttuyn), but this species is extremely variable in morphology and can be confused with R. x bohemica Chrtek & Chrtková and R. sachalinensis (Schmidt) Nakai, which are also both present in Normandy. Genetic analysis would be required for a more rigorous identification. As a consequence, the term knotweed designates Reynoutria spp. throughout the manuscript. Four sites were in a forested area (F1 to F4) and three were in a meadow (M1 to M3; Table 1) in order to compare two contrasting habitats with different functioning. In each site, sampling was done in three knotweed cover classes in order to have a gradient of knotweed density with potentially significant changes in plant communities within a few metres (~ 2–4 m). The three cover classes included the following: high (monospecific stands of knotweed), mid (knotweed cover between 50–90% mixed with native plants, suggesting a front of knotweed colonisation) and not-invaded control plots (with only native vegetation). These seven sites were chosen among a total of 1123 sites in Normandy where knotweed was recorded. We selected sites according to a set of criteria: sites in riparian areas, presence of the three knotweed cover classes with a surface > 60 m2, knotweed patches that were 10–20 years old and an absence of management for at least 7 years. For forest sites, we excluded sites with knotweed located at the border of stands, and we only sampled patches located in the core of riparian forests. In these invaded forests, knotweed gradually replaces stands and establishes important gaps in the forest with a high density of knotweed. To ensure comparisons, control plots (i.e. not invaded) were also positioned in natural forest gaps. Within each site, sampling was done in three plots of 2 m2 within each of the cover classes (control, mid and high), except for F4, where only two plots were sampled due to the small size of the knotweed patches. Thus, a total of 60 plots were sampled overall.
Vegetation survey
Plant communities were sampled in June 2017 in the 2 m2 quadrats. The abundance of each plant species was determined based on the Braun-Blanquet scale (Braun-Blanquet et al. 1952) and then converted into plants cover percentage using the median value of each cover class. For forest sites, we only sampled understory plant communities since no trunks were located in the quadrats.
To identify and quantify shifts in plant strategies with increasing knotweed cover, we selected the three key traits related to the leaf-height-seed (LHS) plant ecology strategy scheme proposed by Westoby (1998), i.e. height, surface leaf area (SLA) and seed mass (Table 2). We also selected leaf area and leaf N content to respectively capture the competition for light and for nitrogen (Table 2) that could be expected with knotweed presence. Lastly, we also included palatability traits LDMC (leaf dry matter content) and C/N ratio that could impact detritivore communities through quality litter properties (Table 2). All trait values came from the database TRY (Kattge et al. 2011). As much as possible, we only used data that came from Western Europe (France, Belgium, Germany, the Netherlands, and the United Kingdom). When trait data was not available for a species, we measured these traits (see Appendix S1, Table S1). The list of the references used to construct the trait matrix is available in Appendix S2. The mean value of the trait for a species was used in the analyses.
Invertebrates data
Macro-invertebrates were sampled in spring 2017, which represented a period of high biological activity in Normandy and, above all, of vegetation development. A sample consisting of a 25 × 25 cm humic epipedon monolith with the floor and 25 cm topsoil layers was excavated in the centre of the 2 m2 quadrat for each of the 60 plots. The macro-invertebrates were hand sorted in the field. All specimens were immediately stored in a 95% ethanol solution and counted in the laboratory. All invertebrates (mainly Arthropoda, Gasteropoda and Lumbricidae) were identified at a species level or the highest possible taxonomic level (Appendix S1, Tables S3). Taxa that could not be identified at the species level were identified as morphospecies (i.e. they were regrouped as taxonomic units based on shared morphological characteristics). Overall, 145 specimens were identified as morphospecies, representing 10% of our specimens. Of these, 69 were identified at genus level. The principal families concerned are Staphylinidae (49), Linyphiidae (28), Stratiomyidae (10), Limoniidae (8) and Sciaridae (8). In these families, morphospecies were determined based on general appearance (all), mandibular and head shape (Staphylinidae), chelicerae shape and chaetotaxy (Linyphiidae), posterior spiracles and anal lobes (Limoniidae) (see Appendix S3 for more details). When the identification at the morphospecies level was considered unreliable (i.e. for many immature Lumbricidae, Enchytraeidae and damaged specimens), they were removed from the species matrix. Species associated with aboveground vegetation, such as caterpillars feeding on green leaves, which were caught only once or twice overall, were considered as accidental catch and were removed from the species matrix. The mean abundance of arthropods was calculated for each knotweed cover class in a site and was used in the analysis.
Invertebrate traits were selected to represent their biotic interaction (feeding and defence) and moving abilities (Table 2). We selected feeding traits that were previously shown to influence resource selection of arthropods. Biting force represents a limitation to the toughness of used resources (Brousseau et al. 2018b; Le Provost et al. 2017), while biomass represents a limitation to prey size for predators (Gravel et al. 2013). For predators, we also included the hunting strategy (active or use of a web) and the use of poison by spiders and centipedes, which increases the range of prey use (Enders 1975). Antipredation defence was included in the form of cuticular toughness (Brousseau et al. 2018b) and presence/absence of physical (spines, case, hairs) and chemical defence. Finally, the structure of soil litter can influence moving/hunting and hiding ability; we reflect this aspect by considering the body shape and leg length (Kaspari and Weiser 2007). Biomass was measured on at least one specimen per species in each sample. Other morphological traits were measured on up to ten specimens. The biting force at the tip of the mouthpart was measured based on the formula h × b/c, where h is the width of the head behind the eyes (or the size of the left chelicera for arachnids), b is the basal width of the mandible (or movable digit) between the upper condyle and the insertion point of the adductor muscle and c is the length from the upper condyle to the tip (See Brousseau et al. 2019 for more details). It was not possible to calculate a biting force for piercing mouthparts, some Diptera larvae, earthworms and Gasteropoda with this formula. Thus, these species were not considered when calculating the community weight mean (CWM) of the biting force in the analysis. The length of the legs was measured on the second left leg of insects, the third left leg of arachnids and a leg in the middle of the body for Myriapoda. The ratio of the leg length and the body width at the middle of the body was used in the analysis. The shape represents the ratio between body width and body length. Specimen biomass was determined with allometric equations found in Smock (1980) (Diptera larvae), Collins (1992) (Gasteropoda and Lumbricidae), Hódar (1996) (Insecta, Isopoda and Myriapoda) and Höfer and Ott (2009) (Arachnida). The cuticular toughness and physical defence were evaluated visually, while the chemical defence was determined based on data in the Pherobase (El-Sayed 2019). Trait value can be consulted in Appendix S1, Table S4.
Soil characteristics
In each plot, the thickness of the litter layer was measured. Then, 500 g of soil on ~ 5 cm depth was collected for measuring edaphic properties the same day the soil fauna was sampled (i.e. early spring). The fresh soil was sieved through a 2 mm sieve in the laboratory to perform the following standard methods of soil analyses. Relative soil humidity was determined after drying aliquots of 20 g of sieved fresh soil at 105 °C for 48 h. Sieved soil was used to measure microbial biomass, ergosterol and the ammonium and nitrate content. Microbial biomass was determined based on chloroform fumigation of the soil (Jenkinson and Powlson 1976). Microbial C was extracted from fumigated and non-fumigated soil samples with K2SO4 using a Shimadzu TOC-L analyser (Shimadzu Corporation SL, Japan). Four grams of sieved fresh soil was used to measure soil ergosterol content using the method proposed by Gong et al. (2001) and was used as a proxy of soil fungal biomass. Ammonium (NH4+) and nitrate (NO3−) content in the soil were quantified by calorimetry with a Gallery analyser (Thermo Fisher Scientific, Waltham, USA). After air-drying, soil pH was measured in a suspension with 1 mol/L of potassium chloride (1:5, w/v) using a FiveEasy pH meter (Mettler Toledo, USA). Total carbon and nitrogen contents were measured in an elemental analyser (CHN Flash 2000 Thermo Scientific, Milan, Italy) after grinding of dry soil material (mixer mill MM 200, Retsch).
Statistical analysis
All analyses were performed with R v. 3.5.1 (R Core Team 2018). Our first objective was to determine if knotweed cover impacts its edaphic environment and the taxonomic communities of soil macro-invertebrates. We described the edaphic conditions in the different knotweed cover classes with a principal component analysis (PCA). The normality distribution of soil variables (pH, NH4+, C/N ratio, humidity, ergosterol, microbial biomass and litter thickness) was checked with a Shapiro test before the PCA. When necessary, values were log transformed. The pH was removed from further analysis as its distribution was not normal. Two correspondence analyses (CA) were performed for the invertebrate data: the first one on the detritivores and the second one on the predators. Species caught only once were removed from the CA after confirming that this has a low impact on the total inertia and the first eigenvalues (Legendre and Legendre 2012). A generalised linear model (GLM) was fitted to our multivariate data to determine if knotweed cover class, habitat (forest vs. meadows) and sampled sites significantly influenced the distribution of the plots in the CA analysis. The interaction between the knotweed cover class and habitat was also tested. We applied a negative binomial regression, as the mean–variance of our abundance data followed a quadratic relationship (Warton et al. 2012). Residual permutation was used as the resampling method to calculate the p-values. The GLM was executed with the function manyglm of the library mvabund (Wang et al. 2012).
The second objective was to determine if knotweed cover modifies the functional composition of plants, detritivores and predators. A PCA was constructed for each trophic level on the standardised community weight mean (CWM) of their respective functional traits calculated with the function functcomp of the library FD (Laliberté et al. 2014). The CWM for the biomass of earthworms and other detritivores was calculated separately due to the large difference between the two groups (33–64 g for earthworms and 0.02–14 g for the others). The normality distribution of each trait was verified with a Shapiro test before PCA construction. When necessary, the value was log or arcsin transformed. If the transformation did not improve the normality, the trait was removed from further analysis: this included the height of plants and the physical defence of detritivores and predators. We determined if the functional composition was related to knotweed cover class, habitat (forest vs. meadows) and sampled sites by fitting a linear model to our multivariate data. The interaction between knotweed cover class and habitat was also tested. As all kept traits followed normality, we fitted a Gaussian model with the function manylm of the library mvabund (Wang et al. 2012).
Two-way ANOVAs were used to check if knotweed cover had an impact on the richness and the functional diversity of each trophic level (plants, detritivores, predators) and if the impact was stronger in meadows or forested habitats. Type-III sums of squares were used, as our design was unbalanced between forested (four sites) and closed habitats (three sites). For each trophic level, we first measured the species richness as the total number of species sampled in each knotweed cover class (high, mid and control) of a site. Three aspects of functional diversity were measured: Rao's entropy representing the diversity in trait value, functional richness representing the range of trait value and functional dispersion representing the distance of trait value from the mean value (Laliberté and Legendre 2010). Multiple comparisons of knotweed cover classes were assessed with a Tukey's range test.
The final objective was to determine if knotweed cover class interferes with trophic interactions. To do so, the Rao's entropy was calculated for the palatability traits of the resources (plants and detritivores) and for the feeding traits of the consumers (detritivores and predators). Rao's entropy was selected over other diversity indices, as it can be calculated with three or fewer traits, can handle binomial and categorical traits, and is highly related to functional dispersion (Botta-Dukát 2005; Laliberté and Legendre 2010). We evaluated the relationship between the functional diversity of the different trophic levels with linear models. We ran separate analyses on 1) the palatability traits of plants (C/N ratio, LDMC and SLA) and feeding traits of detritivores (biomass, biting force and leg length) and 2) the palatability traits of detritivores (biomass, cuticular toughness, legs length and chemical and physical defence) and the feeding traits of predators (biomass, biting force, hunting strategy, legs length and use of poison). For each pair of trophic levels, we ran separate analyses for (1) all plots, (2) control plots alone and (3) plots with knotweed (mid- and high-cover classes together).
Results
Environment
The PCA on the environmental variables did not show any clear separation between the three knotweed cover classes (Fig. 1a). Nonetheless, high-cover classes in four sites were characterised by a high C/N ratio and low microbial biomass. The linear model showed that the environmental conditions varied between meadows and forests (Dev = 28.6, P < 0.01) and across sites (Dev = 24.7, P < 0.01) but not between knotweed cover classes (Table 3).
Principal component analysis (PCA) on a soil characteristics and b plant functional communities structure based on the community weight mean (CWM) of six traits at three knotweed cover classes. Green = control, yellow = mid, red = high, circle = meadows, triangles = forests. Site numbers refer to Table 1
Plants
Overall, 65 species of plants (including knotweed) were identified, but only ten (five unique to forests, two to meadows and three common to both) were found in plots with high knotweed cover (Appendix S1, Table S2). Only knotweed was found in the high-cover patches of site M1, which impeded calculation of the functional diversity in this plot, so it was removed from some analysis. The PCA on the functional structure of plant communities revealed no clear pattern, although sites with high knotweed cover tend to be characterised by extreme trait values (Fig. 1b). Site M2 was associated with small seeds, leaf area and SLA, while plots in mid- and high-knotweed cover classes in sites F1 and F4 were characterised by heavier seeds and higher LDMC than in their control plots. Few variations were observed within control plots. The functional structure varied only between sites (Dev = 8.2, P < 0.05) based on the linear model.
Detritivores
A total of 1012 detritivores belonging to 84 species were sampled. From the species with an abundance ≥ 5, only the rove beetle Homalota sp. and the earthworm Aporrectodes caliginosa (Savigny) were exclusively found in control plots (Appendix S1, Table S3). Only the earthworm Murchieona muldali (Omodeo) was exclusive to high-knotweed cover plots, but five species (two earthworms, a snail, a millipede and a rove beetle) were found in mid- and high-knotweed cover plots but not in control plots. All plots in the high-knotweed cover class but one forested plot (F1) had similar detritivore communities characterised by the earthworms Dendrodrilus rubidus (Savigny) and M. muldali, the woodlice Haplophthalmus mengei (Zaddach) and Porcelio scaber Latreille and the millipedes Leptoiulus belgicus (Latzel), Ophyiulus pilosus (Newport) and Polydesmus inconstans Latzel (Fig. 2a). The communities in the control and mid-knotweed cover classes were more diversified and better characterised by the sampled sites than the knotweed cover class itself. The GLM also reveals that detritivore communities varied based on the site (Dev = 249.5, P < 0.001), but also according to the interaction between the habitat and knotweed cover class (Dev = 38.7, P < 0.001) (Table 3). The detritivore communities appeared to be more different between knotweed cover classes in open (i.e. meadows) than in closed (i.e. forests) habitats as also observed in the CA (Fig. 2a).
Multivariate analyses on detritivore and predator taxonomic and functional community structures (based on the community weight mean (CWM) of six traits) at three knotweed densities. a Correspondence analysis (CA) detritivore taxonomic structure, b Principal component analysis (PCA) on detritivore functional structure: BiomassEW = earthworms biomass, BiomassD = biomass of other detritivores. c CA on predator taxonomic structure. d PCA on predator functional structure. Green = control, yellow = mid knotweed cover class, red = high knotweed cover class, circle = meadows, triangles = forests. Site numbers refer to Table 1
The functional structure of detritivores was highly related to the sampled sites irrespective of knotweed cover class (Fig. 2b). This observation is confirmed by the linear model showing that the functional structure of detritivores varied between sites (Dev = 23.4, P < 0.01) but not with knotweed cover class (Dev = 12.3, P > 0.05).
Predators
A total of 476 predators belonging to 105 species were sampled. Within the species with an abundance ≥ 5, three ant species (Myrmica rubra (L.), Myrmica vandeli Bondroit and Temnothorax sordidulus Müller) were exclusively found in control plots, and two species (the centipede Geophilus insculptus Attems and the spider Microneta viaria (Blackwell)) were found in mid- and high-knotweed density plots but not in controls (Appendix S1, Table S3). Forest sites (closed habitat) are spread along the first axis irrespective of knotweed cover class but have a low variation along the second axis (Fig. 2c). Meadow sites (open habitats) are separated along the second axis principally based on the presence of the ant species M. rubra and M. vandeli (only found in control plots) and Lasius platythorax Seifert (39 out of 42 specimens found in mid- and high-knotweed cover classes). Within meadows, plots with mid- and high-knotweed cover classes where L. platythorax was absent were more similar to forest plots than to control plots. The GLM shows that predator communities varied both according to the site (Dev = 194.2, P < 0.001) and to the interaction between the habitat and knotweed cover class (Dev = 27, P < 0.001) (Table 3), with a higher variation between knotweed cover classes in meadows than in forested habitats.
The functional structure in high-knotweed cover plots was characterised by extreme trait values compared to control plots (Fig. 2d). Most control plots were centred on the first two axes of the PCA, while high- and mid-knotweed density plots were often placed in the periphery. Based on the linear model, the functional structure of predators was not significantly different between knotweed cover classes, habitats or sites (Table 3).
Functional diversity
The species richness and all aspects of functional diversity (Rao's entropy, functional richness and functional dispersion) of plants were lower in high-knotweed cover plots than in controls (Table 4). Furthermore, this negative impact was more marked in meadows than in forested areas for the species and functional richness. No differences were observed for detritivores and predators.
No correlations were observed between the functional diversity (Rao's entropy) of the plants and the detritivores at any knotweed cover classes (Appendix S4, Figure S7). Including only the detritivores found in the litter (e.g. by excluding those found in the soil per se) did not change the results (result not shown). The regression between the functional diversity of the detritivores and the predators reveals a significantly positive but weak regression when all plots were included in the model (R2 = 0.26, P = 0.01) (Fig. 3). When only considering control plots (i.e. without knotweed), the strength and significativity of the positive regression strongly increased (R2 = 0.77, P = 0.006). The regressions were no more significant when testing only knotweed plots, either mid- or high-cover classes separately or together (results not shown).
Discussion
Our results showed that the lower species and functional diversity of plants in high-knotweed cover class plots did not markedly cascade to the macro-detritivores and predators at either the taxonomic or the functional level. Nonetheless, we observed that detritivore and predator communities tend to vary more across knotweed cover classes in meadows than in forests. Furthermore, the presence of knotweed (in mid and high density) strongly reduced the strength of the correlation between the functional diversity of detritivores and predators.
Cascading effect
Contrary to our hypothesis, the decrease in functional diversity in plant communities did not cascade at the other trophic levels. Previously, Milcu et al. (2013) observed that the functional diversity of plants was a good predictor of the species richness of soil detritivores. Such a relationship could be explained by a covariation between the diversity of the palatability traits of the plants and the diversity of the feeding traits of the detritivores (Brousseau et al. 2019). However, too few studies were conducted on the subject to determine the general mechanism responsible for the relationship between plant and detritivore functional diversity. The principal limit is that the feeding traits of many soil organisms are still undetermined or are poorly documented (Moretti et al. 2017; Brousseau et al. 2018a, b). In this study, we were unable to include any feeding traits of earthworms and Gasteropoda, so the functional diversity of the detritivore was biased towards the arthropods. Also, we used traits measured on living plants, while detritivores are in contact with dead and partially decomposed leaves. Thus, it is hard to say if the absence of a cascading effect between plants and detritivores is real or if we were unable to detect it due to the limitations of the traits of these trophic levels.
Nonetheless, we observed a strong positive relationship between the functional diversity of detritivores and predators in control plots (i.e. without knotweed). This supports the idea that undisturbed sites favour a functional trophic connection between detritivores and predators. The loss of connection (i.e. an insignificant regression) in the presence of knotweed suggests a modification of the trophic interactions within the soil ecosystem. Experimentally, Abgrall et al. (2018) reported that knotweed changes the soil food web through allelopathic compounds or at least secondary metabolites. In their study, like in ours, trophic interactions were also more affected than the abundance of the different trophic groups. As food-web structure is directly related to energy flux and nutrient cycling (Brown et al. 2004; Laigle et al. 2018b), changes in its structure can affect the functioning of the ecosystem (Albouy et al. 2014). However, the exact consequences are hard to predict, as they will depend on the structure of the food web (Gravel et al. 2016). We expect that similar results could be observed with other invasive plants. In our study, no changes in species richness and only a small modification in species composition for detritivores and predators were observed. This could imply that the predators rely more on other sources of food, such as aboveground herbivores or intra-guild predation in the presence of knotweed, although our data do not allow speculation on this aspect. Further studies are required, particularly in conjuncture with decomposition, as changes in prey choice have been proven to impact organic matter decomposition rate (Gessner et al. 2010). Experiments relying on techniques such as DNA metabarcoding of gut content of predators (e.g. Kamenova et al. 2018) in natural sites versus sites invaded by an exotic plant would be required to answer this question.
Functional traits
The results at the functional level tend to show that knotweed does not have a direct filtering effect on the traits that we included in our analysis for any of the trophic levels investigated; i.e. none of the traits varied in function of the knotweed cover class. For plants, the main competition with knotweed in spring could occur underground, while all our traits were on aboveground parts. Large reserves in the rhizomes and allelopathic compounds could provide knotweed with a competitive advantage upon native plant species in spring and partly explain this result. However, it is noteworthy that the allelopathic effect of knotweed is rather inconsistent in the literature (Murrell et al. 2011; Parepa and Bossdorf 2016; Moravcová et al. 2011). This lack of a consistent pattern may arise from the use of many different protocols to simulate allelopathy effects (e.g. use of leachates, synthetic chemical compounds, transplantations of living knotweed individuals or pre-trained soil) and detect responses (e.g. germination or seedling growth on a population or community level) using a single substrate or different substrates. The allelopathic potential of the invasive knotweed is surely context-dependent due to climatic and substrate conditions (Parepa and Bossdorf 2016). Moreover, native plants may also respond differently to the presence of allelopathic compounds (e.g. Moravcová et al. 2011). While there is a clear gap in knowledge on how knotweed invasion may rely on novel weapons (i.e. allelochemicals), we cannot exclude that this mechanism may have played a role in our sites. Identifying traits thwarting allelopathic compounds would increase our ability to predict the impact of knotweed invasion on local ecosystems and help to restore invaded sites (Dommanget et al. 2014).
The traits of the invertebrates were selected to represent their ability to move/hide/hunt and their feeding strategies (Table 2). We hypothesised that knotweed would influence these traits by reducing the quality of leaf litter and simplifying its structure (Mincheva et al. 2014). Our results do not suggest that these aspects played an important role in structuring macro-invertebrate communities in knotweed-invaded sites. Other aspects, such as dispersion ability and response to abiotic constraints, could have played an important role, but traits representing these aspects are unfortunately still missing for soil invertebrates (Moretti et al. 2017; Brousseau et al. 2018a, b). Most of our taxa are expected to have low dispersal ability and are generalist feeders (Scheu 2002; David and Handa 2010). Consequently, they could be slower to react to plant invasion. When considering the 66 species caught more than five times, only eight were absent in control plots (none with an abundance > 10). The main difference between densities was in relative abundance, which goes with the sense of a slow replacement in the invaded plots that were 10–20 years old. Alternatively, the knotweed could simply have a low impact on soil macro-invertebrates, at least in spring.
Community structure
The small impact observed on the soil invertebrate communities is concordant with previous studies (Kappes et al. 2007; Topp et al. 2008), but we demonstrated it with a more diverse community including macro-arthropods, earthworms and gasteropods. Very few species were excluded from any of the knotweed density classes, and the differences between density classes were generally due to variations in the relative abundance of few species. Some interesting results are observed, with ant species M. rudra and M. vandeli being exclusively found in control plots in meadows, while the species L. platythorax was mainly found in knotweed-invaded plots (39/42 specimens). As omnivores, ants could be affected by different ecological filters than strict predators, such as centipedes and spiders. For exemple, it was shown that species in the genus Lasius are commonly attracted by the flowers of knotweed in Italy, while this is a rare occurrence for species in the genus Myrmica (Giuliani et al. 2019). Thus, the attraction to knotweed flowers could partly explain the distribution of ants in our sites. Further studies are required to better understand the impact of knotweed invasion on ant assemblages.
Finally, our results showed that the habitat (meadows vs. forests) determines the structuring impact of the knotweed cover class on the macro-invertebrates at the taxonomic level but not at the functional level. The large amount of litter produced by knotweed (Mincheva et al. 2014) could be more contrasting in meadows than in forest sites areas where trees already provide a high amount of leaf litter. This could be partly supported by our results, as the predator communities in several mid- and high-cover classes plots in the meadows tend to be similar to communities in forested habitats. Abgrall et al. (2019) also observed a stronger respones in the soil food web in open habitats in a meta-analysis including a variety of invasive plants. In contrast, McCary et al. (2016) observed a stronger impact in woodlands, although 82% of the invasive plants included in their study in open habitats were herbaceous plants producing a low quantity of litter.
Conclusion
Our results bring new knowledge about the impact of knotweed invasion on soil macro-invertebrates. Principally, we show that the presence of knotweed modifies the connection between the functional diversity of detritivores and predators in a soil ecosystem. A strong positive regression between predators and detritivores was found only in uninvaded sites. This result could mean that feeding interactions are an important feature for determining community structure in control plots but that other factors are more important in the presence of knotweed (cf. Le Provost et al. 2017). Consequently, it can be hypothesised that knotweed presence simplifies the structure of the food web by decreasing the probability of interactions between co-occurring species (cf. Gravel et al. 2016). In contrast, no correlation was observed between plants and detritivores at any knotweed densities despite the strong decrease in plants richness and functional diversity at high densities. This could mean that feeding interaction is not a primary driver of the detritivore communities in studied sites.
Nonetheless, the impact of invasive plants on food-web structure remains largely unknown. Our results suggest a modification in the interaction between predators and detritivores. However, the current state of knowledge in the literature makes it hard to identify the consequence of the change in food-web structure. Food-web structure was identified as an important factor influencing ecosystem functioning (Laigle et al. 2018b; Valiente-Banuet et al. 2015), but determining the actual structure is generally impossible in the context of most studies. For this reason, we need tools to approximate the food-web structure, such as functional diversity and trait-matching. Recent studies helped to identify important traits related to species interaction and food-web structure in soil (Brousseau et al. 2018b; Laigle et al. 2018a) as well as other compartments of ecosystems (Garibaldi et al. 2015; Ibanez et al. 2013), but very few studies relate functional diversity, food-web structure and ecosystems functioning (but see Dehling et al. 2014; Laigle et al. 2018b). Developing such knowledge is required to make applicable the food-web theories in the context of species invasion and global changes.
Availability of data and materials
All data will be available on Mendeley Data. https://doi.org/10.17632/rf7wdssr9t.1
Code availability
No.
References
Abgrall C, Forey E, Mignot L, Chauvat M (2018) Invasion by Fallopia japonica alters soil food webs through secondary metabolites. Soil Biol Biochem 127:100–109. https://doi.org/10.1016/j.soilbio.2018.09.016
Abgrall C, Forey E, Chauvat M (2019) Soil fauna responses to invasive alien plants are determined by trophic groups and habitat structure: a global meta-analysis. Oikos. https://doi.org/10.1111/oik.06493
Aguilera AG, Alpert P, Dukes JS, Harrington R (2010) Impacts of the invasive plant Fallopia japonica (Houtt.) on plant communities and ecosystem processes. Biol Invasions 12:1243–1252. https://doi.org/10.1007/s10530-009-9543-z
Albouy C, Velez L, Coll M, Colloca F, Le Loc’h F, Mouillot D, Gravel D (2014) From projected species distribution to food-web structure under climate change. Glob Chang Biol 20:730–741. https://doi.org/10.1111/gcb.12467
Bardgett RD, Shine A (1999) Linkages between plant litter diversity, soil microbial biomass and ecosystem function in temperate grasslands. Soil Biol Biochem 31:317–321. https://doi.org/10.1016/s0038-0717(98)00121-7
Bartomeus I, Gravel D, Tylianakis JM, Aizen MA, Dickie IA, Bernard-Verdier M (2016) A common framework for identifying linkage rules across different types of interactions. Funct Ecol 30:1894–1903. https://doi.org/10.1111/1365-2435.12666
Bischoff PJ, Connington K (2016) Winter abundances of naked amoebae in the soil system of the invasive species Japanese knotweed (Fallopia japonica) with comparative data from adjacent sites. Acta Protozool 55:155–160. https://doi.org/10.4467/16890027ap.16.015.5747
Bonkowski M, Roy J (2012) Decomposer community complexity affects plant competition in a model early successional grassland community. Soil Biol Biochem 46:41–48. https://doi.org/10.1016/j.soilbio.2011.11.013
Botta-Dukát Z (2005) Rao’s quadratic entropy as a measure of functional diversity based on multiple traits. J Veg Sci 16:533–540. https://doi.org/10.1111/j.1654-1103.2005.tb02393.x
Braun-Blanquet J, Roussine N, Nègre R, Emberger L (1952) Les groupements végétaux de la France méditerranéenne. CNRS, Paris
Brousseau P-M, Gravel D, Handa IT (2018a) On the development of a functional trait approach for studying terrestrial arthropods. J Anim Ecol 87:1209–1220. https://doi.org/10.1111/1365-2656.12834
Brousseau P-M, Gravel D, Handa IT (2018b) Trait-matching and phylogeny as predictors of predator-prey interactions involving ground beetles. Funct Ecol 32:192–202. https://doi.org/10.1111/1365-2435.12943
Brousseau P-M, Gravel D, Handa IT (2019) Traits of litter-dwelling forest arthropod predators and detritivores covary spatially with traits of their resources. Ecol. https://doi.org/10.1002/ecy.2815
Brown JH, Gillooly JF, Allen AP, Savage VM, West GB (2004) Toward a metabolic theory of ecology. Ecol 85:1771–1789. https://doi.org/10.1890/03-9000
Bultman TL, Uetz GW (1984) Effect of structure and nutritional quality of litter on abundances of litter-dwelling arthropods. Am Midl Nat 111:165–172. https://doi.org/10.2307/2425555
Collins PT (1992) Length-biomass relationships for terrestrial Gastropoda and Oligochaeta. Am Midl Nat 128:404–406. https://doi.org/10.2307/2426474
Dassonville N, Vanderhoeven S, Gruber W, Meerts P (2007) Invasion by Fallopia japonica increases topsoil mineral nutrient concentrations. Ecoscience 14:230–240. https://doi.org/10.2980/1195-6860(2007)14[230:ibfjit]2.0.co;2
David JF, Handa IT (2010) The ecology of saprophagous macroarthropods (millipedes, woodlice) in the context of global change. Biol Rev 85:881–895. https://doi.org/10.1111/j.1469-185X.2010.00138.x
Davis ES, Kelly R, Maggs CA, Stout JC (2018) Contrasting impacts of highly invasive plant species on flower-visiting insect communities. Biodivers Conserv 27:2069–2085. https://doi.org/10.1007/s10531-018-1525-y
Dehling DM, Toepfer T, Schaefer HM, Jordano P, Boehning-Gaese K, Schleuning M (2014) Functional relationships beyond species richness patterns: trait matching in plant-bird mutualisms across scales. Glob Ecol Biogeogr 23:1085–1093. https://doi.org/10.1111/geb.12193
Dommanget F, Evette A, Spiegelberger T, Gallet C, Pacé M, Imbert M, Navas M-L (2014) Differential allelopathic effects of Japanese knotweed on willow and cottonwood cuttings used in riverbank restoration techniques. J Environ Manag 132:71–78. https://doi.org/10.1016/j.jenvman.2013.10.024
El-Sayed AM (2019) The pherobase: database of pheromones and semiochemicals. http://www.pherobase.com/
Enders F (1975) The influence of hunting manner on prey size, particularly in spiders with long attack distances (Araneidae, Linyphiidae, and Salticidae). Am Nat 109:737–763. https://doi.org/10.2307/2459867
Garibaldi LA et al (2015) Trait matching of flower visitors and crops predicts fruit set better than trait diversity. J Appl Ecol 52:1436–1444. https://doi.org/10.1111/1365-2664.12530
Gessner MO, Swan CM, Dang CK, McKie BG, Bardgett RD, Wall DH, Hattenschwiler S (2010) Diversity meets decomposition. Trends Ecol Evol 25:372–380. https://doi.org/10.1016/j.tree.2010.01.010
Giuliani C, Lastrucci L, Cresti L, Santini G, Foggi B, Lippi MM (2019) The morphology and activity of the extrafloral nectaries in Reynoutria × bohemica (Polygonaceae). Plant Biol 21:975–985. https://doi.org/10.1111/plb.13004
Gong P, Guan X, Witter E (2001) A rapid method to extract ergosterol from soil by physical disruption. Appl Soil Ecol 17:285–289. https://doi.org/10.1016/S0929-1393(01)00141-X
Gravel D, Poisot T, Albouy C, Velez L, Mouillot D (2013) Inferring food web structure from predator-prey body size relationships. Methods Ecol Evol 4:1083–1090. https://doi.org/10.1111/2041-210x.12103
Gravel D, Albouy C, Thuiller W (2016) The meaning of functional trait compositions of food webs for ecosystem functioning. Philos Trans R Soc B Biol Sci 371:1–14. https://doi.org/10.1098/rstb.2015.0268
Hejda M, Pyšek P, Jarošík V (2009) Impact of invasive plants on the species richness, diversity and composition of invaded communities. J Ecol 97:393–403. https://doi.org/10.1111/j.1365-2745.2009.01480.x
Hódar JA (1996) The use of regression equations for estimation of arthropod biomass in ecological studies. Acta Oecol 17:421–433
Höfer H, Ott R (2009) Estimating biomass of Neotropical spiders and other arachnids (Araneae, Opiliones, Pseudoscorpiones, Ricinulei) by mass-length regressions. J Arachnol 37:160–169. https://doi.org/10.1636/t08-21.1
Ibanez S, Lavorel S, Puijalon S, Moretti M (2013) Herbivory mediated by coupling between biomechanical traits of plants and grasshoppers. Funct Ecol 27:479–489. https://doi.org/10.1111/1365-2435.12058
Jenkinson DS, Powlson DS (1976) The effects of biocidal treatments on metabolism in soil—I. Fumigation with chloroform. Soil Biol Biochem 8:167–177. https://doi.org/10.1016/0038-0717(76)90001-8
Kamenova S, Bretagnolle V, Plantegenest M, Canard E (2018) DNA metabarcoding diet analysis reveals dynamic feeding behaviour and biological control potential of carabid farmland communities. bioRxiv. https://doi.org/10.1101/332312
Kappes H, Lay R, Topp W (2007) Changes in different trophic levels of litter-dwelling macrofauna associated with giant knotweed invasion. Ecosyst 10:734–744. https://doi.org/10.1007/s10021-007-9052-9
Kaspari M, Weiser M (2007) The size–grain hypothesis: do macroarthropods see a fractal world? Ecol Entomol 32:279–282. https://doi.org/10.1111/j.1365-2311.2007.00870.x
Kattge J et al (2011) TRY—a global database of plant traits. Glob Chang Biol 17:2905–2935. https://doi.org/10.1111/j.1365-2486.2011.02451.x
Laigle I, Aubin I, Digel C, Brose U, Boulangeat I, Gravel D (2018a) Species traits as drivers of food web structure. Oikos 127:316–326. https://doi.org/10.1111/oik.04712
Laigle I, Aubin I, Gravel D (2018b) Species traits and community properties explain species extinction effects on detritus-based food webs. ArXiv:1809.03614v03611
Laliberté É, Legendre P (2010) A distance-based framework for measuring functional diversity from multiple traits. Ecol 91:299–305. https://doi.org/10.1890/08-2244.1
Laliberté É, Legendre P, Shipley B (2014) FD: measuring functional diversity from multiple traits, and other tools for functional ecology, R package version 1.0–12.
Lavoie C (2017) The impact of invasive knotweed species (Reynoutria spp.) on the environment: review and research perspectives. Biol Invasions 19:2319–2337. https://doi.org/10.1007/s10530-017-1444-y
Le Provost G, Gross N, Börger L, Deraison H, Roncoroni M, Badenhausser I (2017) Trait-matching and mass effect determine the functional response of herbivore communities to land use intensification. Funct Ecol 31:1600–1611. https://doi.org/10.1111/1365-2435.12849
Legendre P, Legendre L (2012) Numerical ecology, 3rd edn. Elsevier, Amsterdam
McCary MA, Mores R, Farfan MA, Wise DH (2016) Invasive plants have different effects on trophic structure of green and brown food webs in terrestrial ecosystems: a meta-analysis. Ecol Lett 19:328–335. https://doi.org/10.1111/ele.12562
Milcu A et al (2013) Functionally and phylogenetically diverse plant communities key to soil biota. Ecol 94:1878–1885. https://doi.org/10.1890/12-1936.1
Mincheva T, Barni E, Varese GC, Brusa G, Cerabolini B, Siniscalco C (2014) Litter quality, decomposition rates and saprotrophic mycoflora in Fallopia japonica (Houtt.) Ronse Decraene and in adjacent native grassland vegetation. Acta Oecol 54:29–35. https://doi.org/10.1016/j.actao.2013.03.010
Moravcová L, Pyšek P, Jarošik V, Zákravský P (2011) Potential phytotoxic and shading effects of invasive Fallopia (Polygonaceae) taxa on the germination of native dominant species. NeoBiota 9:31–47. https://doi.org/10.3897/neobiota.9.1266
Moretti M et al (2017) Handbook of protocols for standardized measurement of terrestrial invertebrate functional traits. Funct Ecol 31:558–567. https://doi.org/10.1111/1365-2435.12776
Murrell C, Gerber E, Krebs C, Parepa M, Schaffner U, Bossdorf O (2011) Invasive knotweed affects native plants through allelopathy. Am J Bot 98:38–43. https://doi.org/10.3732/ajb.1000135
Parepa M, Bossdorf O (2016) Testing for allelopathy in invasive plants: it all depends on the substrate! Biol Invasions 18:2975–2982. https://doi.org/10.1007/s10530-016-1189-z
Podgaiski LR, Joner F, Lavorel S, Moretti M, Ibanez S, Mendonça MdS, Jr., Pillar VD, (2013) Spider trait assembly patterns and resilience under fire-induced vegetation change in south Brazilian grasslands. PLoS ONE 8:e60207. https://doi.org/10.1371/journal.pone.0060207
R Core Team (2018) R: A Language and Environment for Statistical Computing, 3.5.1. R Foundation for Statistical Computing, Vienna, Austria
Rumlerová Z, Vilà M, Pergl J, Nentwig W, Pyšek P (2016) Scoring environmental and socioeconomic impacts of alien plants invasive in Europe. Biol Invasions 18:3697–3711. https://doi.org/10.1007/s10530-016-1259-2
Scheu S (2002) The soil food web: structure and perspectives. Eur J Soil Biol 38:11–20. https://doi.org/10.1016/S1164-5563(01)01117-7
Skubala P, Mierny A (2009) Invasive Reynoutria taxa as a contaminant of soil. Does it reduce abundance and diversity of microarthropods and damage soil habitat? Pestycydy 1–4:57–62
Smock LA (1980) Relationships between body size and biomass of aquatic insects. Freshw Biol 10:375–383. https://doi.org/10.1111/j.1365-2427.1980.tb01211.x
Topp W, Kappes H, Rogers F (2008) Response of ground-dwelling beetle (Coleoptera) assemblages to giant knotweed (Reynoutria spp.) invasion. Biol Invasions 10:381–390. https://doi.org/10.1007/s10530-007-9137-6
Valiente-Banuet A et al (2015) Beyond species loss: the extinction of ecological interactions in a changing world. Funct Ecol 29:299–307. https://doi.org/10.1111/1365-2435.12356
Wang Y, Naumann U, Wright ST, Warton DI (2012) mvabund– an R package for model-based analysis of multivariate abundance data. Methods Ecol Evol 3:471–474. https://doi.org/10.1111/j.2041-210X.2012.00190.x
Wardle DA, Bardgett RD, Klironomos JN, Setälä H, van der Putten WH, Wall DH (2004) Ecological linkages between aboveground and belowground biota. Science 304:1629–1633. https://doi.org/10.1126/science.1094875
Wardle DA, Bardgett RD, Callaway RM, Van der Putten WH (2011) Terrestrial ecosystem responses to species gains and losses. Science 332:1273–1277. https://doi.org/10.1126/science.1197479
Warton DI, Wright ST, Wang Y (2012) Distance-based multivariate analyses confound location and dispersion effects. Methods Ecol Evol 3:89–101. https://doi.org/10.1111/j.2041-210X.2011.00127.x
Westoby M (1998) A leaf-height-seed (LHS) plant ecology strategy scheme. Plant Soil 199:213–227. https://doi.org/10.1023/A:1004327224729
Wolkovich EM, Bolger DT, Holway DA (2009) Complex responses to invasive grass litter by ground arthropods in a Mediterranean scrub ecosystem. Oecologia 161:697–708. https://doi.org/10.1007/s00442-009-1425-7
Acknowledgements
PMB was supported by a Grant from the University of Rouen-Normandie. The study was funded by the "Agence de l’eau Seine-Normandie". We thank the Ecodiv lab members for technical assistance and fruitful discussions. We also thank anonymous reviewers which helped improve the manuscript.
Funding
The study was funded by the "Agence de l’eau Seine-Normandie".
Author information
Authors and Affiliations
Contributions
The idea and field work methodology were developed by EF, MC and TDA. Data were collected by TDA, MC, EF and PMB (invertebrate taxonomy) and analysed by PMB. PMB led the writing and all authors contributed critically.
Corresponding author
Ethics declarations
Conflict of interest
The authors declares that they have no conflict of interest statements.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Brousseau, PM., Chauvat, M., De Almeida, T. et al. Invasive knotweed modifies predator–prey interactions in the soil food web. Biol Invasions 23, 1987–2002 (2021). https://doi.org/10.1007/s10530-021-02485-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-021-02485-9