Abstract
Green Iguanas (Iguana iguana) are invasive in Puerto Rico due to a variety of negative economic effects, yet we know very little about their ecological impacts. Because they are herbivorous, defecate intact seeds, move through the forest, and have long gut-passage times, Green Iguanas may affect seed germination and seed dispersal. In summer 2013, a total of 258 Green Iguana scat samples were collected at the Humacao Natural Reserve in southeastern Puerto Rico. Seeds extracted from scat and collected from fruit were planted under common garden conditions using experimental treatments designed to tease apart the effects of feces, fruit, and ingestion on seed germination. Green Iguanas decreased the time for seeds to germinate in Ficus spp. by removing fruit pulp, but had no effect on germination of native Annona glabra seeds. For non-native P. pterocarpus and Pterocarpus spp., Green Iguanas produced conflicting results, decreasing the percentage of seeds germinating, but at the same time, reducing the time for seeds to germinate. Green Iguanas likely disperse most seeds beyond the canopies of parental tree at our site. Government and economic resources are being used to eradicate Green Iguana populations in Puerto Rico, but the lack of consistent effects of Green Iguanas on seed germination for the plant species consumed at our site complicates generalizing about their ecological effects and developing management plans that minimize negative effects for native plant communities. We recommend additional studies that target both species of particular concern, such as threatened native or invasive species, as well as studies of sensitive habitats in Puerto Rico.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Green Iguana (Iguana iguana) has been introduced to Puerto Rico, Florida, and many islands in the Lesser Antilles and Pacific, primarily as a result of the exotic pet trade (Rivero 1998). Its native range extends from Mexico to tropical/subtropical South America (Rivero 1998). Green Iguanas are widespread and abundant in their introduced range with some studies estimating a threefold higher abundance compared to their native range (López-Torres et al. 2012; but see Arce-Nazario and Carlo 2012). Green Iguanas are sometimes associated with agricultural loss, commercial and residential landscape damage, erosion hazards, salmonella infection, and are a threat to aviation because they bask on runways (López-Torres et al. 2012; but see García-Quijano et al. 2011). Because of these negative impacts on the economy, the government of Puerto Rico has classified Green Iguanas as a nuisance pest. Despite these well-known economic costs, we know little about potential ecological effects of Green Iguanas. Introduction of this large herbivore might affect the diversity and abundance of native and non-native plant species through direct herbivory as well as its effect on seed dispersal and germination, which could influence community composition.
Some studies have characterized the distribution, diet, and reproduction patterns of Green Iguanas in Puerto Rico (García-Quijano et al. 2011; Govender et al. 2012; López-Torres et al. 2012), but no study has assessed effects on seed germination and the potential for seed dispersal by Green Iguanas. This species feeds primarily on vegetation, specifically leaves, flowers and fruits (Rand et al. 1990; Lara-López and González-Romero 2002; Figueiredo-de-Andrade et al. 2011; Govender et al. 2012). Green Iguanas have a number of characteristics that should make them successful seed dispersers, including consumption of fruits, relatively long passage times, microbial activity in the intestinal tract, and the ability to swallow food in large portions, which allows them to process seeds without destroying them by chewing (Troyer 1984a,b; van Marken 1992; Schupp 1993). Green Iguanas do not generally have extensive home ranges, varying between 0.65 and 0.95 ha (Rand et al. 1989), and daily movements can average 10 m or less (Escobar et al. 2010). However, their ability to move across the landscape creates the potential for seed dispersal to environments not otherwise easily accessed, such as upstream habitats or dense forests that limit dispersal through the air, and they make some longer-distance movements associated with seasonal reproduction.
Effects of digestion on seed germination are common in reptile, mammal, and bird seed dispersers, both enhancing and inhibiting seed germination (Traveset 1998). For example, frugivores potentially enhance germination by removing pulp from the seeds, which can contain germination inhibitors or support potentially infectious fungi and bacteria (Traveset 1998). Green Iguanas are known to decrease the time to germination under experimental conditions for some plant species in their native range (Morales-Mávil 1997; Benítez-Malvido et al. 2003). Whether Green Iguanas have a significant effect on seed germination in Puerto Rican plant communities composed of both native and non-native plant species is not known.
In this study, we assessed the potential for invasive Green Iguanas to effect seed germination and seed dispersal in a mesic, mangrove habitat in Puerto Rico. We experimentally tested for possible seed germination effects for several tropical plant species that are naturally consumed by Green Iguanas. We also quantified minimum dispersal distances for the most common seeds consumed by Green Iguanas. Because Green Iguanas are the largest and most abundant vertebrate frugivore in Puerto Rican mangrove forests, their impact on seed germination may be important for plant community structure (Iverson 1985). Previous studies of Green Iguana diet in Puerto Rico have found they consume plant material from both native and non-native species, including seeds of the highly invasive Brazilian pepper, Schinus terebinthifolius (Govender et al. 2012). If Green Iguanas promote the germination of non-native species compared to native species, then they could pose a threat to native communities by facilitating the spread of potentially invasive species.
Methods
We conducted this study at the Natural Reserve of Humacao (NRH) in southeastern Puerto Rico. Within the NRH, we concentrated our efforts near the Palmas Lagoon, a mangrove forest with white mangroves (Laguncularia racemosa) and successional vegetation on higher elevation substrate. We collected Green Iguana scat samples from mid-June through July 2013 in ~0.15 km2 of the 10.5 km2 reserve, focusing primarily along hiking paths in the forest. Samples were found primarily on the ground, and locations of most scat samples were recorded using GPS. We were careful to collect all adjacent pellets as a single sample. We also recorded GPS coordinates for all mature trees of our focal species in the study area.
Green Iguana scat samples were stored out of the sun in plastic bags at room temperature, and dissected less than 1 week after collection. We used a dissecting microscope and magnifying glass to extract seeds from the scat sample; some small seeds may have been below detection limits using this method. No water was used because it could initiate the germination process prior to planting the seeds. We identified seeds in scat samples from four main tree species—Annona glabra, Ficus spp., Peltophorum pterocarpum, and Pterocarpus spp.—by comparing them to reference samples from fruits collected at our site (see details in the Supplementary Materials). For germination trials, we used these seeds and others collected from 3 to 10 individuals of each tree species for control treatments.
Our germination experiment consisted of four treatments under common growth conditions: (1) seeds that have not passed through the Green Iguana gastrointestinal tract without fruit pulp; (2) seeds that have not passed through the Green Iguana gastrointestinal tract with 0.5 g fruit pulp; (3) seeds that have passed through the Green Iguana gastrointestinal tract mixed with 0.5 g fecal material; and (4) seeds that have passed through the Green Iguana gastrointestinal tract without fecal material. These four treatments allow us to separate the effects of gut-passage, fruit pulp removal, and the presence of feces (Samuels and Levey 2005), giving us a robust way to evaluate the overall effect of Green Iguanas on seed germination.
Seeds were planted individually in clean commercial soil in plastic germination domes as they were found in scat samples, alternating treatments. Up to 14 seeds from each scat sample were planted with an equivalent number of control seeds. For A. glabra, individual unaltered seeds were used because this species produces discrete units of pulp with each seed. For treatments that included clean seeds, tissue paper was used to clean the seeds, but not water. All treatments were not possible for Peltophorum pterocarpum and Pterocarpus spp. because feces could not be completely cleaned from the seeds, so the treatment consisting of ingested seeds without feces was not possible. Additionally, the treatment of seeds with fruit pulp was not possible because both species have fruits that lack pulp. Domes were housed in a covered, well-lighted area near the University of Puerto Rico Rio Piedras, exposed to natural light cycles, and rotated daily to account for possible microclimate differences. Seeds were watered daily using a pressure sprayer for a period of 60 days and checked daily for germination. Successful germination was scored when shoots emerged from the soil.
To estimate the minimum dispersal distance of seeds, we calculated the difference between the mean overstory radius for a sample of trees from each focal species and the mean distance from scat samples containing seeds to the nearest seed-producing tree of the same species. We considered this a conservative estimate of seed dispersal distance by Green Iguanas at our study site (Moura et al. 2015).
We measured two responses in the germination experiment, germination percentage (i.e., the number of germinating seeds out of the total number planted) and time to germination (i.e., the number of days to germination). We used analysis of variance (ANOVA) to test for differences among the treatments for (log) time to germination, and Tukey’s Honestly Significant Difference (HSD) tests to determine differences among treatment levels. We used a General Linear Model (GLM) to test for differences among the treatments for germination percentage, following Crawley (2007) but implemented in JMP v. 11 (SAS Institute, Inc 2014). Because germination percentage was calculated as a proportion, the results were bounded (0–1). We used a binomial distribution using a binomial denominator, which is a two-vector response that accounts for the number of germinated seeds versus the number of planted seeds. We used analysis of variance (ANOVA) to test for differences among the focal species in (log) minimum distance of scat samples from the nearest parental tree, and Tukey’s HSD tests to determine differences among the four focal species. We used JMP v. 11 to conduct all statistical analysis (SAS Institute, Inc 2014).
Results
We collected a total of 258 Green Iguana scat samples, of which 122 (47 %) contained seeds. Most scat samples contained only one species of seed (68 %), but some contained two (27 %) or three (5 %) different species of seeds with an average of 8.2 seeds per scat (Table 1). Annona glabra was the most common species found in scat at 36 % of samples, followed by Ficus spp. at 21 %, an unidentified single species ‘Unknown’ at 21 %, P. pterocarpum at 13 %, and Pterocarpus spp. at 11 % (Table 1). In terms of the total numbers of seeds in scat, A. glabra seeds were most common at 49 %, followed by Ficus spp. at 17 %, P. pterocarpum at 4 %, and Pterocarpus spp. at 3 % (Table 1).
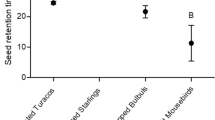
There were no significant differences in germination percentage among treatments for A. glabra (Χ2 = 1.09, df = 3, P = 0.77) or Ficus spp. (Χ2 = 1.26, df = 3, P = 0.73; Table 2). In contrast, a greater percentage of uningested seeds without fruit pulp than ingested seeds with feces germinated for P. pterocarpum (Χ2 = 4.90, df = 3, P = 0.03) and Pterocarpus spp. (Χ2 = 6.97, df = 3, P = 0.008; Table 2), but few total seeds germinated for these species. Time to germination did not differ among treatments for A. glabra (F3,19 = 2.01, P = 0.48; Table 2). In contrast, germination times differed among treatments for Ficus spp. (F3,116 = 3.72, P = 0.01), P. pterocarpum (F1,16 = 9.68, P = 0.007), and Pterocarpus spp. (F1,17 = 10.6, P = 0.005; Table 2; Fig. 1). Green Iguana effects on seed germination differed among the four focal plant species, decreasing the percentage of seeds germinating in some instances (P. pterocarpum and Pterocarpus spp.) and reducing the number of days to germination in others (Ficus spp., P. pterocarpum, and Pterocarpus spp.).
The minimum distance between Green Iguana scat samples containing seeds of a focal species and the nearest mature tree of the same species differed among the four focal species (F3,50 = 6.84, P = 0.0006). Tukey’s HSD tests showed that A. glabra seeds (mean = 33.0 m) were found significantly farther from the nearest tree than Ficus spp. seeds (mean = 9.9 m; Fig. 2; Supplementary Table 1). For all species, the average minimum dispersal distance exceeded the mean canopy shadow as measured by the radius of the overstory canopy (Supplementary Table 1), suggesting a role for Green Iguanas in seed dispersal regardless of their effects on seed germination.
Frequency of minimum distances of scat samples to seed-producing trees of the same species (m) for a Annona glabra (n = 23) and b Ficus spp. (n = 21) seeds found in Green Iguana scat samples. Minimum dispersal distances should be discounted by taking into account the overstory canopy radius for each tree species, which is on average 2.2 m for Annona glabra and 0.8 m for Ficus spp.
Discussion
Green Iguanas in Puerto Rico consumed fruits from both native and non-native plant species and these seeds were likely dispersed beyond the canopies of parent trees in most cases. Effects on seed germination varied among tree species as well as between measures of seed germination, which makes predicting the community-wide impacts of fruit consumption by invasive Green Iguanas difficult.
Ficus spp. can be a keystone species for herbivores due to their abundant year round fruit presence (Santinelo Pereira et al. 2007). Yet, native range studies have found no evidence of Ficus seed consumption by Green Iguanas (Rand et al. 1990; Lara-López and González-Romero 2002; Benítez-Malvido et al. 2003). In contrast, Green Iguanas in Puerto Rico had Ficus spp. seeds in 21 % of scat samples and fruit pulp removal led to a 24–35 % reduction in the number of days for Ficus spp. seeds to germinate. Whether these effects on seed germination and dispersal by Green Iguanas are positive or negative for native plant communities in Puerto Rico depends on whether seeds are from native F. citrifolia or non-native F. benjamina, which we were unable to differentiate after Green Iguanas consumption.
Our two measures of germination success differed for P. pterocarpus and Pterocarpus spp. Ingestion by Green Iguanas reduced the number of days to germination for P. pterocarpum by 53 % and for Pterocarpus spp. by 63 %, but also decreased the percentage of seeds germinating in both species. Consumption and dispersal of seeds from the year round fruiting, non-native P. pterocarpum could make this species a high risk to native plant communities. However, these potential negative effects are tempered in this case because P. pterocarpum seeds account for only 3.5 % of the total number of seeds in Green Iguana scat. The seeds of other non-natives, such as the invasive Brazilian pepper (Schinus terebinthifolius) may be also affected by Green Iguana ingestion and dispersal, however, we could not determine the effect on this species because it was not present at our study site.
For native A. glabra, ingestion of seeds by Green Iguanas does not appear to affect germination; however, seed germination was overall very low for A. glabra as in previous studies (Infante Mata and Moreno-Casasola 2005; Table 2). Given that A. glabra seeds represented almost half of all seeds in feces (Table 1), Green Iguanas at our site may heavily use these fruits during summer months. This differs from previous studies in Puerto Rico and the native range where Green Iguanas occupy A. glabra trees, but have not been observed feeding on this species (Lara-López and González-Romero 2002; Figueiredo-de-Andrade et al. 2011). Annona glabra is a major component of the diet of the Puerto Rican slider turtle (Trachemys stejnegeri stejnegeri), which is a near threatened species that is listed as a critical element of Puerto Rico’s fauna (Tortoise and Freshwater Turtle Specialist Group 1996). The Puerto Rican Department of Natural Resources (Departamento de Recursos Naturales y Ambientales 2009) lists the seasonally available fruit (from March to November) of A. glabra as an important component of the conservation of Puerto Rican sliders. Green Iguana consumption of A. glabra fruit might negatively affect Puerto Rican sliders by decreasing this food source. In contrast, Green Iguanas may have a positive effect by dispersing native A. glabra seeds, particularly to environments not otherwise easily accessed, such as upstream habitats and non-flooded inland habitats. However, whether these seeds are deposited in suitable microenvironments is unknown. Understanding the relative importance of positive and negative effects of Green Iguanas on A. glabra is an important area of future study.
Although eradication and control efforts of Green Iguanas to address potential economic loss and various hazards are already occurring in Puerto Rico, lack of information on specific negative ecological effects of this invasive species limit efforts to reduce ecological impacts. Our study shows that Green Iguanas effect seed germination and dispersal of both native and non-native plant species. Given these species-specific effects, it is difficult to predict the overall effects of Green Iguanas on mangrove forests and other communities in Puerto Rico. Therefore, future ecological research should target specific plant species and communities to assess the longer-term effects of Green Iguanas on seed germination and dispersal, particularly on plant species available at different times of the year.
References
Arce-Nazario JA, Carlo TA (2012) Iguana iguana invasion in Puerto Rico: facing the evidence. Biol Invasions 14:1981–1984
Benítez-Malvido J, Tapia E, Suazo I, Villaseñor E, Alvarado J (2003) Germination and seed damage in tropical dry forest plants ingested by iguanas. J Herpetol 37:301–308
Crawley MJ (2007) The R book. Wiley, West Sussex, pp 511–526
Departamento de Recursos Naturales y Ambientales (2009) Plan de manejo: Reserva natural el Pantano Bosque Pterocarpus y Lagunas Mandry y Santa Teresa en Humacao, Technical Report
Escobar RA, Besier E, Hayes WK (2010) Evaluating headstarting as a management tool, post-release success of green iguanas (Iguana iguana) in Costa Rica. Int J Biodivers Conserv 2:204–214
Figueiredo-de-Andrade CA, Montoya-Ospina RA, Voltolini JC, Ruiz-Miranda CR (2011) Population biology and behaviour of the alien species Iguana iguana (Linneaeus, 1758) on a restored wetland in Puerto Rico. Herpetol Notes 4:445–451
García-Quijano CG, Carlo TA, Arce-Nazario J (2011) Human ecology of a species introduction: interactions between humans and introduced Green Iguanas in a Puerto Rico urban estuary. Hum Org 70:164–178
Govender Y, Muñoz M, Ramírez L, Puente-Rolón A, Cuevas E (2012) An isotopic study of diet and muscles of the Green Iguana (Iguana iguana). J Herpetol 46:167–170
Infante Mata D, Moreno-Casasola P (2005) Effect of in situ storage, light, and moisture on the germination of two wetland tropical trees. Aquat Bot 83:206–218
Iverson JB (1985) Lizards as seed dispersers? J Herpetol 19:292–293
Lara-López M, González-Romero A (2002) Alimentación de la Iguana verde Iguana iguana (Suqamata: Iguanidae) en la Mancha, Veracruz, Mexico. Acta Zool Mex 85:139–152
López-Torres AL, Claudio-Hernández HJ, Rodríguez-Gomez CA, Longo AV, Joglar RL (2012) Green Iguanas (Iguana iguana) in Puerto Rico: is it time for management? Biol Invasions 14:35–45
Morales-Mávil JE (1997) Viabilidad de semillas ingeridas por la iguana verde (Iguana iguana L.) en la zona de la Palma, region de Los Tuxtlas, Veracruz. Unpubl. Master’s thesis, Instituto de Neuroe-tologia, Universidad Veracruzana, Xalapa, Veracruz, Mexico
Moura AC, Cavalcanti L, Leite-Filho E, Mesquita DO, McConkey KR (2015) Can green iguanas compensate for vanishing seed dispersers in the Atlantic forest fragments of north-east Brazil? J Zool 215:189–196
Rand AS, Font E, Ramos D, I-Werner D, Bock BC (1989) Home range in Green Iguanas (Iguana iguana) in Panama. Copeia 1989:217–221
Rand AS, Dugan A, Monteza H, Vianda D (1990) The diet of a generalized folivore: Iguana iguana in Panama. J Herpetol 24:211–214
Rivero JA (1998) The amphibians and reptiles of Puerto Rico, 2nd edn. Editorial de la Universidad de Puerto Rico, Puerto Rico
Samuels IA, Levey DJ (2005) Effects of gut passage on seed germination: do experiments answer the question they ask? Funct Ecol 19:365–368
Santinelo Pereira RA, Rodrigues E, Oliverira-Menezes A Jr (2007) Phenological patterns of Ficus citrifolia (Moraceae) in a seasonal humid-subtropical region in Southern Brazil. Plant Ecol 188:265–275
SAS Institute, Inc. (2014) JMP 11.1 Cary, NC
Schupp W (1993) Quantity, quality and the effectiveness of seed dispersal by animals. Vegetatio 107(108):15–29
Tortoise and Freshwater Turtle Specialist Group (1996) Trachemys stejnegeri. In: IUCN Red List of Threatened Species. Version 2014.2. Accessed 12 November 2014. www.iuncredlist.org
Traveset A (1998) Effects of seed passage through vertebrate frugivores’ guts on germination: a review. Perspect Plant Ecol Evol Syst 1:151–190
Troyer K (1984a) Structure and function of the digestive tract of a herbivorous lizard Iguana iguana. Physiol Zool 57:1–8
Troyer K (1984b) Diet selection and digestion in Iguana iguana: the importance of age and nutrient requirements. Oecologia 61:201–207
van Marken L (1992) Digestion in an ectotheric herbivore, the Green Iguana (Iguana iguana): effect of food composition and body temperature. Physiol Zool 65:649–673
Acknowledgments
We would like to thank Mahmoud Abouhkeir and James Ackerman from the University of Puerto Rico Rio Piedras Museum of Zoology for providing laboratory equipment, and Luis Encarnación and his staff at the Puerto Rico Department of Natural Resources Humacao Natural Reserve for providing useful information and assistance. This work was conducted with permission from the Puerto Rican Department of Natural Resources permit number 2013-IC-027 and approved by the University of Rhode Island Institutional Animal Care and Use Committee (AN13-03-011). We thank Evan Preisser, Nancy Karraker and Tomás Carlo for valuable suggestions on the experimental design, statistical analyses, and text of the manuscript; Luis López, Javier López, and Jose, Clarisa and Francis Burgos-Rodríguez for help in the field; and four anonymous reviewers and the Associate Editor for helpful comments that improved the manuscript. The University of Rhode Island provided financial support.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Burgos-Rodríguez, J.A., Avilés-Rodríguez, K.J. & Kolbe, J.J. Effects of invasive Green Iguanas (Iguana iguana) on seed germination and seed dispersal potential in southeastern Puerto Rico. Biol Invasions 18, 2775–2782 (2016). https://doi.org/10.1007/s10530-016-1190-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-016-1190-6