Abstract
The environmental and social impacts of Phragmites australis invasion have been extensively studied in the eastern United States. In the West where the invasion is relatively recent, a lack of information on distributions and spread has limited our ability to manage invasive populations or assess whether native populations will experience a decline similar to that in the East. Between 2006 and 2015, we evaluated the genetic status, distribution, and soil properties (pH, electrical conductivity, and soil texture) of Phragmites stands in wetlands and riparian systems throughout the Southwest. Native (subspecies americanus), Introduced (haplotype M), and Gulf Coast (subspecies berlandieri) Phragmites lineages were identified in the survey region, as well as watershed-scale hybridization between the Native and Introduced lineages in southern Nevada. Two Asian haplotypes (P and Q) that were previously not known to occur in North America were found in California. The Native lineage was the most frequent and widespread across the region, with four cpDNA haplotypes (A, B, H, and AR) occurring at low densities in all wetland types. Most Introduced Phragmites stands were in or near major urban centers and associated with anthropogenic disturbance in wetlands and rivers, and we document their spread in the region, which is likely facilitated by transportation and urban development. Soil pH of Native and hybrid stands was higher (averaging 8.3 and 8.6, respectively) than Introduced stands (pH of 7.5) and was the only soil property that differed among lineages. Continued monitoring of all Phragmites lineages in the Southwest will aid in assessing the conservation status of Native populations and developing management priorities for non-native stands.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Human-linked environmental stressors are causing rapid declines in indigenous species worldwide. These byproducts of human society, such as habitat loss, degradation (Blair 2001), or introduction of exotic species (Vitousek et al. 1997; Walck et al. 1999) are often cited as primary causes of native species declines, but frequently, multiple factors (Trentanovi et al. 2013) can impact biological communities simultaneously (Czech and Krausman 1997). Compared with natural ecosystems, urban centers tend to have disproportionately higher numbers of non-native species (Kowarik 1995; Trentanovi et al. 2013), and when coupled with other associated anthropogenic disturbances, overwhelm the ability of native species to persist (McKinney 2002). Successful conservation strategies for native plant populations depend on accurate identification and assessment of population trends in the face of environmental change. A critical challenge for plant conservation is obtaining adequate biological and environmental information necessary to evaluate the status of vulnerable species or determine if protection is needed (Schemske et al. 1994). Moreover, misidentification of members of cryptic species complexes during rare species assessment or pest management efforts can have serious unintended effects on native plant populations (Bickford et al. 2007). Collection and dissemination of accurate biogeographical data are essential for timely responses to threats to imperiled species and effective conservation actions.
Wetlands in the Southwest United States are hotspots of biodiversity, and at the same time, are among the most rare and imperiled systems in North America (Hendrickson and Minckley 1985; Unmack and Minckley 2008; Ball-Damerow et al. 2014). These habitats include ephemeral riparian zones, isolated springs and seeps, and less frequently, low gradient rivers with perennial flows. Many of these wetland types (except for perennial rivers) are supported by artesian spring features created where aquifers meet the ground surface or along geologic faults and fractures (Sivinski and Tonne 2011). The most abundant plant species common to these systems include reed grasses (Phragmites australis and Arundo donax), sedges (Schoenoplectus americanus and S. acutus), willows (Salix spp.), Fremont cottonwood (Populus fremontii), seep willow (Baccharis salicifolia), mesquite (Prosopis spp.), arroweed (Pluchea sericia), and Chenopods (Atriplex spp.) (Hendrickson and Minckley 1985).
Because of the harsh environmental conditions and isolation of desert wetlands, associated species tend to have narrow geographic ranges and high degrees of endemism (Tiner 2003). These species also tend to have low abundances, which increases their risk of extinction from environmental change (Gaston 1998). This risk is exacerbated by rapid urbanization and groundwater depletion, as well as climate change and encroachment by non-native species occurring in this region (Deacon et al. 2007; Unmack and Minckley 2008). Wetland systems are particularly vulnerable to plant invaders because they are often the ultimate repository of plant propagules from upstream sources (Stohlgren et al. 1998; Zedler and Kercher 2004). Several of the worst invasive plant species are non-native macrophytes that form monocultural stands and reduce native floral and faunal diversity of the wetlands they invade (Daehler and Strong 1996; Dudley 2000; Shafroth et al. 2005). One of the most extensive invasion processes in North America involves various genetic forms of Phragmites australis Cav, (Trin.) ex. Steud. (common reed; Saltonstall 2002).
Phragmites is one of the most prevalent species in North American wetlands and riparian systems, with a complex of native and introduced lineages occurring across the continent. The native lineage (P. australis subsp. americanus; hereafter Native Phragmites) is genetically diverse and the most widespread lineage throughout the West (Saltonstall 2002; Meyerson et al. 2010; Kettenring et al. 2012; Kettenring and Mock 2012) where it typically occurs at low densities in mixed wetland plant communities. An invasive European lineage (chloroplast DNA haplotype M; hereafter Introduced Phragmites) was introduced into eastern North America at least 150 years ago, and by the 1960s, was widespread in East Coast salt marshes and wetlands (Saltonstall 2002). This lineage has since spread south to the Gulf Coast and west to the Pacific Coast (Saltonstall 2002; Meyerson et al. 2010), but has likely been present in western urban centers for only about 25 years (but see Smith and Kadlec 1983). Hybridization between Native and Introduced Phragmites haplotypes has been detected infrequently along the East Coast (Saltonstall et al. 2014; Wu et al. 2015), but remains rare on the continental scale (Saltonstall et al. 2016). Other non-native haplotypes such as M1, which originates from the Mediterranean, continue to be identified as more populations across the continent are analyzed (Hauber et al. 2011; Lambertini et al. 2012). A third lineage, Gulf Coast P. australis subsp. berlandieri (hereafter Gulf Coast Phragmites), is found in the southern United States from Florida to California and also extends into Central and South America. It is unknown whether Gulf Coast Phragmites is native or introduced to the region, and is therefore considered cryptogenic (Saltonstall 2002, 2003a, b). Lambertini et al. 2012 suggests that many of the Phragmites populations present along the Gulf Coast states appear to be hybrids of Gulf Coast Phragmites and P. mauritianus, and Gulf Coast Phragmites and non-native haplotypes M and M1 (but see Hauber et al. 2011). Saltonstall (2003a) provides a continental-scale description of the genetic structuring of Phragmites haplotypes in North America.
Phragmites australis invasion and impacts have been extensively studied in eastern North America, but have received little attention in the West because this species has been a relatively minor problem compared to other invasive riparian and wetland plants, such as Tamarix spp. and Arundo donax, that have long histories in this region (Shafroth et al. 2005; Lambert et al. 2010a). However, several studies have focused on the genetic diversity and reproductive strategies of Native and Introduced Phragmites populations in the Great Salt Lake region, the site of one of the most extensive invasions in the West, and other areas of Utah and southern Idaho where only native haplotypes occur (Kettenring et al. 2012; Kettenring and Mock 2012). It is unclear if the recent Phragmites invasion in the West will reach the same magnitude as in the East given the differences in climate and edaphic properties between regions. Recent studies have begun to link biogeographic patterns and latitudinal gradients in Native and Introduced Phragmites distribution to community interactions (Chow 2008; Cronin et al. 2015), climate, and anthropogenic disturbance (Hughes et al. 2016).
It is not known if western systems will be as vulnerable to invasion or what role abiotic differences could play in establishment and spread. The temperate climate of eastern North America is similar to that of Europe where Introduced Phragmites is native, but a strong contrast to the semi-arid to arid climate of southwestern North America. Soil properties are also dissimilar among regions with acidic, high organic content soils in the East and alkaline, sandy soils with relatively low organic content in the West. The purpose of this study was to (1) evaluate the genetic status (including hybridization events) of Phragmites stands in wetlands and riparian systems throughout the arid Southwest, (2) document the distribution of Native Phragmites populations across the region, especially in isolated wetlands, (3) assess whether Introduced Phragmites is spreading from sites of apparent introduction into ecologically sensitive natural habitats where their impacts could warrant enhanced management efforts, and (4) begin to assess the environmental factors that may influence the distribution patterns of the different Phragmites lineages in the southwestern United States.
Materials and methods
Surveys of Phragmites populations
Locations of Phragmites populations were identified through online herbarium database searches, analysis of aerial imagery in Google Earth (© Google Inc. 2015), and information from scientists and land managers with botanical knowledge of wetland plants in the region. Phragmites can be distinguished from other vegetation in aerial imagery by its lighter yellow–green color and linear stem architecture. The authors and collaborators work extensively in riparian areas and wetlands in the region, and surveyed Phragmites populations over a 9 year period (2006–2015). Field observations were commonly made along roadways and public right of ways, but extensive segments of riparian corridors were also visited to survey for presence of Phragmites plants. Isolated wetlands were surveyed where herbarium records documented Phragmites presence or if potential habitat was identified through aerial imagery analysis. If populations were abundant in a given area, as much of the accessible area as possible was surveyed and samples were collected from any stands that appeared to differ morphologically. Over 400 locations with appropriate riparian or wetland habitat were surveyed, with Phragmites stands detected and sampled in 97 of these locations (some locations had multiple stands).
Stem density, percent cover, and presence of other species growing alongside Phragmites were also recorded for a subset of stands to assist with describing growth habits and relative dominance among the Phragmites lineages. Stem density and percent cover were measured in 0.25 m2 quadrats placed along a transect run as close to the center of the stand as possible. At least seven evenly spaced quadrats were measured in each stand with all quadrats placed at least 5 m apart (average distance of transects depended on stand size and physical barriers).
Genetic analysis/haplotype determination
Green leaf tissue samples were collected from stands throughout Arizona, California, Colorado, Nevada, and Utah during the growing season by the authors and collaborators. Tissue samples were either air dried or dried using silica gel and stored at –70 °C in the laboratory until genetic analysis. Intensive collection occurred across Clark County, Nevada area where hybridization had been earlier detected (Saltonstall et al. 2016). A total of 177 samples were collected and provenance was determined using DNA sequencing and/or microsatellites (151 samples), Restriction Fragment Length Polymorphism (RFLP) analysis (14 samples), or morphological characteristics (12 samples).
DNA for genetic analyses was extracted using a modified 2 % CTAB extraction protocol (Saltonstall 2002). The lineage of the majority of samples was determined by sequencing two non-coding chloroplast DNA (cpDNA) regions on an ABI 3130XL sequencer (Applied Biosystems) as in Saltonstall (2002). Sequences were aligned using Sequencher 4.1 (GeneCodes Corp.) and compared with known Phragmites haplotypes (Saltonstall 2002). Eight microsatellite regions (GT4, GT8, GT9, GT11, GT13, GT14, GT16, GT22) were amplified using the protocols of Saltonstall (2003b), with multiplexing of primer sets to reduce the number of PCR reactions required. As primer set GT22 does not amplify well in Native samples, this locus was only used when comparing Introduced with Gulf Coast samples. Samples were genotyped on an ABI 3130XL sequencer using LIZ 500 as a size standard and allele sizes were estimated using GeneMapper version 3.7 (Applied Biosystems). Previous work (Saltonstall 2003b; Saltonstall, unpublished data) has identified expected microsatellite allele frequencies in the Phragmites lineages defined by cpDNA haplotypes and these data were used as a reference. Samples were assigned to a lineage using two methods: Bayesian clustering, as implemented in Structure 2.3.3 using the admixture model (Pritchard et al. 2000; Falush et al. 2007) and Principle Coordinates Analysis based on the band-sharing Lynch distance metric (Lynch 1990), as implemented in the R package Polysat (Clark and Jasieniuk 2011). As Phragmites is an allo-polyploid, microsatellite profiles are hereafter referred to as allele phenotypes (Saltonstall 2003b).
Some samples were analyzed using RFLP following the methods of Saltonstall (2003c) to confirm the provenance of plants with distinct morphological characters when genetic sequencing was not available. This test can differentiate between the Native, Introduced, and Gulf Coast lineages and confirm the origin of the maternal parent (seed), but cannot detect hybrids. Any samples that had ambiguous or hybrid characteristics were included in the sequencing analysis above if the DNA was not degraded.
A suite of morphological characters has been developed to distinguish among the three lineages and are reasonably reliable for determining status when molecular methods are not available (Blossey 2015; Saltonstall et al. 2004; Swearingen and Saltonstall 2010). However, stressful environmental conditions can cause variation in several of the stem characters (A. Lambert, personal observation), so caution was used in evaluating character states. Thirteen stands were identified using only the morphological characters described by Blossey (2015) as high quality DNA could not be extracted from them. We excluded any stands where morphological characters were variable or not definitive.
Soil properties
Soil samples were collected from a subset of sites and analyzed to determine if differences in edaphic properties exist among the habitats where the Native, Introduced, and Hybrid plants occurred. Soil samples were not collected from Gulf Coast Phragmites populations. Because soil samples were taken across a broad geographical range potentially of different parent materials and over multiple years, resulting data provide only a coarse assessment of potential differences in soil characteristics among sites that may influence Phragmites lineage distribution. However, soils are generally stable in this arid region when soil moisture is low (Hultine et al. 2015). We also collected soil samples from several of the same sites over multiple years to evaluate temporal changes in measurements and found that within-site variation in pH and electrical conductivity was very low between years. Soil cores (5 cm diameter × 20 cm depth) were collected from 33 populations during the dry season, although the soils in several of the sites were moist or saturated. Three cores, spaced approximately 1 m apart, were taken in each stand as close as possible to the midpoint of the stand and samples were placed in paper bags for transport. Samples were dried at 60 °C for 2 days, then sieved through mesh to remove particles greater than 2 mm. Particle size (texture) was measured using the hydrometer method in Gee and Bauder (1979). Total soluble salt concentration (salinity) was determined by measuring electrical conductivity (Rhoades 1996). To determine electrical conductivity, 60 ml of 0.1 M calcium chloride was added to 20 g of soil (3:1mixture) and mixed on an orbit shaker for 30 min. Conductivity was measured at 21.0 ± 0.5 °C using an EC Testr 11 + meter (Eutech Instruments Pte LTD.). To determine soil pH, 30 g of soil were mixed with 30 ml deionized water (1:1 mixture) and mixed on an orbit shaker for 30 min. pH was measured with an YSI pH 10 m.
Statistical analysis
To assess variation at the regional level and between wetland types, we focused on cpDNA haplotype diversity as microsatellite profiles showed clear distinction between the Native, Introduced, and Gulf Coast lineages. When determining the percentage of stands from each lineage, locations with multiple samples were only counted once. Soil data were analyzed using a one-way ANOVA to determine if there were detectable differences in soil properties (texture, pH, and electrical conductivity) among the three lineages and hybrid populations. Differences in stem density and percent cover among lineages were also analyzed using a one-way ANOVA. Tukey’s test was used for post hoc comparisons of significant main effects.
Results
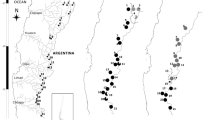
In general, Phragmites was encountered infrequently in Southwest wetland systems and its dominance (stem density or cover) varied considerably among sites (Table 1; Fig. 1). Genetic diversity was high with a total of eight cpDNA haplotypes of native and non-native origin detected in the region. Four Native haplotypes were identified (haplotypes A, B, H, and AR). Introduced haplotype M was the most abundant non-native haplotype, but two previously unidentified haplotypes (P and Q) were also found in California. Gulf Coast Phragmites (haplotype I) was the most common haplotype in the southern portion of the sampling area. Hybrids of the Native and Introduced lineages were found in Las Vegas, NV (Saltonstall et al. 2016) but no evidence for hybridization was found between the Introduced (Hap. M) and Gulf Coast (Hap. I) lineages (Fig. 2). Small and ephemeral wetlands tended to have mixed vegetation communities with low Native Phragmites densities, while urban wetlands with high levels of disturbance and nutrient rich wastewater inputs had larger and dense monoculture stands of non-native haplotypes and hybrid populations.
Native Phragmites australis subspecies americanus lineage
Across the region, Native Phragmites was the most common and geographically widespread lineage, accounting for 63 % of the populations sampled (n = 101 samples). It was found in all wetland types and was the only lineage outside of urban areas or in remote locations where human disturbance was low. It was often associated with surface hydrologic features fed by groundwater, including alkaline marshes, seeps and springs, with 33 % of Native populations occurring in these habitats. Native stands occurred in mixed plant communities with relatively low cover compared to Introduced or hybrid stands (Fig. 3). However, the Native stands that were associated with anthropogenic disturbance, especially sewage treatment, were more robust (Fig. 3).
(Top) Native Phragmites australis stand at Little Caliente Hot Springs in the Los Padres National Forest, Santa Barbara County, California. This population typifies the size and density of native stands observed in the southwest. (Bottom) Native Phragmites stand along the Las Vegas Wash, Clark County, Nevada. The density and robust size of this stand is atypical of southwest populations and is most likely facilitated by the nutrient-rich effluent in which it grows
Native haplotype H was the most abundant haplotype across the region and occurred in all wetland types typically at elevations below 800 m. It was also found in strongly alkaline (pH > 9.0) soils in Death Valley National Park and the Mojave Desert sink. Haplotype B occurred infrequently and was primarily associated with isolated systems and higher elevation mountain springs and creeks. Interestingly, we found two variants of Haplotype B that can have either 10 or 11 A’s in the third microsatellite region of the trnT-trnL locus (Saltonstall et al. 2016). A novel variant of haplotype B was found in samples from California and southern Utah, although this haplotype has been found in samples from six other states (Saltonstall 2003a, this study). Haplotypes A and AR were found in the eastern portion of the sampling area along the upper Colorado River and in Phoenix, Arizona. Haplotype AR is a previously unidentified haplotype (T10/R2; GenBank Accession No. AF457397/AY016333), but is closely related to Haplotypes A, B, and H.
Non-native Phragmites australis haplotypes
Introduced Phragmites stands (n = 26) were associated with wetland modification and disturbance in or near urban centers, and often with systems where wastewater effluent provided permanent flows in historically ephemeral rivers. While it was rare throughout much of the study area, extensive monocultures were found throughout the San Francisco Bay Delta and northern San Joaquin Valley in California. The San Francisco area populations were most extensive (covering many hectares) in the highly disturbed brackish marshes of the Delta. Since the beginning of this study, Introduced Phragmites has been spreading south in the San Joaquin Valley, especially in areas where riparian restoration is occurring (J. Rentner, personal communication). In 2006, a relatively small population (less than 0.25 ha) was found to the south of this region near a sewage treatment facility in the Salinas River, Atascadero, CA. In 2014, new populations were identified in previously surveyed areas 20 km away growing along the banks of man-made reservoirs in San Luis Obispo, CA. In 2007, the San Diego, CA, population was localized to a small island near the mouth of the San Diego River, but new populations have recently established along coastal rivers and marshes to the north (J. Rebman, personal communication). Several Introduced populations were found along the Virgin River, a tributary to the lower Colorado River. A small stand was present in Saint George, Utah in 2010 and additional stands were found in 2014. In 2014, a large stand was identified in Pine Creek (tributary to the Virgin River) in Zion National Park that was not present at the same location during a survey of the area in 2007. Two Introduced Phragmites populations were found in Las Vegas, Nevada, very close to each other on debris and fill at a new housing development. An extensive Introduced population was identified south of Las Vegas along the Colorado River in Needles, California. These populations are linked by the Colorado River, but no additional Introduced populations were detected between Las Vegas and Needles, or south of the Needles population, although there are inaccessible parts of the river in these locations.
Two haplotypes that are native to Asia were identified for the first time in North America. Haplotype P was found in the Mojave Narrows along the Mojave River in Victorville, CA. Several robust populations occurred along this wet river reach, but no other populations were found in the dry reaches to the north or south. All four of the unique stands with this cpDNA haplotype that we tested had the same microsatellite phenotype suggesting that the lineage is spreading clonally along the river. It also appears that this haplotype is octoploid, based on its microsatellite phenotype, which showed four alleles at locus GT4. In addition, two samples identified as haplotype Q were collected from large stands in Bayland Park, Palo Alto, California. These stands were considered invasive by park staff and treated with herbicide in 2007, however recent aerial imagery shows that the stands continue to expand. The two samples that we tested had unique, but closely related, microsatellite phenotypes also suggestive of the plants being octoploid.
Hybrids in the Las Vegas Wash watershed
Hybrid Phragmites populations were widespread in the Las Vegas, Nevada area, and are likely first-generation hybrids based on their microsatellite allele phenotypes which displayed alleles common to both the Native and Introduced lineages at nearly all loci (Saltonstall et al. 2016; Fig. 2). Most of these hybrids had cpDNA Haplotype M (n = 34 samples), indicating that their maternal parent was an Introduced plant. These plants were extremely robust and found growing along the lower reaches of the Las Vegas Wash, as well as in surrounding remnant creeks and drainage channels. Two hybrid samples had cpDNA Haplotype H (Native maternal parent) and microsatellite profiles suggesting that they might be first-generation hybrids as well. These haplotype H hybrids were localized in the upper Wash and were smaller in stature than other hybrids, but still grew in large patches. All hybrid populations were found predominately in areas with heavy soil disturbance, including residential developments, within the Las Vegas Wash and its tributaries, and upper Lake Mead where extensive flood control and riparian restoration projects are occurring. An in-depth analysis of the hybridization we documented in Las Vegas is provided by Saltonstall et al. (2016).
Gulf Coast Phragmites australis subspecies berlandieri lineage
The Gulf Coast lineage (n = 10) was restricted to latitudes below 33.8°N and was generally associated with agricultural canals and modified wetlands linked to the lower Colorado River. A very small stand was found in Cottonwood Creek, a dry river wash north of Phoenix, AZ. All samples from this lineage shared Haplotype I and have unique allele phenotypes across the majority of microsatellite loci, suggesting that they are hexaploid. No evidence for hybridization with the Introduced lineage was detected in either the Structure (results not shown) or PCoA analyses (Fig. 2).
Soil properties
Soil properties were variable across the survey area and among habitat types, with only soil pH showing consistent differences among lineages. Soil pH of Native (mean 8.3 ± 0.7 [SD]) and Hybrid (mean 8.6 ± 0.6) stands was generally higher than that of soils collected in Introduced stands (mean 7.5 ± 1.5), although this result was not significant (F2,52 = 1.47, p = 0.24; Table 2). Electrical conductivity varied substantially among sites, but no significant difference among lineages was detected. Soil texture (% sand/silt/clay) was highly variable and did not differ significantly among lineages, although sand constituted the majority fraction (at least 48 %) of the soil volume for all samples.
Stem density and percent cover
Stem density was significantly different among lineages (F(3,87) = 15.09, p < 0.001; Table 3). Introduced, hybrid, and haplotype H hybrid stands contained 71, 63, 51 % (respectively) more stems per meter than Native stands.
Percent cover was also significantly different among lineages (F(3,87) = 28.02, p < 0.001; Table 3). Introduced and hybrid stands had 109 and 100 % greater cover, respectively, than Native stands. However, the haplotype H hybrid had a similar cover to that of Native stands.
Discussion
Phragmites has been a component of southwestern US wetland plant communities for thousands of years (Goman and Wells 2000; Hansen 1978; Kiviat and Hamilton 2001). Today, wetlands in the Southwest face multiple threats from urbanization and associated reductions in water availability, especially through groundwater overdraft, that have caused regional declines in wetland extent and dependent vegetation (Patten et al. 2008). The future of plant populations, including the Native Phragmites lineage, in these systems is of conservation concern, particularly when considering the fragmented nature of wetland habitats in the xeric habitats of the Southwest. There is a need for ecological and distributional data for these communities at the regional level, yet to date, little information is available. Here, we show broad patterns of regional overlap among Native, Introduced, and Gulf Coast Phragmites lineages in the Southwest, which is the only region of the United States where the three lineages co-occur (Saltonstall 2002, 2003a; Saltonstall et al. 2004; Meyerson et al. 2010). Native Phragmites has high genetic diversity, as we found four cpDNA haplotypes including one new one, which may also reflect the high diversity of habitats in the region. We also document two novel introductions and hybridization between the Native and Introduced lineages. These findings suggest that (1) Native Phragmites remains widely distributed across wetland habitats and is maintaining its genetic diversity; (2) Introduced Phragmites is uncommon but spreading, and where found, is associated with disturbed and urbanized wetlands or those adjacent to transportation corridors; (3) Native and Introduced Phragmites coexist at many sites, but appear genetically isolated everywhere except in southern Nevada where hybrids are common at the watershed scale; (4) Gulf Coast Phragmites is restricted to wetlands associated with human-modification along the lower Colorado River and shows no evidence for hybridization with Introduced Phragmites; and (5) Two haplotypes likely originating from Asia have been introduced to California, but thus far appear to be restricted to two river drainages.
Native Phragmites australias subspecies americanus lineage
Native Phragmites was the most common lineage detected, but generally at low densities. This may reflect the rarity of appropriate wetland habitat types and severity of the edaphic conditions in the region. However, genetic diversity of Native Phragmites in the Southwest region is higher than in the Midwest and eastern parts of North America (Saltonstall 2003a, b) and displays many unique haplotypes and allele phenotypes as well. This high diversity is perhaps due to its long history in the region, as well as adaptation to the relictual nature of the wetlands it inhabits (Minckley et al. 2013). In another study of Phragmites populations in Utah and southern Idaho, Kettenring and Mock (2012) found that Native clones had lower genetic diversity than Introduced clones, possibly due to a greater dependency of Introduced populations on establishment by seed rather than clonal expansion.
Native stands were associated with all wetland habitats and over the range of human disturbance, and was the only lineage present in locations away from urban centers or transportation corridors. It appears that Native Phragmites is the only lineage currently associated with the isolated seeps, springs, and oases in the Southwest, which provide critical habitat for wildlife (Fleishman and Murphy 2005; Fensham et al. 2011). These remote stands had low stem densities and were always mixed with other native wetland plant species.
Non-native Phragmites australis haplotypes
It is unknown how long the Introduced lineage has been present in the western United States or whether multiple introductions have occurred, but it is generally accepted that populations were established in this region in the late twentyth century whereas the eastern invasion began in the 1800’s (Saltonstall 2002). The oldest sample in our dataset was collected in August, 1995 in San Diego, CA below an Interstate highway 5 overpass (D. Hauber pers. comm). Introduced Phragmites is already widespread and expanding in some western systems, including the San Francisco Bay Delta (Grossinger et al. 1998) and around the Great Salt Lake (Kulmatiski et al. 2010; Kettenring et al. 2012; Kettenring and Mock 2012). Kulmatiski et al. (2010) dated the first Introduced Phragmites herbarium samples in the Salt Lake City, Utah area to 1993, and found that current populations expanded to cover 56 % of the extensive wetlands within 27 years. We found the Introduced lineage primarily associated with urban wastewater and highly impacted wetlands in the San Francisco Bay Delta. However, the two populations in Zion National Park and along the Colorado River in Needles, California are in locations with relatively low human disturbance (but near major roads) suggesting that invasion is possible away from urban centers, although it is unclear if alterations occurred in these areas that may have led to establishment. We identified Introduced populations in the Virgin River and tributaries in Southwest corner of Utah and suspect that these represent relatively new establishment events likely facilitated by transport (see Brisson et al. 2010) in the Interstate 15 highway corridor, a major route between Salt Lake City and Las Vegas, Nevada, as well as channel modification for flood control. Kettenring et al. (2012) provide a similar explanation for the widespread Phragmites invasion around the Great Salt Lake in northern Utah. In 2014, we found a new population along the main corridor through nearby Zion National Park, which was not present when we surveyed the area in 2007. Similar range expansions are occurring in coastal California south of San Francisco and in San Diego which suggests that this is an ongoing invasion and expansion into new habitats will continue. There is also concern that Introduced Phragmites will continue to expand its range as water resources are modified along with the growing human population, as well as replace other invasive riparian plants that are primary targets for eradication (Lambert et al. 2010b; Meyerson et al. 2010). The presence and continued spread of the Introduced lineage is a previously unrecognized threat to isolated wetlands in the region, but it is unclear if this lineage can successfully invade these systems, which have substantially different abiotic (especially soil) properties than the temperate regions of Europe where it is native or the Northeastern United States where it has reached its greatest extent.
Hybrids in the Las Vegas Wash watershed
Previously, hybridization between Native and Introduced lineages had only been detected in eastern North America and appeared to occur as infrequent and localized events (Saltonstall et al. 2014; Wu et al. 2015). Saltonstall (2003a, b, c) found no evidence for hybridization across North America and Kettenring and Mock (2012) did not find evidence of hybridization in their analysis of Native and Introduced Phragmites populations in Utah and southern Idaho. The hybrid stands we found in the Las Vegas area of southern Nevada are the first documented evidence that hybridization is occurring at the landscape or watershed level. Further, their abundance and propensity to spread with human disturbance is concerning. Hybrid stands were observed throughout the Las Vegas Wash, an effluent discharge system for regional wastewater that was once an ephemeral wash. Extensive hybrid populations grow immediately adjacent to the river banks, while Native Phragmites is limited to higher terrace locations, and dispersal of clonal fragments appears to be a major source of spread along these rivers. Hybrid stands have also been detected in newly constructed artificial wetlands in the area, but it is unclear if establishment occurred by the wind-borne seeds or movement of rhizomes during construction. It is very possible that hybrids will continue to spread throughout the lower Colorado River Basin. Saltonstall et al. (2016) more fully describe the Phragmites distribution patterns and hybridization observed in southern Nevada.
Gulf Coast Phragmites australis subspecies berlandieri lineage
The geographical origin and taxonomic designation of the Gulf Coast lineage (subspecies berlandieri) has been the subject of much debate (Saltonstall 2002; Jones et al. 1997; Saltonstall and Hauber 2007; Ward 2010; Lambertini et al. 2012), and although it is considered potentially native in the very southern portion of our sampling area (Saltonstall 2002; Saltonstall and Hauber 2007), it may have been introduced to the habitats in which we document it. We found this lineage restricted to the lower Colorado River and canal systems that convey water for agricultural use in southern California and Arizona. Continued population expansion associated with water management is considered a significant concern for resource managers and agricultural interests (C. Bell, personal communication) as these stands appear large and grow as dense monocultures (Lambert and Saltonstall, Personal observation). For example, Phragmites from the Gulf Coast lineage was planted at Yuma Crossing, Arizona over 20 years ago for erosion control, but is now the target of control efforts in that area and much of the lower Colorado River because of its rapid spread and facilitation of fire in riparian corridors (Fred Phillips Consulting 2011).
Soil properties
We expect that differences in soil properties between the eastern and western United States will influence the relative scope of the invasion in the Southwest. The Introduced lineage has evolved under a temperate, high precipitation climate in Europe, and appears capable of invading the majority of wetlands in eastern and central North America with a similar climatic regime. In the West, it appears to be most abundant where excess fresh water (and nutrients) is added to wetland systems and/or where human activities have created a disturbance. The pH of soils collected from Introduced populations was less than 7.6, below the average pH levels of the sites with Native and hybrid stands, although more data are necessary to confirm this trend (we sampled all possible Introduced stands in our study). The highly basic pH of desert wetlands, which at some of our sites exceeded 9.0, may limit or even prevent the spread of the Introduced lineage, but not necessarily hybrid populations, which may have inherited genetic material from their Native parent making them pre-adapted to the desert climate. Kettenring et al. 2012, suggest that other climatic factors, such as the elevated carbon dioxide and temperature conditions expected in the Southwest under a climate change scenario could also facilitate colonization of saline habitats by invasive genotypes.
Conclusions
Although desert wetland ecosystems have been recognized as critical habitats for protecting biodiversity, they are underrepresented as conservation targets (Minckley et al. 2013). Further, the paucity of ecological and environmental information for these habitats contributes to a lack of awareness of the threats of invasive species and human disturbance to associated biota. Native Phragmites is still the most common lineage in the Southwest, but it is unclear how invasion of non-native Phragmites haplotypes in this region will ultimately affect wetland habitats, or whether the scale of invasion and spread occurring in the Great Salt Lake (Kulmatiski et al. 2010; Kettenring et al. 2012; Kettenring and Mock 2012) and the San Francisco Estuary (Grossinger et al. 1998), and most recently the Las Vegas area, will continue across this arid region. It is also disturbing that we found two novel introductions in California that appear to be spreading vegetatively. We suggest that these stands should be a priority for control efforts as they currently are isolated to certain watersheds and it may be possible to eradicate them at this time before they spread. Continued monitoring of Native population trends and spread of Introduced and hybrid populations is critical for determining population trajectories, as well as assessing whether the Native lineage requires management or protected status in the Southwest.
References
Ball-Damerow J, M’Gonigle L, Resh V (2014) Changes in occurrence, richness, and biological traits of dragonflies and damselflies (Odonata) in California and Nevada over the past century. Biodivers Conserv 23:2107–2126
Bickford D, Lohman DJ, Sodhi NS et al (2007) Cryptic species as a window on diversity and conservation. Trends Ecol Evol 22:148–155
Blair RB (2001) Birds and butterflies along urban gradients in two ecoregions of the United States: is urbanization creating a homogeneous fauna? In: Lockwood J, McKinney M (eds) Biotic Homogenization. Springer, New York, pp 33–56
Blossey B (2015) Morphological differences between native and introduced genotypes. In. http://www.invasiveplants.net/phragmites/phrag/morph.htm Accessed: 5 Nov 2015
Brisson J, de Blois S, Lavoie C (2010) Roadside as invasion pathway for common reed (Phragmites australis). Invasive Plant Sci Manag 3:506–514
Chow AK (2008) Effects of latitude on the competitive ability of native and invasive genotypes of Phragmites australis. Masters Thesis Louisiana State University, p 43
Clark LV, Jasieniuk M (2011) POLYSAT: an R package for polyploid microsatellite analysis. Mol Ecol Resour 11:562–566
Cronin JT, Bhattarai GP, Allen WJ et al (2015) Biogeography of a plant invasion: plant–herbivore interactions. Ecology 96:1115–1127
Czech B, Krausman PR (1997) Distribution and causation of species endangerment in the United States. Science 277:1116–1117
Daehler CC, Strong DR (1996) Status, prediction and prevention of introduced cordgrass Spartina spp. invasions in Pacific estuaries. USA Biol Conserv 78:51–58
Deacon JE, Williams AE, Williams CD et al (2007) Fueling population growth in Las Vegas: how large-scale groundwater withdrawal could burn regional biodiversity. Bioscience 57:688–698
Dudley TL (2000) Noxious wildland weeds of California: Arundo donax. In: Bossard C, Randall J, Hoshovsky M (eds) Invasive plants of California’s wildlands. University of California Press, Berkeley
Falush D, Stephens M, Pritchard JK (2007) Inference of population structure using multilocus genotype data: dominant markers and null alleles. Mol Ecol Notes. doi:10.1111/j.1471-8286.2007.01758.x
Fensham RJ, Silcock JL, Kerezsy A et al (2011) Four desert waters: setting arid zone wetland conservation priorities through understanding patterns of endemism. Biol Conserv 144:2459–2467
Fleishman E, Murphy DD (2005) Biodiversity patterns of spring-associated butterflies in a Mojave Desert mountain range. J Lepid Soc 59:89–95
Fred Phillips Consulting (2011) Yuma East Wetlands AHA 68 acre revegetation project final report. Prepared for The Arizona Water Protection Fund and Yuma Crossing National Hertiage Area. p 156
Gaston KJ (1998) Ecology: rarity as double jeopardy. Nature 394:229–230
Gee GW, Bauder JW (1979) Particle size analysis by hydrometer: a simplified method for routine textural analysis and a sensitivity test of measurement parameters. Soil Sci Soc Am J 43:1004–1007
Goman M, Wells L (2000) Trends in river flow affecting the northeastern reach of the San Francisco Bay estuary over the past 7000 Years. Quat Res 54:206–217
Grossinger RM, Alexander J, Cohen AN et al (1998) Introduced tidal marsh plants in the San Francisco Estuary. San Francisco Estuary Institute, Richmond
Hansen RM (1978) Shasta ground sloth food habits, Rampart Cave, Arizona. Paleobiology 4:302–319
Hauber DP, Saltonstall K, White DA et al (2011) Genetic variation in the common reed, Phragmites australis, in the Mississippi River Delta marshes: evidence for multiple introductions. Estuar Coast 34:851–862
Hendrickson DA, Minckley WL (1985) Cienegas—Vanishing climax communities of the American Southwest. Desert Plants 6:131–175
Hughes AR, Schenck FR, Bloomberg J et al (2016) Biogeographic gradients in ecosystem processes of the invasive ecosystem engineer Phragmites australis. Biol Invasions. doi:10.1007/s10530-016-1143-0
Hultine K, Dudley T, Koepke D et al (2015) Patterns of herbivory-induced mortality of a dominant non-native tree/shrub (Tamarix spp.) in a southwestern US watershed. Biol Invasions 17:1729–1742
Jones SD, Wipff JK, Montgomery PM (1997) Vascular plants of Texas. University of texas Press, Austin
Kettenring K, Mock K (2012) Genetic diversity, reproductive mode, and dispersal differ between the cryptic invader, Phragmites australis, and its native conspecific. Biol Invasions 14:2489–2504
Kettenring KM, de Blois S, Hauber DP (2012) Moving from a regional to a continental perspective of Phragmites australis invasion in North America. AoB Plants. doi:10.1093/aobpla/pls040
Kiviat E, Hamilton E (2001) Phragmites use by Native North Americans. Aquat Bot 69:341–357
Kowarik I (1995) On the role of alien species in urban flora and vegetation. In: Pysek P, Prach K, Rejmánek M, Wade P (eds) Plant invasions; general aspects and special problems. SPB Academic, Amsterdam, pp 85–103
Kulmatiski A, Beard KH, Meyerson LA et al (2010) Nonnative Phragmites australis Invasion into Utah Wetlands. Western North Am Nat 70:541–552
Lambert AM, Dudley T, D’Antonio CM (2010a) Invasive species and fire in California. Fremontia 38:29–36
Lambert AM, Dudley TL, Saltonstall K (2010b) Ecology and impacts of the large-statured invasive grasses Arundo donax and Phragmites australis in North America. Invasive Plant Sci Manag 3:489–494
Lambertini C, Mendelssohn IA, Gustafsson MHG et al (2012) Tracing the origin of Gulf Coast Phragmites (Poaceae): a story of long-distance dispersal and hybridization. Am J Bot 99:538–551
Lynch M (1990) The similarity index and DNA fingerprinting. Mol Biol Evol 7:478–484
McKinney ML (2002) Urbanization, biodiversity, and conservation: the impacts of urbanization on native species are poorly studied, but educating a highly urbanized human population about these impacts can greatly improve species conservation in all ecosystems. Bioscience 52:883–890
Meyerson LA, Lambert AM, Saltonstall K (2010) A tale of three lineages: expansion of common reed (Phragmites australis) in the U.S. southwest and gulf coast. Invasive Plant Sci Manag (in press)
Minckley TA, Turner DS, Weinstein SR (2013) The relevance of wetland conservation in arid regions: a re-examination of vanishing communities in the American Southwest. J Arid Environ 88:213–221
Patten D, Rouse L, Stromberg J (2008) Isolated spring wetlands in the Great Basin and Mojave Deserts, USA: potential response of vegetation to groundwater withdrawal. Environ Manag 41:398–413
Pritchard JK, Stephens M, Donnelly P (2000) Inference of population structure using multilocus genotype data. Genetics 155:945–959
Rhoades JD (1996) Salinity: electrical conductivity and total dissolved solids. In: Sparks DL, Page AL, Helmke PA, Loeppert RH (eds) Methods of soil analysis. Part 3 chemical methods. Soil Science Society of America and American Society of Agronomy, Madison, pp 417–435
Saltonstall K (2002) Cryptic invasion by a non-native genotype of the common reed, Phragmites australis, into North America. P Natl Acad Sci USA 99:2445–2449
Saltonstall K (2003a) Genetic variation among North American populations of Phragmites australis: implications for management. Estuar Coasts 26:444–451
Saltonstall K (2003b) Microsatellite variation within and among North American lineages of Phragmites australis. Mol Ecol 12:1689–1702
Saltonstall K (2003c) A rapid method for identifying the origin of North American Phragmites populations using RFLP analysis. Wetlands 23:1043–1047
Saltonstall K (in review) The naming of Phragmites haplotypes. Biol Invasions
Saltonstall K, Hauber D (2007) Notes on Phragmites australis (Poaceae: arundinoideae) in North America. J Bot Res Inst Texas 1:385–388
Saltonstall K, Peterson PM, Soreng RJ (2004) Recognition of Phragmites australis subsp. americanus (Poaceae: Arundinoideae) in North America: evidence from morphological and genetic analyses. SIDA 21:683–692
Saltonstall K, Castillo HE, Blossey B (2014) Confirmed field hybridization of native and introduced Phragmites australis (Poaceae) in North America. Am J Bot 101:211–215
Saltonstall K, Lambert AM, Rice N (2016) What happens in Vegas, better stay in Vegas: Phragmites australis hybrids in the Las Vegas Wash. Biol Invasions. doi:10.1007/s10530-016-1167-5
Schemske DW, Husband BC, Ruckelshaus MH et al (1994) Evaluating approaches to the conservation of rare and endangered plants. Ecology 75:584–606
Shafroth PB, Cleverly JR, Dudley TL et al (2005) Control of Tamarix in the western United States: implications for water salvage, wildlife use, and riparian restoration. Environ Manag 35:231–246
Sivinski B, Tonne P (2011) Survey and assessment of aridland spring cienegas in the southwest region. Unpublished report prepared for NM Energy, Minerals and Natural Resources Department and USDI-Fish & Wildlife Service, Region 2
Smith LM, Kadlec JA (1983) Seed banks and their role during drawdown of a North American marsh. J Appl Ecol 20:673–684
Stohlgren TJ, Bull KA, Otsuki Y et al (1998) Riparian zones as havens for exotic plant species in the central grasslands. Plant Eco 138:113–125
Swearingen J, Saltonstall K (2010) Phragmites field guide: distinguishing native and exotic forms of common reed (Phragmites australis) in the United States. Plant Conservation Alliance, Weeds Gone Wild. http://www.nps.gov/plants/alien/pubs/index.htm
Tiner RW (2003) Geographically isolated wetlands of the United States. Wetlands 23:494–516
Trentanovi G, von der Lippe M, Sitzia T et al (2013) Biotic homogenization at the community scale: disentangling the roles of urbanization and plant invasion. Divers Distrib 19:738–748
Unmack PJ, Minckley WL (2008) The demise of desert springs. In: Stevens LE, Meretsky VJ (eds) Aridland springs in North America: ecology and conservation. University of Arizona Press, Tucson, pp 11–34
Vitousek PM, D’Antonio CM, Loope LL et al (1997) Introduced species: a significant component of human-caused global change. N Z J Ecol 21:1–16
Walck JL, Baskin JM, Baskin CC (1999) Effects of competition from introduced plants on establishment, survival, growth and reproduction of the rare plant Solidago shortii (Asteraceae). Biol Conserv 88:213–219
Ward DB (2010) North America has two species of Phragmites (Gramineae). Castanea 75:394–401
Wu CA, Murray LA, Heffernan KE (2015) Evidence for natural hybridization between native and introduced lineages of Phragmites australis in the Chesapeake Bay watershed. Am J Bot 102:805–812
Zedler JB, Kercher S (2004) Causes and consequences of invasive plants in wetlands: opportunities, opportunists, and outcomes. CRC Crit Rev Plant Sci 23:431–452
Acknowledgments
We thank J. Andre (University of California, Riverside), A. LaVoie and A. Manwaring (United States Department of Fish and Wildlife), D. Keil (California Polytechnic University, San Luis Obispo), J. Rebmen (San Diego Natural History Museum), N. Rice (Southern Nevada Water Authority), G. Short (Center for Natural Lands Management), M. Walgren (California Department of Parks and Recreation), C. Bell (University of California Cooperative Extension), and the Tamarisk Coalition for collecting specimens and information on Phragmites locations, and A. Lambert, K. Arakawa, M. Taguchi, J. Roberts, and C. Ta for assistance with sample preparation and processing. Partial funding was provided by the Southern Nevada Water Authority.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest editors: Laura A. Meyerson and Kristin Saltonstall/Phragmites invasion.
Rights and permissions
About this article
Cite this article
Lambert, A.M., Saltonstall, K., Long, R. et al. Biogeography of Phragmites australis lineages in the southwestern United States. Biol Invasions 18, 2597–2617 (2016). https://doi.org/10.1007/s10530-016-1164-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-016-1164-8