Abstract
Arundo donax and Phragmites australis are two of the most aggressive invasive grasses worldwide, both are associated with wetlands and can be very abundant, becoming dominant in these ecosystems. These two species are common in northern Mexico. Genetic and ecological characterization of A. donax in two populations from the state of Coahuila (North of Mexico) indicate that they are less clonal and more variable, as well as with a higher genetic diversity compared to populations in other parts of the world and suggest that their genotypes are adapted to different environmental conditions and may represent independent introductions. On the other hand, genealogical analyses show that two independent lineages of P. australis are present in Mexico, the Gulf Coast subspecies, P. australis ssp. berlandieri, found across Mexico, including the state of Coahuila, and the endemic native subspecies, P. australis ssp. americanus, found in a population from Cuatro Ciénegas Basin (CCB) (Coahuila, Mexico). Here, we conduct a review of the genetic and ecological characteristics of both species in the Chihuahuan Desert, mainly focusing in CCB. The aim is to provide a better understanding in the evolutionary ecology of these two closely related and ecologically similar species and determine if these species of grasses represent a risk for the ecosystem and the valley’s biota.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
The Biology of Invasive Plants
The classification of plants as either native or introduced is a fundamental biological distinction, as it determines virtually all aspects of how the organisms are treated in scientific and applied contexts. For example, in ecological studies, native species are treated as vital participants in natural services and processes, whereas ecological studies of invasive plants focus on their harmful effects (D’Antonio and Vitousek 1992; Kolar and Lodge 2001; Sakai et al. 2001; Lambrinos 2004; Mack et al. 2000, and references therein). If the species is considered native, then extraction efforts will inevitably require management with a view toward conservation. On the other hand, if it is known to be invasive, then intensive use and even eradication are usually viewed as permissible and even desirable (Rejmánek 2000; D’Antonio and Meyerson 2002; Vilà et al. 2011; Simberloff et al. 2013; Blackburn et al. 2014; Latombe et al. 2016, and references therein).
It is well known that the genetic characteristics of populations will determine their capacity of establishment and range expansion (Mooney and Cleland 2001; Sakai et al. 2001; Lee 2002; Allendorf and Lundquist 2003; Dlugosch and Parker 2008; Suarez and Tsutsui 2008). Since species can evolve during their initial establishment and during their range expansion (Sakai et al. 2001), knowledge of the levels of diversity and of the genetic structure in the native and introduced range can help us to better understand the underlying demographic processes and the adaptive evolution that lead to the invasion (Mooney and Cleland 2001; Sakai et al. 2001; Lee 2002; Allendorf and Lundquist 2003; McCauley et al. 2003; Trewick et al. 2004; Kang et al. 2007; Prentis et al. 2008). That is, the genetic reshuffle at founding will determine the outcome and future impact for non-native populations (Mooney and Cleland 2001; Lee 2002; Novak and Mack 2005; Prentis et al. 2008).
Molecular markers (genetic data from the nuclear and organellar genomes) are critical to identify and study invasive species, pinpointing areas of origin of invaders, distinguishing among species in groups that are difficult to differentiate morphologically (i.e., identification of cryptic species), understanding types and distance of migration, tracking dispersal and spread, determining whether or not hybridization is occurring, and detecting introgression (Hufbauer 2004; Booth et al. 2007; Ward et al. 2008), which are crucial aspects of the basic biology of invaders that we need to know to develop an appropriate management and control strategy of these species.
Among the great diversity of flowering plants, grasses constitute a major group of invasive plants and their negative impact (e.g., they can dramatically alter native plant community structure and ecosystem processes such as fire frequency, nutrient cycling, and water circulation) has long been under great scrutiny by ecologists and conservation scientists (D’Antonio and Vitousek 1992; Strauss et al. 2006).
The Protected Area of Cuatro Ciénegas and Two Potentially Invasive Grasses
Protected natural areas constitute a valuable instrument of environmental policy to safeguard biological richness and to carry out actions for the preservation of biodiversity (SEMARNAP 1997; INE 1999; CONANP 2018). In Mexico there are 182 protected natural areas that are subject to special protection, conservation, restoration, and development regimes (CONANP 2016). However, the management of wild habitats depends almost entirely on whether dominant species are regarded as important native keystones to be protected or harmful exotics to be eradicated (Dudley and Collins 1995; SEMARNAP 1997; INE 1999; Mack et al. 2000; D’Antonio and Meyerson 2002; Hendrickson and McGaugh 2005; Vilà et al. 2011; Simberloff et al. 2013; CONABIO 2018; CONANP 2018). Despite the importance of the native versus naturalized distinction for science and applied situations, the status of many species remains ambiguous, even for some species that dominate vast areas of habitat.
The Cuatro Ciénegas Basin (CCB) (Coahuila, Mexico) is considered the most important wetland in the Chihuahuan Desert, and one of the most important wetlands in Mexico (INE 1999). At the international level, it is classified as a RAMSAR site and is considered a priority wetland for the world (INE 1999), an area of protection of flora and fauna under Mexican government, and a priority site for Conservation of Nature by the World Wildlife Fund for Nature and UNESCO (INE 1999; Souza et al. 2012). CCB is a remarkable area, having the highest endemism than any other place in North America (Stein et al. 2000), and much of the valley’s biota is classified either as endangered, threatened, or in special protection status by the Mexican government and the Convention on International Trade of Endangered Species (CITES) (Minckley 1992; INE 1999; Souza et al. 2004; Souza et al. 2006; Instituto Nacional de Ecología y Cambio Climático 2016). Current threats to its biodiversity include water exploitation, species invasions, industrial development, rapidly increasing tourism and population growth. Therefore, actions have been implemented to promote the conservation of terrestrial and aquatic ecosystems in the region. One of these actions is the control and eradication of invasive species (INE 1999; Souza et al. 2004; Hendrickson and McGaugh 2005; Instituto Nacional de Ecología y Cambio Climático 2016).
The giant reed, Arundo donax and the common reed, Phragmites australis are considered two of the most aggressive invasive grasses worldwide and the negative ecological impact of these species is well known (Perdue 1958; Dudley and Collins 1995; Bell 1997; Dudley 2000; Saltonstall 2002; Saltonstall and Hauber 2007; Saltonstall and Stevenson 2007; Lambert et al. 2010; Meyerson et al. 2010). These two species of grasses are associated with wetlands and can be very abundant and become dominant in these ecosystems. Both species are common in northern Mexico; however, the more ecological approach has left out many of the genetic or evolutionary aspects of their introduction in the region.
In this chapter we carry out a review of the genetic and ecological characteristics of A. donax and P. australis in general and in particular for the ecological region of the Chihuahuan Desert, with special focus in the CCB. The aim is to provide a better understanding of the evolutionary ecology of these two related and ecologically similar species, and determine if these grass species represent a risk for the ecosystem and the valley’s biotic diversity.
The Complex Case of the Invasive Arundo donax
Systematics and distribution of Arundo donax. Arundo L. (Poaceae, tribe Arundineae) is a cosmopolitan genus that includes three to five taxa distributed from tropical Asia to the Mediterranean Basin (Conert 1961; Bell 1997; Grass Phylogeny Working Group 2001; Danin 2004). The species Arundo donax (Fig. 15.1 a, b, and c) is the largest species in the genus and is one of the tallest herbaceous grasses (up to 10 m tall). Since giant reed has been cultivated in Asia, southern Europe, North Africa, and the Middle East for thousands of years (Perdue 1958; Zohary 1962; Bell 1997), the native range is a matter of speculations because the biogeographic and evolutionary origin of this species has been obscured through ancient and widespread cultivation, and has been variously reported as southern Asian (Bell 1997; Dudley 2000), eastern Asian (Polunin and Huxley 1987), and from countries surrounding the Mediterranean Sea, where it occurs along the other Arundo species, A. plinii Turra, A. collina Tenore and A. mediterranea Danin (Perdue 1958). Nevertheless, a more recent study has provided evidence that A. donax likely originated in Asia and subsequently spread into the Mediterranean region (Mariani et al. 2010). The ancient spread of A. donax has been accounted mainly due to its multiple uses, including the making of baskets, mats, fishing rods, walking-sticks, fences, roof thatching, plant stakes, musical instruments such as the reeds for clarinets and saxophones, shading or as ornamental, and more recently for erosion control, paper, pulp, and rayon (viscose) (Perdue 1958; Zohary 1962). Therefore, it has become widely dispersed by humans and it is currently found growing into all of the tropical-subtropical and warm-temperate areas of the world (Dudley 2000; Lewandowski et al. 2003; Khudamrongsawat et al. 2004; Ahmad et al. 2008; Mariani et al. 2010; Tarin et al. 2013).
In the USA, giant reed is believed to have been initially introduced into southern California from the Mediterranean in the early 1800s for erosion control, with later introductions being made in Texas and Florida as late as the 1940s (Bell 1997; Perdue 1958). It was also used for roof thatching and widely cultivated for the production of reeds for musical instruments (Perdue 1958; Bell 1997). Since its introduction, A. donax has escaped cultivation and become a major invasive weed of riparian habitats, such as in Southern California, Florida, and along the Rio Grande in the border between Texas and Mexico (Bell 1997; Dudley and Collins 1995; Dudley 2000), where it not only displaces native species but also dramatically modifies ecological and successional processes (Bell 1997; Dudley 2000).
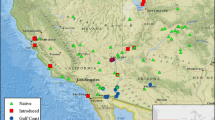
In Mexico, A. donax (Fig. 15.1 a, b, and c) is a common introduced species, growing in a variety of climates and habitats, including disturbed marshes, wetlands, rivers, lakes, riparian zones, and along roads. In the CCB, A. donax has been recently identified and efforts to document its invasion began in 2004 (Hendrickson and McGaugh 2005). Although it is not clear when Arundo arrived to the area, at present it has been documented to occur in a variety of mostly small to relatively large stands widely scattered throughout the CCB and surrounding areas (Fig. 15.2) (Hendrickson and McGaugh 2005). Nowadays, Arundo is regarded as one of the major threats to native riparian and aquatic ecosystems in the CCB (Hendrickson and McGaugh 2005; CONABIO 2018), and control measures have been implemented in the valley, including control using chemical herbicides, cutting and removing biomass, as well as prescribed fire (Hendrickson and McGaugh 2005; Contreras-Arquieta and Cruz-Nieto 2007).
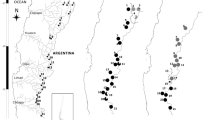
Genetic characterization. In Mexico, Colin et al. (unpublished) used Inter Simple Sequence Repeats (ISSRs) to estimate the current geographic distribution of genetic diversity of A. donax in 20 natural populations across different geographic regions, and found a total of 77 different genotypes (clones) along the Mexican territory evidencing that all the Mexican populations are multiclonal (including from 3 to 9 different genotypes). In the case of northern Mexico, they analyzed 60 individuals from two populations of the state of Coahuila (CCB, N = 32 and Valle Cruya, N = 28), detecting 13 unique genotypes not found in any other populations (five and eight genotypes in the CCB and Valle Cruya, respectively; Fig. 15.3). In addition, they found high values of clonal diversity (Table 15.1), as suggested by the proportion of distinguishable genotypes (G/N, where G= unique genotypes, and N= number of analyzed individuals (Ellstrand and Roose 1987)). We can conclude that Mexican populations are less clonal and genetically more variable than many populations in other parts of the world (Table 15.1).
Reproduction. Arundo donax has been observed flowering annually between August and October, and although it produces abundant flowers, viable seeds have not been observed in most areas where it has been introduced (Perdue 1958), including North America, Europe, and Australia (Dudley 2000; Di Tomaso and Healey 2003; Lewandowski et al. 2003; Williams et al. 2008). Thus, asexual propagation through stem layering and rhizome proliferation is believed to be the primary mode of reproduction (Dudley 2000; Boland 2006; Johnson et al. 2006; Williams et al. 2008; Mariani et al. 2010; Haddadchi et al. 2013; Lewandowski et al. 2003).
In Mexico, genetic analyses of random mating by evaluating linkage disequilibrium among loci showed evidence of linkage disequilibrium for the population of CCB, suggesting an asexual mode of propagation (Colin et al. unpublished), in accordance to findings in previous studies (Khudamrongsawat et al. 2004; Ahmad et al. 2008; Mariani et al. 2010; Haddadchi et al. 2013). In contrast, no evidence of linkage disequilibrium among loci was found in Valle Cruya, being consistent with (at least partially) sexual recombination (Colin et al. unpublished). Although the frequency at which rare sexual reproductive events may occur in A. donax remains unclear, viable seeds (Perdue 1958) have been reported in Asian populations (Afghanistan, South Western Pakistan, and Iran), and more recently, Johnson et al. (2006) found a low frequency of ovules that may have been viable in florets collected in the USA (California, Nevada, Colorado, New Mexico, Texas, Georgia, and Washington D. C.), as well as in northern Mexico, particularly in the state of Nuevo Leon. Thus, the evidence for sexual reproduction that was detected in Valle Cruya is not surprising, but it should be taken with caution (Colin et al. unpublished), since there is no knowledge about the biological reproduction in the Mexican A. donax populations.
Eventually, it will be important to conduct studies on the fruit production, seed set rates, viability, and germination of seeds as well as on their ability to spread in the different Mexican populations of A. donax. These analyses of reproductive biology along with molecular markers should allow us to better disentangle the roles of seed and asexual propagation, and to understand the importance of the reproductive systems in governing the organization and levels of genetic diversity within the species.
Genetic structure and genetic relationships. It is important to describe the genetic differentiation (also called genetic structure) of populations because it reflects the biological processes of the past that modeled the evolution of the species (Templeton 2006; Pleines et al. 2009; Zhao et al. 2013). In addition, analyzing how variation is partitioned within and between populations in introduced species is critical to understand their potential to establish and spread in a novel range (Sakai et al. 2001; Facon et al. 2006; Marrs et al. 2008).
Genetic structure analyses of A. donax in Mexico, carried out by means of several methods (i.e., principal coordinate analysis, agglomerative hierarchical clustering analysis, and AMOVA), suggest the existence of four genetic groups showing a clear genetic differentiation (coancestry coefficient θ = 0.830), and low levels of gene flow between clusters (Colin et al. unpublished). In the particular case of the populations from the state of Coahuila, the analysis indicates that the Valle Cruya population is more similar to populations near the coasts of the Pacific and of the Gulf of Mexico, while all the plants from CCB remained separated, forming a distinct group (Colin et al. unpublished). In addition, these authors also identify that these two populations are genetically very different, as they do not share genotypes between them (Fig. 15.3) (Colin et al. unpublished).
Ecological and genetic characterizations. To evaluate the role of environmental conditions and to detect climate differences and/or similarities between the two populations located in the state of Coahuila, a canonical correspondence analysis was carried out using the genotype distribution data and the environmental variables (Hijmans et al. 2005) (Colin et al. unpublished). The ecological characterization indicates that the distribution and abundance of genotypes are influenced by environmental factors. Temperature seasonality appeared as the strongest environmental variable correlated with the North region, and it is directly associated with genotypes distributed in the area, suggesting that the Northern genotypes are adapted to stronger seasonality with scarce rainfall.
On the other hand, the comparisons in the global distribution of the species indicate that the Valle Cruya population is associated with the bioclimatic space occupied by the invasive occurrence records from the USA and some areas from Europe, while the population of Cuatro Ciénegas was more similar to the environmental space occupied by native records from Asia and some areas from Australia and Africa (Colin et al. unpublished).
General observations and perspectives on Arundo donax. The apparently obligate asexual propagation of A. donax in introduced ranges of Mediterranean region, Europe, and the USA may keep their genetic diversity low (Ahmad et al. 2008). On the other hand, in the case of Mexican A. donax populations, the higher genetic diversity could be likely maintained by different modes of dispersal (i.e., asexual propagation by means of broken stems or rhizome fragments, and some levels of sexual reproduction), suggesting that the frequency at which rare sexual reproductive events may occur in A. donax may be variable among populations (Colin et al. unpublished).
Another alternative to sexual reproduction that can explain the level of variation observed in A. donax could be multiple introductions from different source regions, as suggested by Khudamrongsawat et al. (2004) and evidenced by Tarin et al. (2013) in the USA. Somatic mutations could also be contributing to the genetic variation in A. donax (Khudamrongsawat et al. 2004) and to determine the importance of this type of mutations on the genetic variation found in the Mexican populations of Arundo, it would be necessary to compare the genetic composition of plants with known rhizome connections (Khudamrongsawat et al. 2004; Haddadchi et al. 2013).
However, the differences in the values in a given geographic area may be simply due to different sampling schemes and the use of different genetic markers (Mohammadi and Prasanna 2003; Zhao et al. 2006). It will be important to use a standardized method to study the species worldwide (Colin et al. unpublished). It is also important to include in the future large samples from more areas in Asia, where the species apparently originated (Mariani et al. 2010) and that have been poorly studied.
As mentioned above, multiple introductions or a single introduction of multiple genotypes from diverse source populations can result in enhanced genetic diversity in the introduced range (e.g., Simberloff 2009; Lavergne and Molofsky 2007; Kelager et al. 2013), and this may result in the development of locally adapted genotypes (or ecotypes) through natural selection (Sexton et al. 2002; Lavergne and Molofsky 2007; Prentis et al. 2008). In relation to this, the ecological characterization of genotypes of A. donax in the North region of Mexico suggests that the genotypes in these populations are influenced by fluctuations in temperature and altitude ranges (Colin et al. unpublished), and point out that A. donax may have been introduced different times from disjunct regions (perhaps from the USA and Asia).
The use of genetic data to reconstruct invasion histories, and reveal how a nonnative plant adapts and expands into new territory, can also lead to more effective management strategies (Sakai et al. 2001; Allendorf and Lundquist 2003; Prentis et al. 2008). That is, populations having high genetic variation may be more difficult to control because of naturally variable genotypes within the introduced population or the possibility of newly emerged resistant plants, as a result of ongoing natural selection (Sexton et al. 2002; Sterling et al. 2004; Prentis et al. 2008).
In recent years efforts have been made to identify specific phytophagous insects in giant reed as biocontrol agents in the USA (Goolsby and Moran 2009; Goolsby et al. 2009; Goolsby et al. 2013; Goolsby et al. 2015). However, the high regional differentiation of giant reed in Mexico and the multiple genotypes detected in the North region of Mexico imply that these diverse populations may show different levels of susceptibility or resistance to pathogen or other biocontrol agents (Burdon et al. 1981; Sexton et al. 2002; Bruckart et al. 2004; Sterling et al. 2004; Prentis et al. 2008). Therefore, the genetic diversity among populations may be sufficiently great to warrant different control strategies. Differential responses to the same management method have been observed in genetically diverse populations (Sexton et al. 2002; Sterling et al. 2004; Goolsby et al. 2006), so knowledge of existing genetic diversity in the populations provides further insight into the responses of populations to specific management strategies, including the use of chemical control and biocontrol programs (Burdon and Marshall 1981; Chapman et al. 2004; Gaskin et al. 2005; Ward et al. 2008).
Further research of biogeographic relationships in A. donax should include populations across a broader range from North America (USA and Mexico), Europe (mainly from Mediterranean basin), and Asia in order to address the question of multiple introductions. Other molecular tools, for instance, chloroplast DNA, could be used in conjunction with ISSR or microsatellite data (Tarin et al. 2013) coupled with ecological analysis to identify if there are different lineages through the global distribution and to determine the possible divergences between those lineages. Additionally, potential source populations of the invader can also be identified, and these populations may help to further locate associated natural enemies, which may be useful later as biological control agents (Ellstrand and Schierenbeck 2000; Clark et al. 2013; Kelager et al. 2013; Ndlovu et al. 2013).
Phragmites australis, an Important Native Species with Invasive Potential
Systematics and distribution of Phragmites australis The genus Phragmites Adans. (Poaceae, tribe Arundineae) has a worldwide distribution, from the Arctic regions to the tropics (Den Hartog et al. 1989). Historically a number of species, subspecies, and varieties in the genus have been described; currently four species are recognized: P. australis (Cav.) Trin. ex Steud., P. karka (Retz.) Trin. ex Steud., P. mauritianus Kunth and P. japonicus Steud (Clevering and Lissner 1999; Saltonstall et al. 2004; Lambertini et al. 2006).
Within the genus, the species Phragmites australis (Fig. 15.1 d and e) is the most widely distributed, as it is found in Europe, Asia, Africa, America, and even Australia (Conert 1961; Bjôrk 1967; Clevering and Lissner 1999; Saltonstall 2003a). Phragmites australis has been present for at least 40,000 years in southwestern United States (Hansen 1978) and chloroplast genetic data from (cpDNA) indicate that three distinct lineages are found in North America. P. australis ssp. americanus (comprising thirteen native North American haplotypes: A–H, AA, AB, AC, S, and Z) found throughout the USA and called the “Native North America” lineage (Saltonstall et al. 2004). P. australis ssp. berlandieri, with only one haplotype I, that grows in Southern United States (from Florida to California), Mexico, Central America, and Asia, named the “Gulf Coast” lineage, (Saltonstall 2002; Saltonstall and Hauber 2007; Saltonstall and Stevenson 2007); this lineage is usually not considered invasive (Meyerson et al. 2009; Meyerson et al. 2010). The third lineage is the haplotype M of P. australis, considered an invasive lineage that was probably introduced from Eurasia to North America since ca. 1600 and now is the most common P. australis found from the south of Canada and most USA (Saltonstall 2002, 2003a, 2003b; Saltonstall et al. 2004; Saltonstall and Hauber 2007; Saltonstall and Stevenson 2007; Meyerson et al. 2010).
Phragmites australis in Mexico (Fig. 15.1 d and e) is a common species, growing in different environments from disturbed to well-preserved, along roads rivers, marshes, wetlands, and lakes. In the CCB P. australis (Fig. 15.1 d and e) is a very common species, however, as in Arundo, it is not clear when Phragmites arrived to the region. Presently, it is found in different stands widely scattered throughout the CCB (Fig. 15.2), and it is considered a native species of the valley (Colin and Eguiarte 2016).
Genetic characterization. Two lineages of Phragmites are present along Mexico: The Gulf Coast lineage, P. australis subsp. berlandieri found across of Mexico, and the Native North America lineage P. australis subsp. americanus, found in north of Mexico, in the state of Coahuila (Colin and Eguiarte 2016).
Phragmites australis subsp. berlandieri has been characterized by a single cpDNA haplotype (haplotype I) and low genetic diversity in the USA, Central America, and northern South America (Saltonstall 2002, 2003a). Nevertheless, 13 new haplotypes (BH, BM- BX) were found in Mexico, strongly suggesting expansion and diversification in this country (Colin and Eguiarte 2016). From this subspecies four populations were analyzed in the CCB, (Mojarral, Vereda, Desviación, and Mezquite) for a total of 58 individuals (Colin and Eguiarte 2016). Chloroplast haplotype diversity (Hd) in these populations ranged from zero to 0.5333, and nucleotide diversity (π) among these populations ranged from zero to 0.0003 (Table 15.2).
Phragmites australis subsp. berlandieri populations in CCB are characterized by a lower genetic variation when compared with central and southern regions of Mexico (Table 15.2), and by the presence of two haplotypes (BH and BM). The Mojarral population presented only one haplotype (BH; Fig. 15.4), whereas the remaining populations had two haplotypes, with greater predominance of haplotype BM in the populations Desviación and Mezquite, while the haplotype BH was more frequent in the Vereda population (Fig. 15.4). It is interesting to mention that the haplotype I has been reported in the Texas side of the Rio Grande and even in the Cuatro Ciénegas Valley (K. Saltonstall, Smithsonian Tropical Research Institute, personal communication). Nevertheless, Colin and Eguiarte (2016) sampling did not detect this haplotype in the CCB (Fig. 15.4), and it was detected only in populations from southern and central Mexico, being more common in the southern areas.
Populations of Phragmites australis depicting the frequency of haplotypes found in Cuatro Ciénegas Basin (CCB). Haplotypes BH and BM (violet and red colors, respectively) belong to Phragmites australis ssp. berlandieri. Haplotypes BI, BJ, BK, and BL (green color gradient) depicting haplotypes that were more closely related to the native North American lineage Phragmites australis ssp. americanus. Data were obtained from Colin and Eguiarte (2016)
On the other hand, P. australis subsp. americanus includes thirteen native North American cpDNA haplotypes (A–H, AA, AB, AC, S, and Z), showing high genetic diversity (Saltonstall 2002, 2003a, 2003b). In Mexico, four new haplotypes (BI–BL) were found in the Poza X population from CCB (Fig. 15.4); these haplotypes are related to the native lineage of North America, particularly to haplotype B (Saltonstall 2002, 2003a), increasing the range of P. australis subsp. americanus (Colin and Eguiarte 2016). As in the USA, this Mexican population (Poza X) showed high genetic variation compared with the populations of P. australis subsp. berlandieri (Table 15.2). The finding of these haplotypes in the CCB may be related to the high diversity and levels of endemism of the basin (Souza et al. 2012; Carson et al. 2015).
Reproduction. Previous studies in P. australis (Rice et al. 2000; Ishii and Kadono 2002; Saltonstall 2002, 2003a; Brisson et al. 2008; Howard et al. 2008; Fer and Hroudova 2009; Baldwin et al. 2010; Belzile et al. 2010; McCormick et al. 2010; Kirk et al. 2011) have documented both sexual and asexual (e.g., lateral rhizome extension, rhizome fragments, and tillering) propagation. Flowers are monoecious and wind-pollinated. Each infructescence can contain thousands of seeds, which have long silky hairs on the rachilla, facilitating wind dispersal. It was originally suggested that the species is self-incompatible (Clevering and Lissner 1999), but more recent evidence suggests that self-pollination is possible (Lambert and Casagrande 2007). In addition, environmental conditions can affect production and seed viability; seeds require high exposure to light, as well as diurnal temperature fluctuations (range 10° to 30° C) to germinate (Marks et al. 1994; Campbell 2007; Meyerson et al. 2010).
Phragmites australis subsp. berlandieri flowers annually from late October to November, and although it produces abundant flowers, viable seeds have not been observed in most areas from the USA, Mexico, Central America, and northern South America, and vegetative propagation through stem layering and rhizome proliferation is believed to be the primary mode of reproduction (Pellegrin and Hauber 1999; Saltonstall 2002, 2003a; Meyerson et al. 2009: Meyerson et al. 2010). Colin and Eguiarte (2016), working with populations across the species’ geographical range in Mexico, found that some populations contain only a single haplotype (such as the population Mojarral; see Table 15.2 and Fig. 15.4), suggesting that in some cases the populations may derive from a single founder, and subsequently spread by asexual propagation. In contrast, in the majority of the populations—as in Desviación, Mezquite, and Vereda in CCB (see Table 15.2 and Fig. 15.4)—different haplotypes were detected, indicating that different individuals originated these populations (Colin and Eguiarte 2016).
Phragmites australis subsp. americanus flowers earlier, between June and October (Meyerson et al. 2009; Meyerson et al. 2010). It propagates both by seed, mainly dispersed by wind, and by vegetative propagules likely dispersed by water and maybe by animals. However, apparently this lineage has lower seed germination rates (Meyerson et al. 2010). In Mexico, genetic diversity for this subspecies was high (see Poza X population in Table 15.2 and Fig. 15.4). Again, as in the other subspecies, this indicates either that dispersal events involved multiple founders by vegetative propagules or that dispersal by seeds is important for the establishment of new populations (Colin and Eguiarte 2016).
These findings suggest that in Mexico the genetic composition of the populations from both lineages is likely the result of different modes of spread and dispersal, including at least some sexual reproduction. However, as discussed by Colin and Eguiarte (2016), it is important to conduct future studies including aspects of the reproductive ecology, seed germination, and dispersal that shall help us to determine the importance of reproductive systems in the life history of the species. In addition, it will be relevant to use nuclear DNA (e.g., microsatellites or SNPs) to understand the roles of seed and asexual propagation and to better describe the evolutionary genetics of P. australis in the CCB and Mexico (Colin and Eguiarte 2016).
Genetic structure and genetic relationships. Using a representative set of samples from the Mexican range, Colin and Eguiarte (2016) investigated the genetic structure patterns within and between the introduced P. australis subsp. berlandieri populations by conducting a spatial analysis of molecular variance (SAMOVA). Colin and Eguiarte (2016) found seven genetic groups (K= 7) in Mexico. The CCB was characterized by the presence of two genetic groups (K1 and K2). The first cluster (K1) included the Mojarral and Vereda populations that were grouped together with populations belonging to the states of Michoacán, Veracruz, Tabasco, Jalisco, Campeche, Yucatán, and Quintana Roo. The second cluster (K2) comprises only the Desviacion and Mezquite populations.
The historical demography of these groups was inferred by examining hypotheses of demographic expansion (Colin and Eguiarte 2016). For the first cluster, K1 a recent range expansion was inferred and the estimate of the age of population expansion was older (ca. 0.73 Myr before the present) for K1. In contrast, the analysis for the K2 cluster suggests that these populations have maintained a constant population size (Colin and Eguiarte 2016).
Ecological and genetic characterizations. At present, there is no data available on whether or not environmental factors affect the distribution and abundance of haplotypes for both lineages in the CCB. However, the patterns of genetic diversity found across the species’ geographical range in Mexico suggest that dispersal was from the South towards the North, with a reduction of genetic diversity in the process (Table 15.2) (Colin and Eguiarte 2016). This gradient of genetic diversity suggests that the Pleistocene climatic changes were relevant for the historical dynamics of P. australis in Mexico. In consequence, the populations in the CCB may be relatively recent, and this may also explain why the P. australis populations from Mexico—even in the northern areas— are so different from the populations in the USA (Colin and Eguiarte 2016).
General observations and perspectives on P. australis. As found by Colin and Eguiarte (2016) in the CCB we can find two independent and native lineages of Phragmites: P. australis subsp. berlandieri and P. australis subsp. americanus, we provided insights into the dynamic of the populations of P. australis subsp. berlandieri in the CCB, and Colin and Eguiarte (2016) also demonstrated that the invasive lineage of P. australis (haplotype M) is absent in the CCB.
The CCB is characterized by the presence of two (Fig. 15.4) of the thirteen haplotypes belonging to P. australis subsp. berlandieri found in Mexico, and by a low genetic variation (Table 15.2). The detection of new haplotypes in the Poza X population from P. australis subsp. americanus increased the proposed range of this lineage (Fig. 15.4) (Colin and Eguiarte 2016).
Populations in the CCB have significantly lower genetic variation when compared with populations from the central and southern Mexican regions (Colin and Eguiarte, personal observation). Genetic diversity can be reduced in expanding populations, as only a few individuals contribute with genetic variation to the newly colonized populations (Buckley et al. 2012; Hallatschek and Nelson 2008). Previous studies have shown that there is a correlation between genetic diversity and environmental heterogeneity in P. australis (Curn et al. 2007; Hansen et al. 2007; Engloner 2009), but whether or not climatic factors affect the distribution and abundance of haplotypes in P. australis subsp. berlandieri in Mexico has yet to be determined (Colin and Eguiarte in preparation).
The genetic structure of populations is not always reflected in the geographical proximity of individuals, and individuals with different geographical locations are not necessarily genetically differentiated (Evanno et al. 2005). Among multiple-population clustering of the Mexican population, group K1 included different populations separated by long distances, and this cluster included the Mojarral and Vereda CCBs populations, that were grouped together with populations from Michoacán, Veracruz, Tabasco, Jalisco, Campeche, Yucatán, and Quintana Roo states. In contrast, the cluster K2 that included Desviación and Mezquite was restricted to a small area within CCB (Colin and Eguiarte, personal observation). These results suggest that genetic clustering in P. australis in Mexico is best explained as a function of genetic divergence rather than of geographical distance, in contrast to what happens in the USA (Saltonstall 2003a) and, perhaps in China (An et al. 2012).
Finally, results suggest that haplotype U distributed in Australia and Asia (Saltonstall 2002, 2003a) is the closest relative to haplotypes of P. australis subsp. berlandieri found in Mexico (Colin and Eguiarte 2016); however, their history is complex. Genetically, it has been shown that the haplotype I has affinity to different geographic regions (Saltonstall 2002, 2003a; Lambertini et al. 2006) and has been reported to have a closer relationship with Phragmites in Asia (Saltonstall 2002, 2003a), as well as with the African species P. mauritianus (Lambertini et al. 2006); also morphological analyses indicate that haplotype I may correspond to P. karka, from tropical Africa (Saltonstall and Hauber 2007; Ward 2010). Future genetic and morphological analyses of plants from Africa and South America will be needed to understand the origin and evolution of P. australis in Mexico and of the Phragmites genus in general.
Concluding Remarks: A. donax Is an Aggressive Invasive Plant, But P. australis Is Native to CCB
Here we presented a review of the evolutionary biology and invasive potential of two related and ecologically similar grasses (A. donax and P. australis) in the Chihuahuan Desert, with special emphasis in the CCB. In general, in the state of Coahuila there are 13 genotypes of A. donax and two independent native lineages of Phragmites (P. australis subsp. berlandieri and P. australis subsp. americanus), although the invasive lineage (M) was not found. We hope that this review will shed new light on the understanding of ecological, evolutionary, and biogeographic aspects of the complex studied species and can also assist in the development of conservation, management, and policy strategies of these species in the region, and in particular in the CCB.
The genetic data available for A. donax and P. australis reflect that both sexual and asexual propagation seem to be relevant but the role of each reproductive mode varies in each species. Gaps in our knowledge are particularly apparent in the dispersal and establishment phases of the life cycle of these two species, for example rates of seed set and germination have not yet been determined in Mexico. Indeed, it will be important to conduct studies on the viability and germination of seeds as well as on their ability to spread in native and introduced environments to assess the fecundity of these species and of their different genetic lineages. At the same time, as we mentioned above, it will be relevant to use nuclear DNA (in particular microsatellites or SNPs) markers to better understand the roles of seed and asexual propagation and evaluate their importance to determine genetic diversity within and among populations.
Recent studies have shown that when species with a broad climatic and geographical range colonize or invade a new environment or spread over large geographical ranges, they may come upon suitable habitats to colonize and can establish in contrasting climatic regions, displaying environmental tolerances and physiological plasticities that promote their potential to adapt and invade these environments (Lee 2002; Sax et al. 2007; Vellend et al. 2007; Prentis et al. 2008). Thus, the novel environmental conditions found in the range of introduction may act as strong selection forces and can lead to rapid evolution (in centuries, decades, or even just in years) during the establishment and spread of invasive species (García-Ramos and Rodríguez 2002; Colautti and Lau 2015).
In A. donax the data shown here suggest that the genotypes distributed in the Northern region are adapted to stronger seasonality with scarce rainfall, and point out that A. donax may have been introduced in different occasions from disjunct regions. On the other hand, for P. australis, we cannot say at this time whether the haplotypes are influenced by fluctuations in temperature, rainfall, and/or altitude ranges; however, evidence suggests a post-glacial colonization or recolonization of P. australis in the CCB. Thus, more research is needed to evaluate whether or not the environmental factors play an important factor in the distribution of haplotypes in the region.
Besides natural selection, lack of gene flow between the native and introduced ranges can generate genetic differentiation and divergence between them and may eventually lead to allopatric speciation (García-Ramos and Rodríguez 2002; Lee 2002; Sax et al. 2007; Vellend et al. 2007; Prentis et al. 2008; Futuyma 2013; Colautti and Lau 2015). In Mexico, for A. donax 77 different genotypes were detected by Colin et al. (unpublished), among which 13 unique genotypes were found in northern Mexico (5 and 8 genotypes in the CCB and Valle Cruya, respectively (Fig. 15.3), but we still do not know if they are the same or different haplotypes from those found in the Mediterranean region, Europe, Australia, and/or the USA. Thus, we need to use a standardized method (e. g., chloroplast DNA, in conjunction with microsatellite markers or SNPs) to evaluate if there are different lineages and to determine their biogeographical ranges in the species worldwide distribution.
Phragmites australis in Mexico seems to still be in the process of differentiation and diversification (Colin and Eguiarte personal observation). Of the thirteen (BH, BM- BX) new haplotypes found now in Mexico, two of them (BH and BM) were found in the CCB. Furthermore, 4 new haplotypes belonging to the native North America Lineage were also found in the CCB (Fig. 15.4). Experimental and ecological data including viability and germination of seeds information are needed to determine the importance of the propagation mode (sexual vs. asexual) in the life history of the different lineages of P. australis in Mexico.
Further research in morphology, ecology, and genetics of more samples from A. donax, especially from the USA, Europe, Asia, and the Mediterranean region and from P. australis in particular from South America, and Africa, are still needed to link the observed patterns in genetic variation with the environmental factors in order to determine more clearly the evolutionary processes in both species and address in detail their origin, ecology, and invasive potential in Mexico and in particular in the CCB.
References
Ahmad R, Liow P, Spencer D et al (2008) Molecular evidence for a single genetic clone of invasive Arundo donax in the USA States. Aquatic Botany 88:113–120
Allendorf FW, Lundquist LL (2003) Introduction: population biology, evolution, and control of invasive species. Conservation Biology 17:24–30
An JX, Wang Q, Yang J et al (2012) Phylogeographic analyses of Phragmites australis in China: Native distribution and habitat preference of the haplotype that invaded North America. Journal of Systematics and Evolution 50:334–340
Baldwin AH, Kettenring KM, Whigham DF (2010) Seed banks of Phragmites australis-dominated brackish wetlands: Relationships to seed viability, inundation, and land cover. Aquatic Botany 93:63–169
Bell GP (1997) Ecology and management of Arundo donax, and approaches to riparian habitat restoration in southern California. In: Brock JH, Wade M, Pysek P, Green D (eds) Plant Invasions: Studies from North America and Europe. Backhuys publishers, Leiden, The Netherlands, pp 103–113
Belzile F, Labbe J, Leblanc M et al (2010) Seeds contribute strongly to the spread of the invasive genotype of the common reed (Phragmites australis). Biological Invasions 12:2243–2250
Bjôrk S (1967) Ecologic investigation of Phragmites communis. Studies in the theoretic and applied limnology. Folia Limnologica Scandinavica 14:1–248
Blackburn TM, Essl F, Evans T (2014) A unified classification of alien species based on the magnitude of their environmental impacts. PLoS Biology 12:e1001850
Boland JC (2006) The importance of layering in the rapid spread of Arundo donax (giant reed). Madrono 53:303–312
Booth D, Provan J, Maggs CA (2007) Molecular approaches to the study of invasive seaweeds. Botanica Marina 50:385–396
Brisson J, Paradis E, Bellavance M (2008) Evidence of sexual reproduction in the invasive common reed (Phragmites australis subsp. australis; Poaceae) in eastern Canada: A possible consequence of global warming. Rhodora 110:225–230
Bruckart W, Cavin C, Vajna L et al (2004) Differential susceptibility of Russian thistle accessions to Colletotrichum gloeosporioides. Biol Control 30:306–311
Buckley J, Butlin RK, Bridle JR (2012) Evidence for evolutionary change associated with the recent range expansion of the British butterfly, Aricia agestis, in response to climate change. Molecular Ecology 21:267–280
Burdon JJ, Groves RH, Cullen JM (1981) The impact of biological control on the distribution and abundance of Chondrilla juncea in south-eastern Australia. Journal of Applied Ecology 18:957–966
Burdon JJ, Marshall DR (1981) Biological control and the reproductive mode of weeds. Journal of Applied Ecology 18:649–658
Campbell AL (2007) Sexual Reproduction in Non-Native Common Reed, Phragmites australis. M.S. thesis. Ohio State University. Columbus, OH, USA
Carson EW, Souza V, Espinosa-Perez H et al (2015) Mitochondrial DNA diversity and phylogeography of Lucania interioris inform biodiversity conservation in the Cuatro Ciénegas basin, Mexico. Western North Naturalist 75:200–208
Chapman H, Robson B, Pearson ML (2004) Population genetic structure of a colonising, triploid weed, Hieracium lepidulum. Heredity 92:182–188
Clark LV, Evans KJ, Jasieniuk M (2013) Origins and distribution of invasive Rubus fruticosus L. agg. (Rosaceae) clones in the western United States. Biol Invasions 15:1331–1342
Clevering OA, Lissner J (1999) Taxonomy, chromosome numbers, clonal diversity and population dynamics of Phragmites australis. Aquatic Botany 64:185–208
Colautti RI, Lau JA (2015) Contemporary evolution during invasion: evidence for differentiation, natural selection, and local adaptation. Molecular Ecology 24:1999–2017
Colin R, Eguiarte LE (2016) Phylogeographic analyses and genetic structure illustrate the complex evolutionary history of Phragmites australis in Mexico. American Journal of Botany 103:876–887
CONABIO (2018) Sistema de información sobre especies invasoras en México. Comisión Nacional para el Conocimiento y Uso de la Biodiversidad. http://www.biodiversidad.gob.mx/especies/Invasoras/invasoras.html. Accessed Sep 2018
CONANP (2018) Programa Nacional de Áreas Naturales Protegidas, 2014–2018. Comisión Nacional de Áreas Naturales Protegidas. Mexico City, Mexico
CONANP (2016). https://www.gob.mx/conanp/acciones-y-programas/areas-naturales-protegidas-decretadas Accessed Sep 2018
Conert HJ (1961) Die Systematik und Anatomie der Arundinae. Cramer, Weinheim, West Germany
Contreras-Arquieta A, Cruz-Nieto M (2007) Control de la planta invasora Arundo donax en Cuatro Ciénegas, un centro de gran biodiversidad de especies endémicas de flora y fauna de Norteamérica. Informe técnico final de Pronatura Noreste, AC, US Fish & Wildlife Service. Monterrey, Nuevo León, Mexico
Curn V, Kubatova B, Vavrova P et al (2007) Phenotypic and genotypic variation of Phragmites australis: comparison of populations in two human-made lakes of different age and history. Aquatic Botany 86:321–330
D’Antonio CM, Meyerson LA (2002) Exotic plant species as problems and solutions in ecological restoration: a synthesis. Restoration Ecology 10:703–713
D’Antonio CM, Vitousek PM (1992) Biological invasion by exotic grasses, the grass-fire cycle and global change. Annual Review of Ecology and Systematics 23:63–88
Danin A (2004) Arundo (Gramineae) in the Mediterranean reconsidered. Willdenowia 34:361–369
Den Hartog C, Kvet J, Sukopp H (1989) Reed: a common species in decline. Aquatic Botany 35:1–4
Di Tomaso JM, Healey EA (2003) Aquatic and riparian weeds of the west. University of California—Agriculture and Natural Resources Publication 3421:254–262
Dlugosch KM, Parker IM (2008) Founding events in species invasions: genetic variation, adaptive evolution, and the role of multiple introductions. Molecular Ecology 17:431–449
Dudley TL (2000) A. donax L. In: Bossard CC, Randall JM, Hoshovsky MC (eds) Invasive Plants of California’s Wildlands. University of California Press, Berkeley, CA, USA, pp 53–58
Dudley TL, Collins B (1995) Biological Invasions in California Wetlands: The Impacts and Control of Non-indigenous Species in Natural Areas. Pacific Institute for SIDES, Oakland, CA, USA
Ellstrand NC, Roose ML (1987) Patterns of genotypic diversity in clonal plant species. American Journal of Botany 74:123–131
Ellstrand NC, Schierenbeck KA (2000) Hybridization as a stimulus for the evolution of invasiveness in plants? Proc Natl Acad Sci USA 97:7043–7050
Engloner AI (2009) Structure, growth dynamics and biomass of reed (Phragmites australis) A review. Flora 204:331–346
Evanno G, Regnaut S, Goudet J (2005) Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Molecular Ecology 14:2611–2620
Facon BB, Genton J, Shykoff J (2006) A general eco-evolutionary framework for understanding bioinvasions. Trends in Ecology and Evolution 21:130–135
Fer T, Hroudova Z (2009) Genetic diversity and dispersal of Phragmites australis in a small river system. Aquatic Botany 90:165–171
Futuyma DJ (2013) Evolution, 3rd edn. Sinauer, Sunderland, MA, USA
García-Ramos G, Rodríguez D (2002) Evolutionary speed of species invasions. Evolution 56:661–668
Gaskin JF, Dao-Yuan Z, Bon M-C (2005) Invasion of Lepidium draba (Brassicaceae) in the western United States: distributions and origins of chloroplast DNA haplotypes. Molecular Ecology 14:2331–2341
Goolsby JA, De Barro PJ, Makinson JR et al (2006) Matching the origin of an invasive weed for selection of a herbivore haplotype for a biological control programme. Molecular Ecology 15:287–297
Goolsby JA, Moran PJ (2009) Host range of Tetramesa romana Walker (Hymenoptera: Eurytomidae), a potential biological control of giant reed, Arundo donax L. in North America. Biological Control 49:160–168
Goolsby JA, Moran PJ, Adamczyk JA et al (2009) Host range of the European, rhizome-stem feeding scale Rhizaspidiotus donacis (Leonardi) (Hemiptera: Diaspididae), a candidate biological control agent for giant reed, Arundo donax L. (Poales: Poaceae) in North America. Biocontrol Science and Technology 19:899–918
Goolsby JA, Moran PJ, Racelis AE et al (2015) Impact of the biological control agent Tetramesa romana (Hymenoptera: Eurytomidae) on Arundo donax (Poaceae: Arundinoideae) along the Rio Grande River in Texas. Biocontrol Science and Technology 26:47–60
Goolsby JA, Racelis AE, Goolsby JB et al (2013) Evaluation of biogeographical factors in the native range to improve the success of biological control agents in the introduced range. Biocontrol Science and Technology 23:1213–1230
Grass Phylogeny Working Group (2001) Phylogeny and subfamilial classification of the grasses (Poaceae). Annals of Missouri Botanical Garden 88:373–457
Haddadchi A, Gross CL, Fatemi M (2013) The expansion of sterile Arundo donax (Poaceae) in southeastern Australia is accompanied by genotypic variation. Aquatic Botany 104:153–161
Hallatschek O, Nelson DR (2008) Gene surfing in expanding populations. Theor Popul Biol 73:158–170
Hansen RM (1978) Shasta ground sloth food habits, Rampart Cave, Arizona. Paleobiology 3:302–319
Hansen DL, Lambertini C, Jampeetong A et al (2007) Clone-specific differences in Phragmites australis: effects of ploidy level and geographic origin. Aquatic Botany 86:269–279
Hardion L, Verlaque R, Baumel A et al (2012) Revised systematics of Mediterranean Arundo (Poaceae) based on AFLP fingerprints and morphology. Taxon 61:1217–1226
Hendrickson DA, Mcgaugh S (2005) Arundo donax (Carrizo Grande / Giant Cane) in Cuatro Ciénegas. http://desertfishes.org/cuatroc/organisms/non-native/arundo/Arundo.html. Accessed September 2018
Hijmans RJ, Cameron SE, Parra JL et al (2005) Very high resolution interpolated climate surfaces for global land areas. International Journal of Climatology 25:1965–1978. http://www.worldclim.org Accessed September 2018
Howard R, Travis SE, Sikes BA (2008) Rapid growth of a Eurasian haplotype of Phragmites australis in a restored brackish marsh in Louisiana, USA. Biological Invasions 10:369–379
Hufbauer RA (2004) Population Genetics of Invasions: Can We Link Neutral Markers to Management? Weed Technology 18:1522–1527
INE (1999) Programa de Manejo del Área de Protección de Flora y Fauna Cuatrociénegas. Instituto Nacional de Ecología, Tlacopac, DF, Mexico
Instituto Nacional de Ecología y Cambio Climático (2016). https://www.gob.mx/inecc/acciones-y-programas/analisis-de-la-variacion-del-nivel-de-los-principales-cuerpos-de-agua-de-cuatrocienegas Accessed Sep 2018
Ishii J, Kadono Y (2002) Factors influencing seed production of Phragmites australis. Aquatic Botany 72:129–141
Johnson M, Dudley T, Burns C (2006) Seed production in Arundo donax? California Invasive Plant Council 14:12–13
Kang M, Buckley YM, Lowe AJ (2007) Testing the role of genetic factors across multiple independent invasions of the shrub Scotch broom (Cytisus scoparius). Molecular Ecology 16:4662–4673
Kelager A, Pedersen JS, Bruun HH (2013) Multiple introductions and no loss of genetic diversity: invasion history of Japanese Rose, Rosa rugosa, in Europe. Biol Invasions 15:1125–1141
Khudamrongsawat J, Tayyar R, Holt J (2004) Genetic diversity of giant reed (Arundo donax) in the Santa Ana River, California. Weed Science 52:395–405
Kirk H, Paul J, Straka J et al (2011) Long-distance dispersal and high genetic diversity are implicated in the invasive spread of the common reed, Phragmites australis (Poaceae), in northeastern North America. American Journal of Botany 98:1180–1190
Kolar CS, Lodge DM (2001) Progress in invasion biology: predicting invaders. Trends in Ecology and Evolution 16:199–204
Lambert AM, Casagrande RA (2007) Characteristics of a successful estuarine invader: evidence of self-compatibility in native and non-native lineages of Phragmites australis. Mar. Ecol. Prog. Ser. 337:299–301
Lambert AM, Dudley TL, Saltonstall K (2010) Ecology and Impacts of the Large-Statured Invasive Grasses Arundo donax and Phragmites australis in North America. Invasive Plant Science and Management 3:489–494
Lambertini C, Gustafsson MHG, Frydenberg J et al (2006) A phylogeographic study of the cosmopolitan genus Phragmites (Poaceae) based on AFLPs. Plant Systematics and Evolution 258:161–182
Lambrinos JG (2004) How interactions between ecology and evolution influence contemporary invasión dynamics. Ecology 85:2061–2070
Latombe G, Pyšek P, Jeschke JM et al (2016) A vision for global monitoring of biological invasions. Biological Conservation 213:295–308
Lavergne S, Molofsky J (2007) Increased genetic variation and evolutionary potential drive the success of an invasive grass. Proc Natl Acad Sci USA 104:3883–3888
Lee CE (2002) Evolutionary genetics of invasive species. Trends in Ecology and Evolution 17:386–391
Lewandowski I, Scurlock JMO, Lindvall E et al (2003) The development and current status of perennial rhizomatous grasses as energy crops in the US and Europe. Biomass Bioenergy 25:335–361
Mack RN, Simberloff D, Lonsdale WM (2000) Biotic invasions: causes, epidemiology, global consequences, and control. Ecological Applications 10:689–710
Mariani C, Cabrini R, Danin A et al (2010) Origin, diffusion and reproduction of the giant reed (Arundo donax L.): a promising weedy energy crop. Annals of Applied Biology 57:191–202
Marks M, Lapin B, Randall J (1994) Phragmites australis (P. communis): threats, management, and monitoring. Nat. Areas J. 14:285–294
Marrs RA, Sforza R, Hufbauer RA (2008) When invasion increases population genetic structure: a study with Centaurea diffusa. Biological Invasions 10:561–572
McCauley DE, Smith RA, Lisenby JD et al (2003) The hierarchical spatial distribution of chloroplast DNA polymorphism across the introduced range of Silene vulgaris. Molecular Ecology 12:3227–3235
McCormick MK, Kettenring KM, Baron HM et al (2010) Extent and reproductive mechanisms of Phragmites australis spread in brackish wetlands in Chesapeake Bay, Maryland (USA). Wetlands 30:67–74
Meyerson LA, Lambert AM, Saltonstall K (2010) A Tale of Three Lineages: Expansion of Common Reed (Phragmites australis) in the U.S. Southwest and Gulf Coast. Invasive Plant Science and Management 3:515–520
Meyerson LA, Saltonstall K, Chambers RM (2009) Phragmites australis in eastern North America: A historical and ecological perspective. In: Silliman BR, Grosholz E, Bertness MD (eds) Salt Marshes Under Global Siege. University of California Press, California, USA, pp 57–82
Meyerson LA, Viola DV, Brown RN (2010) Hybridization of invasive Phragmites australis with a native subspecies in North America. Biological Invasions 12:103–111
Minckley WL (1992) Three decades near Cuatro Ciénegas. Mexico: photographic documentation and a plea for area conservation. J Ariz-Nev Acad Sci 26:89–118
Mohammadi SA, Prasanna BM (2003) Analysis of genetic diversity in crop plants—salient statistical tools and considerations. Crop Science 43:1235–1248
Mooney HA, Cleland EE (2001) The evolutionary impact of invasive species. Proceedings of the National Academy of Sciences of the United States of America 98:5446–5451
Ndlovu J, Richardson DM, Wilson JRU et al (2013) Elucidating the native sources of an invasive tree species, Acacia pycnantha, reveals unexpected native range diversity and structure. Ann Bot (Lond) 111:895–904
Novak SJ, Mack RN (2005) Genetic bottlenecks in alien plant species. Influence of mating systems and introduction dynamics. In: Sax DF, Stachowicz JJ, Gaines SD (eds) Species invasions: insights into ecology, evolution and biogeography. Sinauer Associates, Sunderland, MA, USA, pp 201–228
Pellegrin D, Hauber DP (1999) Isozyme variation among populations of the clonal species, Phragmites australis (Cav.) Trin. ex Steudel. Aquatic Botany 63:241–259
Perdue RE (1958) Arundo donax: source of musical reeds and industrial cellulose. Economic Botany 12:368–404
Pilu R, Cassani E, Landoni M et al (2014) Genetic characterization of an Italian Giant Reed (Arundo donax L.) clones collection: exploiting clonal selection. Euphytica 196:169–181
Pleines T, Jakob SS, Blattner FR (2009) Application of non-coding DNA regions in intraspecific analyses. Plant Systematics and Evolution 282:281–294
Polunin O, Huxley A (1987) Flowers of the Mediterranean. Hogarth Press, London, UK
Prentis PJ, Wilson JRU, Dormontt EE et al (2008) Adaptive evolution in invasive species. Trends in Plant Sciences 13:288–294
Rejmánek M (2000) Invasive plants: approaches and predictions. Austral Ecology 25:497–506
Rice D, Rooth J, Stevenson JC (2000) Colonization and expansion of Phragmites australis in upper Chesapeake Bay tidal marshes. Wetlands 20:280–299
Sakai AK, Allendorf FW, Holt JS et al (2001) The population biology of invasive species. Annual Review of Ecology and Systematics 32:305–332
Saltonstall K (2002) Cryptic invasion by a non-native genotype of the common reed, Phragmites australis, into North America. Proc Natl Acad Sci USA 99:2445–2449
Saltonstall K (2003a) Genetic variation among North American Populations of Phragmites australis: implications for management. Estuaries 26:444–451
Saltonstall K (2003b) Microsatellite variation within and among North American lineages of Phragmites australis. Molecular Ecology 12:1689–1702
Saltonstall K, Hauber DP (2007) Notes in Phragmites australis (Poaceae: Arundinoidae) in North America. Journal of the Botanical Research Institute of Texas I(I):385–388
Saltonstall K, Peterson PM, Soreng RJ (2004) Recognition of Phragmites australis subsp. Americanus (Poaceae: Arundinoideae) in North America: evidence from morphological and genetic analyses. Sida 21:683–692
Saltonstall K, Stevenson JC (2007) The effect of nutrients on seedling growth of native and introduced Phragmites australis. Aquatic Botany 86:331–336
Sax DF, Stachowicz JJ, Brown JH et al (2007) Ecological and evolutionary insights from species invasions. Trends in Ecology and Evolution 22:465–471
SEMARNAP (1997) Programa de Conservación de la Vida Silvestre y Diversificación Productiva en el Sector Rural, 1997–2000. Secretaría de Medio Ambiente, Recursos Naturales y Pesca, México, DF, Mexico
Sexton JP, McKay JK, Sala A (2002) Plasticity and genetic diversity may allow salt cedar to invade cold climates in North America. Ecol Appl 12:1652–1660
Simberloff D (2009) The role of propagule pressure in biological invasions. Annu Rev Ecol Evol Syst 40:81–102
Simberloff D, Martin JL, Genovesi P et al (2013) Impacts of biological invasions: what’s what and the way forward. Trends in Ecology and Evolution 28:58–66
Souza V, Escalante AE, Espinoza LE et al (2004) Cuatro Ciénegas: un laboratorio natural de astrobiología. Ciencias 75:4–12
Souza V, Espinosa-Asuar L, Escalante AE et al (2006) An endangered oasis of aquatic microbial biodiversity in the Chihuahuan desert. Proc Natl Acad Sci USA 103:6565–6570
Souza V, Siefert JL, Escalante AE et al (2012) The Cuatro Ciénegas Basin in Coahuila, Mexico: An astrobiological Precambrian Park. Astrobiology 12:641–647
Stein BA, Kutner LS, Adams JS (2000) Precious Heritage: The Status of Biodiversity in the United States. Oxford University Press, Oxford, UK
Sterling TM, Thompson DC, Abbott LB (2004) Implications of invasive plant variation for weed management. Weed Technology 18:1319–1324
Strauss SY, Webb CO, Salamin N (2006) Exotic taxa less related to native species are more invasive. Proc Natl Acad Sci USA103:5841–5845
Suarez AV, Tsutsui ND (2008) The evolutionary consequences of biological invasions. Molecular Ecology 17:351–360
Tarin DA, Pepper E, Goolsby JA et al (2013) Microsatellites Uncover Multiple Introductions of Clonal Giant Reed (Arundo donax). Invasive Plant Science and Management 6:328–338
Templeton AR (2006) Population genetics and microevolutionary theory. John Wiley, Hoboken, NJ, USA
Trewick SA, Morgan-Richards M, Chapman HM (2004) Chloroplast DNA diversity of Hieracium pilosella (ASTERACEAE) introduced to New Zealand: reticulation, hybridization, and invasion. American Journal of Botany 91:73–85
Vellend M, Harmon LJ, Lockwood JL et al (2007) Effects of exotic species on evolutionary diversification. Trends in Ecology and Evolution 22:481–488
Vilà M, Espinar JL, Hejda M et al (2011) Ecological impacts of invasive alien plants: a meta-analysis of their effects on species, communities and ecosystems. Ecol Lett 14:702–708
Ward DB (2010) North America has two species of Phragmites (Gramineae). Castanea 75:394–401
Ward SM, Gaskin JF, Wilson LM (2008) Ecological Genetics of Plant Invasion: What Do We Know? Invasive Plant Science and Management 1:98–109
Ward SM, Reid SD, Harrington J et al (2008) Genetic variation in invasive populations of yellow toadflax (Linaria vulgaris) in the western United States. Weed Sci 56:394–399
Williams CMJ, Biswas TK, Schrale G et al (2008) Use of saline land and wastewater for growing a potential biofuel crop (Arundo donax L.). In: Irrigation Australia 2008 Conference CD of Proceedings, Melbourne, Australia, 20–22 May. Available from: http://irrigation.org.au/publications-resources/2008-irrigation-australia-conference-papers
Zhao R, Cheng Z, Lu W et al (2006) Estimating genetic diversity and sampling strategy for a wild soybean (Glycine soja) population based on different molecular markers. Chinese Science Bulletin 51:1219–1227
Zhao J, Solis-Montero L, Lou A et al (2013) Population structure and genetic diversity of native and invasive populations of Solanum rostratum (Solanaceae). PLoS One 8:e79807
Zohary M (1962) Plant life of Palestiene: Israel and Jordan. Ronald Press, New York, NY, USA
Acknowledgments
The manuscript was written during a sabbatical leave of LEE in the University of Minnesota, Department of Plant and Microbial Biology, in the laboratory of Peter Tiffin, with support of the program PASPA- DGAPA, UNAM. We deeply acknowledge the detailed reviews of the manuscript by Irene Pisanty and by Erika Aguirre Planter. Ricardo Colin Nuñez was a doctoral student from Programa de Doctorado en Ciencias Biomédicas, Universidad Nacional Autónoma de México (UNAM) and received fellowship 215751 from CONACyT. We thank the Programa de Posgrado de Doctorado en Ciencias Biomédicas, Universidad Nacional Autónoma de México (UNAM) and the Laboratorio de Evolución Molecular y Experimental, Instituto de Ecología, UNAM.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Colin, R., Eguiarte, L.E. (2020). Genetic and Ecological Characterization of the Invasive Wetland Grasses Arundo donax and Phragmites australis in the Cuatro Ciénegas Basin. In: Mandujano, M., Pisanty, I., Eguiarte, L. (eds) Plant Diversity and Ecology in the Chihuahuan Desert. Cuatro Ciénegas Basin: An Endangered Hyperdiverse Oasis. Springer, Cham. https://doi.org/10.1007/978-3-030-44963-6_15
Download citation
DOI: https://doi.org/10.1007/978-3-030-44963-6_15
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-44962-9
Online ISBN: 978-3-030-44963-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)