Abstract
Introduced mammalian herbivores are known to be detrimental to native biodiversity and can alter ecosystem processes, by direct and indirect effects. Island systems, with inherently high rates of extinction are particularly susceptible to the impacts of such herbivores. The introduced spotted deer (Axis axis) is a potential threat to native forest floor and semi arboreal lizards in the Andaman Islands. We evaluated the nature and extent of this potential indirect effect on lizards from 2012 to 2014. We sampled for lizard abundance, arthropod abundance and understory vegetative cover on islands with varying intensity of spotted deer use. We inferred that, spotted deer depressed the abundance of forest floor and semi arboreal lizards approximately five fold, by reducing vegetative cover in the understory. The findings reveal a probable indirect effect of spotted deer on reptile abundance mediated by structural changes in the understory vegetation. The study provides evidence and the impetus for conservation of endemic reptiles in small tropical islands by mitigating the impacts of invasive spotted deer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduced mammalian herbivores (IMH) have a global presence owing to both incidental and intentional introductions. They have become subjects of concern and conservation action (Courchamp et al. 2003; Campbell and Donlan 2005) as they impact native ecosystems both directly and indirectly. Foraging and trampling by IMH impact: vegetative survival (Barrios-Garcia et al. 2012), vegetative structure and growth (Relva et al. 2010), plant community composition (Oduor et al. 2010), exotic plant invasion (Parker et al. 2006), presence or density of native vertebrates (North et al. 1994; Smit et al. 2001) and invertebrates (Wardle et al. 2001), soil dynamics (Stritar et al. 2010), and other alterations of ecosystem processes (Vázquez and Simberloff 2003).
The Andaman Islands, in the Bay of Bengal, are a group of tropical islands which have witnessed a spate of introductions of IMH in the last century, including spotted deer. At present, spotted deer (Axis axis) are present throughout the Islands, with the exception of Little Andaman Island and South Sentinel Island (Ali 2004). Negative impacts of the deer on seedling and sapling survival and forest structure have been documented (Ali 2004; Ali and Pelkey 2013). Other IMH include barking deer (Muntiacus muntjac) and hog deer (A. porcinus), but these have restricted distributions. Apart from IMHs, the Andaman wild pig (Sus scrofa andamanensis), an omnivorous endemic race (Srinivasulu and Srinivasulu 2012), is also found in the Islands.
These islands have 53 species of terrestrial herpetofauna (20 lizards, 22 snakes, 11 amphibians) of which 40 % are endemic (Harikrishnan et al. 2010). In this study, we investigated possible indirect pathways of interactions between herbivores and reptiles (Janzen 1976), and hypothesised that herbivory by spotted deer in the Andaman Islands would reduce vegetative cover and/or depress folivorous arthropod abundance. This in turn might lead to a decline of insectivorous lizards, or render the habitat unsuitable for lizards and increase risk of predation.
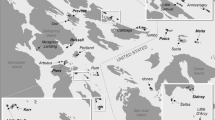
The Andaman Islands are situated between lat 10°30′N and 13°40′N, and long 92°10′E and 93°10′E. This island group is divided into two major parts: Great Andamans and Little Andaman Island separated by a distance of ca. 50 km. The South-West monsoon commencing in May and the North-East monsoon commencing in November, account for the majority of the annual rainfall which ranges from 3000 to 3500 mm. We carried out the study in the evergreen forests of ten uninhabited islands and the uninhabited parts of North Andaman, South Andaman, Rutland and Little Andaman Island (Fig. 1), all of which fall under national parks, reserved forests or tribal reserves. The islands varied in size from 1.45 to 1463.53 km2, and Little Andaman was the only island in the sample that did not have spotted deer (Table 1). These islands were in close proximity (Fig. 1), and experienced similar rainfall patterns. Further, only evergreen forest below the altitude of 100 m was sampled. We conducted the sampling during the dry season (December to April), from 2012 to 2014.
Study area map showing the 14 islands sampled in the Andaman archipelago from 2012 to 2014. The numbers on the figure correspond to the serial number given in Table 1
We considered an island as the experimental unit and sampled for lizard abundance, intensity of deer use, arthropod abundance and understory vegetative cover. We sampled for forest floor and semi arboreal lizards following a modified version of Rodda et al. (2001), a method of total count, in 39 bounded plots of 100 m2 each. These bounded plots could not be placed randomly as the method requires relatively flat terrain, but were representative of the evergreen forest of the islands and were separated from each other by a minimum distance of 100 m. All plots were sampled only once. The number of plots per island was dependent on the island size (Table 1). We demarcated the 10 m × 10 m plots by digging a moat along the perimeter, in which we buried the bottom edge of a 0.5 m high plastic sheet. We did not grease the top of the sheet to deter lizards from escaping, as our study area had only two species with expanded sub-digital lamellae, which were arboreal species, and were recorded rarely within plots. We positioned stakes along the boundary and left the plot undisturbed for about 24 h. The following day, we approached the plot from four sides and quickly raised the plastic sheet, securing the top edge to the stakes so that animals did not escape. We applied a broad strip of smooth duct tape (ca. 70 mm) around the trunk of trees at a height of 2 m from the ground, which effectively prevented all lizards other than two species with expanded sub-digital lamellae from arboreal escape. We captured semi-arboreal agamid lizards in the plots using a fishing line noose at the beginning of the search. We scanned tree trunks up to a height of 2 m, searched and removed leaf litter from the plot. Search inside the plot continued for 0.5 h after the capture of the last individual. Harikrishnan and Vasudevan (2015) provide a detailed description of the sampling method and its efficiency in documenting herpetofaunal community in the Islands.
As we could not obtain density estimates of spotted deer using line transects, due to island size and limited detection, we measured intensity of use. Each island was gridded into 1 km × 1 km grids. We walked four 200 m long trails in each grid, each of which was further divided into four segments of 50 m. We recorded presence (1) and absence (0) of indirect signs of deer use such as hoof marks, pellets, fraying and browse marks for each segment of each trail across 28 grids. A total of 110 trails were walked. The observed scores in each trail, ranging from 0/4 to 4/4, were averaged for the island to come up with a measure of intensity of spotted deer use (Table 1). We also identified plants that were browsed by deer. Other ungulates in the study area included the introduced barking deer on two islands (Bennet and Anderson) and the native Andaman wild pig on two islands (Rutland and Little Andaman). The fecal remains and hoof marks of these ungulates were easily differentiated from that of spotted deer.
We also measured the hypothesised mediator variables viz. folivorous arthropod abundance, litter arthropod abundance and vegetative cover below the maximum deer browse height of 1.5 m. These variables were sampled at the 0th and 200th m of the trails used to sample for intensity of deer use, i.e. a total of eight points per grid. We sampled for vegetative cover by holding a white sheet at 1.5 m and positioning a densitometer (Forestry Suppliers, USA) close to the ground. Four equally spaced points in each grid of the densitometer were marked prior to sampling. The number of points covered by vegetation was recorded. We sampled for folivorous arthropods by the bagging method (Katti and Price 1996), in which, a plastic bag was used to collect branches below the height of 1.5 m at the sampling locations. Cotton balls soaked in chloroform were put inside the bags to anesthetize any arthropods captured. The arthropods were sorted and counted as per their taxonomic order. We measured litter arthropod abundance by collecting and searching leaf litter inside a 0.5 m × 0.5 m quadrat. Weight of collected leaf litter was measured using a Pesola™ spring balance of 1 g accuracy. We calculated the arthropod abundances per 100 g of leaf/litter weight. There was no ethical violation of human and animal rights during this work. In North Andaman and South Andaman, data on intensity of use by spotted deer, vegetation cover and arthropod abundance were not collected.
As the variables sampled in grids (all except lizard density) within an island were auto correlated, they were averaged to the scale of islands. Lizard densities in bound plots of each island were also averaged. All variables were standardized using Z transformations. Generalized Linear Models (GLMs), with Gaussian errors, were constructed to explain the variance in overall and individual reptile species densities. AICc values were used to compare the candidate models (Burnham and Anderson 2002). We calculated the relative importance of predictor variables (parameter weight) by summing AICc weights across all the models which included the variable (Burnham and Anderson 2002). Further, regressions were carried out to test the influence of island area and the effect of intensity of spotted deer use on the mediator variables: total arthropod abundance and vegetative cover. The significance level (α) of linear regressions was set at 0.05. Adjusted R2 values have been reported along with the regression estimates. All analyses were done using the statistical software R (R Core Team 2013).
Density of lizards did not have a significant relationship with area in the 14 islands sampled (R2 = 0.148, β = 0.462, SE = 0.256, p = 0.095). Mean lizard density on islands with and the island without spotted deer was 6.76 (SE = 1.12) and 36.3 per 100 m2 respectively (Table 1). Mean intensity of spotted deer use, measured in 11 islands with spotted deer presence, was 0.83 (SE = 0.08) where, eight islands had values >0.9 on a scale of 0–1 (Table 1). Intensity of spotted deer use was associated with reduced lizard density (R2 = 0.393, β = −0.669, SE = 0.234, p = 0.017). Vegetative cover best explained the variance in overall reptile density as well as in densities of Coryphophylax subcristatus and Lygosoma bowringii (Table 2). Best models for Cyrtodactylus rubidus included a null model (Table 2). As expected, vegetative cover decreased with increase in intensity of deer use (R2 = 0.667, β = −0.835, SE = 0.173, p < 0.001) in the islands. We recorded 31 plants species which were foraged by the spotted deer (Online Resource 1).
Eight species of lizards were recorded in 39 bounded plots, namely, C. subcristatus, Coryphophylax brevicaudus, L. bowringii, C. rubidus, Eutropis andamanensis, Sphenomorphus maculatus, Hemidactylus sp. and Cnemaspis andersoni. Among species that occurred more than ten times in our samples (Fig. 2), C. subcristatus (R2 = 0.481, β = 0.727, SE = 0.217, p = 0.007) and L. bowringii (R2 = 0.446, β = 0.704, SE = 0.224, p = 0.01) were positively related to vegetative cover.
Bar plot with error bars showing the density/bound plot of eight individual reptile species across islands with and without spotted deer. Among species that occurred more than ten times in our samples, Coryphophylax subcristatus, Cyrtodactylus rubidus and Lygosoma bowringii had greater densities on the island without spotted deer than on islands with spotted deer
Studies documenting indirect effects of IMH on island reptiles (North et al. 1994) are rare, but the possibility of such an effect on reptiles in general has been explored (McCauley et al. 2006; Knox et al. 2012). Low densities of lizards associated with islands with spotted deer and an overall negative relationship warrants an explanation. We infer a significant influence of understory vegetative cover on lizards, in contrast to the indistinct influence of arthropod abundance. Competing explanations for the role of vegetative cover in the relationship between lizards and spotted deer are: (1) inter-island variation in understory vegetative cover is associated with high deer use and low reptile densities; (2) deer use reduces vegetative cover, and that in turn, diminishes lizard density.
Vegetation in the Islands is dominated by old growth evergreen and littoral evergreen forest, owing to high rainfall and tropical location (Ali and Pelkey 2013). Open grasslands are absent in small islands and therefore, the spotted deer primarily use the evergreen forest. Spotted deer on the Indian sub-continent show a preference towards relatively sparse understory (Bhat and Rawat 1995). This association could explain the negative relationship between intensity of deer use and vegetation cover, thereby, suggesting a possible bottom up effect on lizard densities. Our observations reveal a stark difference in the understory vegetation cover in islands, with and without spotted deer (Table 1). This is further substantiated with the findings of previous studies documenting a negative impact of the deer on seedling and sapling survival (Ali 2004) and forest structure (Ali and Pelkey 2013). Additionally, we found many evergreen plants foraged by the deer (Online Resource 1), which is otherwise considered as a grazer in its natural range. We found evidence for top down factors regulating lizard densities, but we do not infer a cause–effect relationship at this stage, due to small sample size and constraints of the study design.
Changes in vegetative cover may lead to an alteration of microclimatic factors (Côté et al. 2004) through an increase in penetration of sunlight and changes in soil temperature and texture, which could affect the survival of the sub-terranean eggs laid by agamids (e.g. Coryphophylax spp.) and skinks (e.g. L. bowringii). Reduction in sapling density in the presence of deer could lead to limited perches and roosting sites for the most abundant lizard on the islands, C. subcristatus (44 % of all lizards), which roosts on saplings and understory leaves. Further, a reduction in vegetative cover increases the vulnerability to predation for all semi arboreal and forest floor lizards. Impact of IMH on lizards documented by us is consistent with findings by North et al. (1994).
Additional abiotic and biotic factors that could explain variation in lizard density on the islands were: area, altitude, latitude, rainfall, vegetation type, and natural species composition. As the sampled islands were relatively close to each other, they were similar in terms of the above mentioned factors, except area. The relationship of area with individual species density has been hypothesised to be positive, negative as well as neutral (Connor et al. 2000). Though, in the case of birds and insects the relationship is positive (Connor et al. 2000), a ubiquitous negative relationship is observed in the case of lizards (Buckley and Jetz 2007). In fourteen islands, we did not find a significant effect of area on density of lizards.
Invasive species are known to facilitate the establishment of other exotic species (Parker et al. 2006), a phenomenon referred to as ‘invasional meltdown’ (Simberloff and Von Holle 1999). By opening up of habitat or by selective browsing of understory vegetation, spotted deer could help in the spread and establishment of exotic plants, which could prove detrimental to the survival of natives. Significant alterations in abundance and composition of species could have an effect on ecosystem processes (Simberloff 2011). Considering the plethora of documented direct and indirect effects IMH have on native systems, we could just be beginning to fully unravel the impact of the spotted deer on the Islands’ ecosystem.
Despite several expeditions to document herpetofaunal diversity of the Islands, new species continue to be discovered (Harikrishnan et al. 2012) with several species yet to be described. Owing to the threat posed by the invasive spotted deer to the endemic lizards and their habitat, it is important the deer population in the Islands are actively managed. Management actions against IMH on islands elsewhere have largely been successful (Courchamp et al. 2003; Campbell and Donlan 2005). With the use of modern techniques (Oppel et al. 2010), it is possible to control or eradicate them from small islands. The conservation dividend of freeing any island of IMH is high, aiding possible recovery of several endemic reptiles.
Using a robust measure of lizard density, we conclude that the reduction in density was strongly associated with relative abundance of spotted deer on the Islands. The findings lead us to endorse the examination of potential indirect effects of any invasive species while assessing its impact on native biodiversity. A detailed investigation into the impact of spotted deer on other native taxa through exclusion experiments is necessary. The evidence presented here suggests increase in lizard densities, and a probable reversal in local extinction of some endemic lizards, if spotted deer is removed from the Andaman Islands.
References
Ali R (2004) The effect of introduced herbivores on vegetation in the Andaman Islands. Curr Sci 86:1103–1112
Ali R, Pelkey N (2013) Satellite images indicate vegetation degradation due to invasive herbivores in the Andaman Islands. Curr Sci 105:209–214
Barrios-Garcia MN, Relva MA, Kitzberger T (2012) Patterns of use and damage by exotic deer on native plant communities in northwestern Patagonia. Eur J Wildl Res 58:137–146
Bhat SD, Rawat GS (1995) Habitat use by chital (Axis axis) in Dhaulkhand, Rajaji National Park, India. Trop Ecol 36:177–189
Buckley LB, Jetz W (2007) Insularity and the determinants of lizard population density. Ecol Lett 10:481–489
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach, 2nd edn. Springer, New York
Campbell K, Donlan C (2005) Feral goat eradications on islands. Conserv Biol 19:1362–1374
Connor EF, Courtney AC, Yoder JM (2000) Individuals-area relationships: the relationship between animal population density and area. Ecology 81:734–748
Côté SD, Rooney TP, Jean-Pierre T, Dussault C, Waller DM (2004) Ecological impacts of deer overabundance. Annu Rev Ecol Syst 35:113–147
Courchamp F, Jean-Louis C, Pascal M (2003) Mammal invaders on islands: impact, control and control impact. Biol Rev 78:347–383
Harikrishnan S, Vasudevan K (2015) The devil is in the detail: estimating species richness, density, and relative abundance of tropical island herpetofauna. BMC Ecol 15:18
Harikrishnan S, Vasudevan K, Choudhury BC (2010) A review of herpetofaunal descriptions and studies from Andaman and Nicobar islands, with an updated checklist. In: Ramakrishna, Raghunathan C, Sivaperuman C (eds) Recent trends in biodiversity of Andaman and Nicobar Islands. Zoological Survey of India, Kolkata, pp 387–398
Harikrishnan S, Vasudevan K, Chandramouli SR, Choudhury BC, Dutta SK, Das I (2012) A new species of Coryphophylax Fitzinger in: Steindachner, 1867 (Sauria: Iguania: Agamidae) from the Andaman Islands, India. Zootaxa 3451:31–45
Janzen DH (1976) The depression of reptile biomass by large herbivores. Am Nat 110:371–400
Katti M, Price T (1996) Effects of climate on Palaearctic warblers over-wintering in India. J Bombay Nat Hist Soc 93:411–427
Knox CD, Cree A, Seddon PJ (2012) Direct and indirect effects of grazing by introduced mammals on a native, arboreal gecko (Naultinus gemmeus). J Herpetol 46:145–152
McCauley DJ, Keesing F, Young TP, Allan BF, Pringle RM (2006) Indirect effects of large herbivores on snakes in an African savanna. Ecology 87:2657–2663
North SG, Bullock DJ, Dulloo ME (1994) Changes in the vegetation and reptile populations on Round Island, Mauritius, following eradication of rabbits. Biol Conserv 67:21–28
Oduor AMO, Gómez JM, Strauss SY (2010) Exotic vertebrate and invertebrate herbivores differ in their impacts on native and exotic plants: a meta-analysis. Biol Invasions 12:407–419
Oppel S, Beaven BM, Bolton M, Vickery J, Bodey TW (2010) Eradication of invasive mammals on islands inhabited by humans and domestic animals. Conserv Biol 25:232–240
Parker JD, Burkepile DE, Hay ME (2006) Opposing effects of native and exotic herbivores on plant invasions. Science 311:1459–1461
R Core Team (2013) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/
Relva MA, Nuñez MA, Simberloff D (2010) Introduced deer reduce native plant cover and facilitate invasion of non-native tree species: evidence for invasional meltdown. Biol Invasions 12:303–311
Rodda GH, Campbell EW III, Fritts TH (2001) A high validity census technique for herpetofaunal assemblages. Herpetol Rev 32:24–30
Simberloff D (2011) How common are invasion-induced ecosystem impacts? Biol Invasions 13:1255–1268
Simberloff D, Von Holle B (1999) Positive interactions of non indigenous species: invasional meltdown? Biol Invasions 1:21–32
Smit R, Bokdam J, den Ouden J, Olff H, Schot-Opschoor H, Schrijvers M (2001) Effect of introduction and exclusion of large herbivores on small rodent communities. Plant Ecol 155:119–127
Srinivasulu C, Srinivasulu B (2012) South Asian mammals: their diversity, distribution, and status. Springer, New York
Stritar ML, Schweitzer JA, Hart SC, Bailey JK (2010) Introduced ungulate herbivore alters soil processes after fire. Biol Invasions 12:313–324
Vázquez DP, Simberloff D (2003) Changes in interaction biodiversity induced by an introduced ungulate. Ecol Lett 6:1077–1083
Wardle DA, Barker GM, Yeates GW, Bonner KI, Ghani A (2001) Introduced browsing mammals in New Zealand natural forests: aboveground and belowground consequences. Ecol Monogr 71:587–614
Acknowledgments
N.P.M. was funded by Rufford Small Grants (#14448-1) and Mohammed Bin Zayed Species Conservation Fund (#12053708). K.V. and H.S. were supported by SERB, Department of Science and Technology grant SR/S0/AS-08/2009. We would like to thank the Department of Environment and Forest for permits. Wildlife Institute of India and Andaman and Nicobar Islands Environmental Team are thanked for logistical support. We thank Dr. C. Murugan, Botanical Survey of India, for helping with plant identification. We acknowledge the immense hard work put in by our assistants in the field. We thank three anonymous reviewers for their comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mohanty, N.P., Harikrishnan, S., Sivakumar, K. et al. Impact of invasive spotted deer (Axis axis) on tropical island lizard communities in the Andaman archipelago. Biol Invasions 18, 9–15 (2016). https://doi.org/10.1007/s10530-015-1006-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-015-1006-0