Abstract
The diets of sympatric rodents partially define their realized niches. Identifying items in stomachs of introduced rodents helps determine rodents’ trophic positions and species most at risk of consumption. In the Hawaiian Islands, which lacked rodents prior to human arrival, three rodents (Rattus rattus or black rat, R. exulans or Pacific rat, Mus musculus or house mouse) commonly coexist in native habitats where they consume a wide range of plants and animals. These three rodent species were trapped in montane forest for 2.5 years; their stomach contents were analyzed to determine short-term diets (n = 12–95 indiv. per species), and isotopic fractions of δ15N and δ13C in their bone collagen were analyzed to further estimate their trophic positions (n = 11–20 indiv. per species). For all three species, >75 % of individuals had plants and >90 % had arthropods in their stomachs, and significant differences in mean relative abundances were found for food items in stomachs among all three rodents. Rodents may be dispersing some native and non-native seeds, including the highly invasive Clidemia hirta. Most identifiable arthropods in rodent stomachs were non-native, and no stomachs contained birds, snails, or lizards. The δ15N and δ13C signatures were consistent with trophic feeding differences revealed from stomach contents. Dietary niche differentiation by coexisting rodent species is evident in this forest, with Pacific rats being intermediate between the mostly carnivorous house mouse and the mostly herbivorous black rat; such findings can help forecast rodent impacts and direct management efforts in ecosystems where these invasive animals coexist.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
How closely-related species coexist in a community has long intrigued ecologists. Resource use and resource competition are two of the many ecological factors that influence a species’ niche (Elton 1927; Hutchinson 1957). Theory predicts that animals with similar life-history traits and close phylogenetic associations, such as different species of rodents in the same habitat, are able to coexist because they partition resources across time and space (Gause 1934). However, several factors complicate the ability to determine if resource partitioning is actively practiced by coexisting species, including past and present competition, arrival order, relative abundances, and resistance and resiliency to disturbance. Despite the difficulty in determining the mechanisms of differential resource use, dietary comparisons of similar, coexisting species can help define species niches (Kotler and Brown 1988; Biró et al. 2005).
Introduced animals can disrupt food webs by consuming native species and by altering the realized niches of native competitors (Fritts and Rodda 1998; Fukami et al. 2006). Identifying species consumed by introduced animals provides insight into native species’ vulnerability and can inform strategies for managing native and non-native species (Stapp 2002; Caut et al. 2008a; Bonnaud et al. 2011; St Clair 2011). Rodents (Rattus rattus, black or ship rat; R. norvegicus, Norway rat; R. exulans, Pacific rat; Mus musculus, house mouse) have been introduced to many ecosystems and are among the most widespread and problematic invasive animals affecting islands (Towns et al. 2006; Angel et al. 2009; Drake and Hunt 2009). Introduced rodents may consume a wide variety of food items, including plants (e.g., fruits, seeds, vegetative material) and animals (e.g., arthropods, mollusks, birds; Sugihara 1997; Stapp 2002; Drake et al. 2011), and their diets can shift depending upon a number of factors, including food availability, the chemical and nutritional quality of food items, and the rodents’ competitive ability relative to other animals that coexist in the environment (Clark 1981, 1982; Caut et al. 2008a; Ruffino et al. 2011).
Food consumption by introduced rodents is rarely observed directly, perhaps because they are shy, nocturnal, and often burrow belowground (Lindsey et al. 1999; Shiels 2010). Techniques commonly used to assess the diets of introduced rodents include field observations of partially consumed prey (e.g., seeds, mollusks, arthropods; Norman 1970; McConkey et al. 2003; Meyer and Shiels 2009), captive-feeding trials (Bunn and Craig 1989; Williams et al. 2000; Pérez et al. 2008; Shiels 2011), and stomach content analysis (Clark 1981, 1982; Amarasekare 1994; Pisanu et al. 2011). Additionally, the analysis of naturally-occurring stable isotope ratios (i.e., δ15N and δ13C) of the rodents’ tissues have been widely used to determine the diets and trophic levels at which animals have fed during tissue development; however, interpretations of diet using stable isotopes are complicated by variation in tissue turnover rates among organs (Peterson and Fry 1987; Lajtha and Michener 1994). For example, liver tissue has a higher turnover rate than blood cells or muscle, whereas bone collagen is deposited and modified throughout life, so its isotopic values represent a long-term average of an animal’s diet (Lajtha and Michener 1994). The difference in isotopic composition between a consumer and its food (discrimination values) is presumed to average ca. 3 ‰ for δ15N and 1 ‰ for δ13C (Peterson and Fry 1987; Post 2002; Caut et al. 2009); however, discrimination values can differ widely among food sources (Caut et al. 2009). For example, Post (2002) found that the majority of the δ15N discrimination values in lake organisms were 2–4.5 ‰, and Caut et al. (2008b) determined from lab trials that the δ15N discrimination values for black rats ranged from −1.46 to 4.59 ‰. A disadvantage of stable isotope analysis is that it is often imprecise for identifying specific taxa that have been consumed (see Phillips 2012 for a review). Diet assessments that combine multiple techniques, such as stable isotope analysis with stomach content analysis, generally provide a more complete understanding of an animal’s dietary niche than do assessments using only one technique (Drake et al. 2011).
The objectives of this study are to (1) determine the short-term diets, using stomach content analysis, of black rats, Pacific rats, and mice where they coexist in Hawaiian montane forest, and (2) investigate the trophic positions of the three rodent species via the analysis of stable isotopes occurring in low-turnover tissue (i.e., bone collagen) that has integrated the resources used by rodents over several months. These three rodents are widespread and coexist in many ecosystems in Hawaii from sea level to nearly 3,000 m a.s.l. (Amarasekare 1994; Shiels 2010). The first rodents (Pacific rats) arrived with the first humans ca. 800 years ago (Wilmshurst et al. 2011), and the others arrived with Europeans ca. 230 years ago. These rodents consume both plants and animals in Hawaii (Sugihara 1997; Cole et al. 2000; Shiels and Drake 2011; Pender et al. in press). However, the types of species most at risk of consumption by each of these three rodent species where they occur sympatrically have not been well established; such information can assist in native ecosystem management in areas where these rodents have invaded.
Methods
Study site
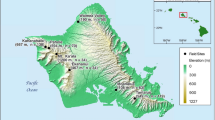
Rodents were obtained from Kahanahaiki Management Unit (21o 32′ N, 158o 11′ W), a 36 ha segment of mesic forest in highly dissected terrain (500-660 m a.s.l.) in the northern Waianae Mountains, on Oahu, Hawaii. Kahanahaiki is managed for native species conservation by the US Army, and the forest was fenced in 1996 to exclude feral goats and pigs. Annual precipitation at the site is approximately 1265 mm (Giambelluca et al. 2011), and the daily air temperature is 16–24 °C (Shiels 2010). The forest is a mixture of native and non-native vegetation. There are >30 tree species common to the forest, and the five dominant tree species include three natives (Diospyros hillebrandii, Psydrax odorata, and Sapindus oahuensis) and two non-natives (Psidium cattleianum and Schinus terebinthifolius; Shiels 2010). Fruit production occurs year-round, with the greatest fruit-fall observed in November-March (fruit numbers) and June–September (biomass) (Shiels 2010).
The black rat, Pacific rat, and house mouse occur at Kahanahaiki; Norway rats are absent from this forest and most others in Hawaii (Shiels 2010). Mean relative abundance (No. indiv. 100 trap nights−1) estimated from bi-monthly mark-and-recapture sampling over 26 months during 2007–2009 were (mean ± SE) 13.5 ± 2.7 for black rats, 0.7 ± 0.4 for Pacific rats, and 7.9 ± 3.3 for house mice (Shiels 2010). Other vertebrate consumers in the forest include native and non-native birds, and non-native reptiles (e.g., Lampropholis delicata), mongooses (Herpestes auropunctatus), and house cats (Felis catus) (Shiels 2010). Invertebrate consumers include native and non-native arthropods and snails, and non-native slugs (Joe and Daehler 2008; Meyer and Shiels 2009).
Stomach content analysis
Black rats (n = 95), Pacific rats (n = 12), and mice (n = 47) were collected from kill-traps (Victor® rat traps) placed on the ground from February 2007 through September 2009. In February 2007, traps were established at 10–25 m intervals along a single 300 m transect and within two 50 × 50 m plots at the ends of the transect (where native tree snails (Achatinella mustellina) were relatively abundant). Each month, 15–32 traps were baited with coconut chunks or peanut butter, set for 2–5 consecutive days, and checked daily. From May to September 2009, approximately 400 kill-traps were added to the 36 ha site and arranged along multiple transects that circled the core interior. Each transect was ca. 50 m from the next closest transect, and trap spacing was 12.5 m along the perimeter and 25 m on all interior transects (Pender et al. in press). We used the same bait as described above, and traps were checked each 1–7 days. Only rodents that were freshly (<24 h) killed, evidenced by lack of obvious decay, were used in this study. These rodent body masses were (mean ± SE) 124 ± 5 g for black rats, 52 ± 4 g for Pacific rats, and 12 ± 1 g for mice; the sex ratio of each species was roughly 50:50. Carcasses were stored in a freezer until analyzed.
Stomach contents were extracted, swirled for 5 min in water and mild detergent (Joy® brand) to separate contents and dissolve gastric juices and oils, sieved through a 0.4 mm sieve, and preserved in 95 % ethanol (Sugihara 1997). A transparent grid (5 × 5 mm for rats; 3 × 3 mm for mice) was positioned beneath a Petri dish containing each sample and then the sample was inspected using a dissecting microscope with 10–20× magnification. Relative abundance (percent) of each food type was determined for each sample by scoring the number of grid-boxes containing a given food type and dividing by the total number of grid-boxes (i.e., 40 grid-boxes). If more than one food type was in a grid-box, the item nearest the center was recorded (Cole et al. 2000). For each rodent species the frequency of occurrence (percent) for each food type was calculated by the presence of each of the food types in a given sample (individual) divided by the total number of samples. There were three major food types: plants, arthropods, and other. Plant food types were further categorized as fruit, seed, and other plant material (including leaves, flowers, stems, wood). Arthropod food types included caterpillar (Lepidoptera larvae), ant (Hymenoptera), burrowing bug (Hemiptera), spider (Araneae), and other arthropod material. The ‘other’ category (major food type) included rodent hair and flesh, and unknown material that did not fit any of the previously listed food types. Food items were classified to the lowest taxonomic level possible using voucher specimens collected from the study site.
Stable isotope analysis
To augment the short-term diet assessment from stomach contents, the trophic positions of the three rodent species were estimated using stable isotope analysis. On a random subset of the trapped rodents (n = 20 black rats, 12 Pacific rats, and 11 mice), bone collagen was extracted and analyzed for δ15N and δ13C values using methods described in Lajtha and Michener (1994) where the femur (plus tibia and fibula for mice) of each individual was excised, cleaned of flesh, and soaked in 0.5 M HCl for 48 h at 4 °C; the remaining sample (now collagen) was rinsed with deionized water, dried at 60 °C for 5 days, and ground to a fine dust. Common food items (fruit, seed, arthropod) were collected from the study site on 15 December 2009 and analyzed for δ15N and δ13C; food items were chosen based on similar species (or life forms) identified in the rodent stomachs and those found to be attractive to rodents at this site during field trials (Shiels and Drake 2011). For plant items (n = 5), one sample from each of the following species was collected for analysis: Alyxia stellata (seed), Clidemia hirta (fruit + seed), Diospyros hillebrandii (seed), P. cattleianum (seed), and Planchonella sandwicensis (fruit). Three samples of each of three herbivorous or detritivorous arthropods were analyzed: caterpillar, isopod, and amphipod. Three predatory arthropods (spiders) were analyzed, including Steatoda capensis and two unknown species. Samples were dried at 60 °C, ground to homogenize either multiple individuals of the same species (e.g., plants, herbivorous/detritivorous arthropods) or single individuals (e.g., spiders), and, like the rodent bone collagen, analyzed isotopically using a Carla Erba elemental analyzer (model NC2500) with an attached mass spectrometer (Finnegan DeltaS with source upgrade). Stable isotope ratios were expressed in δ notation as parts per thousand (‰) deviation from international standards (Lajtha and Michener 1994).
Statistical analysis
The relative abundances of food types were compared among the three rodents by parametric and non-parametric ANOVAs. Fruit and seed met parametric assumptions of ANOVA after arcsine square-root transformations; the remaining comparisons used Kruskal–Wallis tests to assess significant differences among rodents for each food type. Post-hoc Tukey’s tests (for fruit and seed) or Mann–Whitney U tests were applied to assess significance between rodent species; significance was based on P < 0.05 (R Development Core Team 2010).
To test whether the diet of black rats changed during the time period when few (February 2007–April 2009), and the majority (May–September 2009), of Pacific rats and mice were trapped, ANOVAs, after square-root transformations, were used to compare the two time periods for each of three food types: fruit, seed, and arthropod.
Results
Stomach content analysis
All three rodent species consumed both plants and arthropods (Fig. 1). Plant relative abundance in stomachs differed significantly among rodents (P < 0.001; χ2 = 56.7, df = 2), with black rats > Pacific rats > mice (P < 0.015 for each post hoc comparison; Fig. 1). Arthropod mean relative abundance also differed significantly among rodents (P < 0.001; χ2 = 56.7, df = 2), with mice > Pacific rats > black rats (P < 0.035 for each post hoc comparison; Fig. 1). Rodent hair, which dominated the ‘other’ category in Fig. 1, was found in most stomachs of each species (69 % of black rat individuals, 67 % of Pacific rats, and 57 % of mice), and mean relative abundance for rodent hair was not significantly different among rodents (P = 0.775; χ2 = 0.5, df = 2; Table 1). Rodent hair in stomachs probably resulted from grooming; rodent flesh with hair attached was rare (i.e., in n = 1 black rat, and n = 1 Pacific rat stomach). No evidence of birds, reptiles, slugs, snails, or fungi occurred in any stomachs.
Mean relative abundance (%) of major food types found in stomachs of black rats, Pacific rats, and house mice in Hawaiian mesic forest. There were significant (P < 0.05) differences between each species for the two major food types (plant and arthropod). The ‘other’ category is dominated by rodent hair, which was most likely a result of grooming rather than cannibalism
All plant and arthropod food types analyzed were found in stomachs of at least some individuals of all three rodents (Table 1). There were significant differences among rodents for most food types found in stomachs, and burrowing bugs were the only prey whose relative abundance was not significantly different among rodents (Table 1). Fruit comprised the majority of the plant material for both rats, but seed was the most abundant plant material in mice (Table 1). Caterpillars comprised the majority of the identifiable arthropods found in each of the rodents, and were ca. 94 % of the arthropod diet of mice (Table 1).
For each rodent species, >75 % of individuals had plants in their stomachs (Table 2). All black rats and Pacific rats had fruit in their stomachs, and >90 % also had seed. The frequency of mouse stomachs with fruit (40 %) tended to be less than those containing seed (64 %). The majority of seed in all three rodents appeared chewed and was probably destroyed, but intact seeds of some native and non-native species were found in black rats, and all three rodents had intact seeds of the invasive non-native C. hirta. The frequency of other plant material (mostly stems and leaves) was highest in Pacific rats, intermediate in mice, and lowest in black rats.
Arthropods, which mostly appeared as fragments rather than intact animals, were found in nearly all (>90 %) of the rodents examined (Table 2). Only four species of arthropod were found intact in rodent stomachs, and these were Solenopsis papuana (Papuan thief ant, Hymenoptera), Xylosandrus compactus (black twig-borer, Coleoptera), Stelidota geminata (strawberry sap beetle, Coleoptera), and Phthiraptera (rat lice) (Table 2). The two arthropod species that were found in some individuals of all three rodents were S. papuana and Rhytidoporus indentatus (burrowing bug, Hemiptera). Most identifiable arthropods were non-native species; the only identifiable native arthropods found in stomachs were Banza sp. (bush cricket/katydid, Orthoptera), Blackburnia epicurus (ground beetle, Coleoptera), and Rhyncogonus sp. (weevil, Coleoptera) in black rats, and Mecaphesa sp. (crab spider, Araneae) in mice. Unknown species of caterpillars were found in all of Pacific rats, 83 % of mice, and 34 % of black rats (Table 2).
There was no evidence from stomach content analysis indicating a dietary shift in black rats between seasons when few (February 2007–April 2009), and the majority (May–September 2009), of Pacific rats and mice were trapped (P = 0.709 for fruit; P = 0.860 for seed; P = 0.549 for arthropod; Fig. 2). Clear evidence of seasonal patterns in consumed food items were generally absent in our study; fruit and/or seed of some common species at the site (e.g., C. hirta) were found each month in some rodent stomachs, whereas uncommon species (e.g., Cyrtandra dentata and Delissea waianaeensis) were limited to stomachs of single individuals that were recovered during one collection period.
Mean ± SE relative abundance (%) of fruit, seed, and arthropod identified in stomachs of black rats in Hawaiian mesic forest for the time periods when few (February 2007–April 2009), and the majority (May–September 2009), of Pacific rats and mice were trapped. There were no significant differences (P > 0.05) between time periods for any of the food items
Stable isotope analysis
Examining trophic positions via isotopic signatures reveals that black rats have lower δ15N values than Pacific rats and mice (Fig. 3), and therefore appear to generally be feeding at lower trophic levels than are Pacific rats and mice. The Pacific rat and mouse have similar δ15N signatures. Spiders, which prey on arthropods but not plants, are generally feeding at higher trophic levels than all three rodents. Although all three rodents consume plants and animals, the δ15N findings are consistent with the results from stomach contents, which depict black rats as mainly vegetarian and Pacific rats and mice as slightly more carnivorous.
The three rodents appear to form a distinct grouping from their potential prey when δ13C is examined (Fig. 3). Although there is a relatively high amount of variability among Pacific rat and mouse samples for δ13C, these two rodents are nearly equal in δ13C and tend to be slightly higher than the δ13C signature of the black rat (Fig. 3). The herbivorous/detritivorous arthropods are about 1.5 ‰ from plants, but surprisingly the spiders are also aligned with the herbivorous/detritivorous arthropods for δ13C (Fig. 3).
Discussion
Black rats, Pacific rats, and house mice each consume a variety of plants and animals where they coexist in this insular tropical forest. The dietary niches of these three rodents differ such that the house mouse is primarily carnivorous and feeds mainly on arthropods (especially caterpillars), the black rat is primarily vegetarian and feeds mainly on fruit and seed, and the Pacific rat has an intermediate diet that, over its lifetime, is more closely related to the house mouse than to the black rat. An understanding of the trophic level overlap among these introduced rodents, as evidenced by stomach contents and stable isotope analysis, should help identify the types of species that may be vulnerable to rodent consumption and deserving of conservation attention.
Plant and animal tissue fragments, and intact seeds, in the rodent stomachs provided evidence of the taxa that were consumed during the 2.5 year study. Invasive rodents are generally viewed as seed predators (Clark 1981; Towns et al. 2006; Angel et al. 2009), and most seeds consumed by rodents in our study appeared highly vulnerable to predation, having been chewed and fragmented to the point that species identification was impossible. However, some seeds in rodent stomachs were intact and identifiable, such as the highly invasive C. hirta, which was found in some individuals of all three rodents, and could potentially be dispersed by them. A greater range of seed sizes can pass intact through black rats than through the two smaller rodents (Williams et al. 2000). Intact seeds of eight plant species, including at least two endangered natives and two invasive non-natives, occurred in black rats (Table 2) and their small seeds (≤1.5 mm length) would likely be passed intact and germinate (Shiels 2011; Shiels and Drake 2011). Similarly, arthropods were more identifiable in black rats than in the other rodents, perhaps because many of the fragments were slightly larger. True bugs, spiders, ants, crickets and other orthopterans, beetles, and caterpillars are common prey of these three rodents elsewhere (Cole et al. 2000; Innes 2005; Ruscoe and Murphy 2005; St Clair 2011), and they occurred in rodents in our study. Approximately one-third of the identifiable arthropod taxa in black rats were native in our study, but only one native species (Mecaphesa sp.) occurred in the house mouse, and none were identified in Pacific rats. The frequency of native species among total arthropod prey items was low; however, this may be an artifact of the high percentage of prey items, such as caterpillars, whose species-level identity and therefore provenance could not be determined. Stomach content analyses cannot directly yield estimates of population-level impacts on prey species. Nevertheless, the fact that individuals of rare native arthropod species, such as B. epicurus, were found in rodent stomachs suggests that rodents may represent important threats to the long-term viability of such species.
As expected, all three rodent species were highly omnivorous; however, the relative abundances of food items (e.g., fruit, seed, arthropod) and identifiable species consumed differed among rodents. Such niche differentiation is consistent with theory used to explain coexistence between closely related organisms (Gause 1934; Kotler and Brown 1988). Plant material often comprises 75–80 % of black rat diets within and outside of Hawaii, regardless of the types of coexisting rodent species (Kami 1966; Norman 1970; Clark 1981; Cole et al. 2000; Sweetapple and Nugent 2007; this study). In Hawaii, fruit can constitute the bulk of the black rats’ plant diet in mesic forest (55 % relative abundance of stomach contents; this study), arid shrubland (44 %; Cole et al. 2000), and wet forest (23–53 %; Sugihara 1997). When Pacific rats are the only rodents present at insular sites, plants can be 65–90 % of their diet (Wirtz 1972; Mosby et al. 1973; Bunn and Craig 1989), yet fruit consumption by Pacific rats in Hawaii appears more variable (41 % in our study vs. 3–16 % in Sugihara 1997) than it does for black rats. Both rat species consume fruit of problematic invasive species in Hawaii, such as C. hirta and Rubus rosifolius (Beard and Pitt 2006; this study) and P. cattleianum (this study). Interestingly, fruit of R. rosifolius and P. cattleianum were not found in any mice at Kahanahaiki despite being abundant during the time when most mice were trapped. The amount of fruit (11 %) in mouse stomachs was much less than in both rat species in our study, but similar to the 10 % determined by Cole et al. (2000). Fruit was absent from all 25 mouse stomachs analyzed from gulches adjacent to sugar cane fields on Hawaii Island (Kami 1966). Studies within and outside Hawaii suggest that house mice consume relatively small portions of fruit (especially fleshy fruit) compared to seed, vegetative material, and arthropods (Kami 1966; Cole et al. 2000; Angel et al. 2009; this study). It is unclear why fruits and seeds from other common species from Kahanahaiki were not observed in rodent stomachs; it may be a result of food preference or simply a reflection of the difficulty in identifying microscopic material in rodent stomachs.
Arthropods are common in diets of introduced rodents, being found in at least 80 % of stomachs examined from Hawaii and elsewhere (Gales 1982; Amarasekare 1994; Sugihara 1997; Miller and Webb 2001; this study). In a recent review, Angel et al. (2009) found that arthropods were the food of choice for house mice on islands in the Southern Ocean, a pattern consistent with that at Kahanahaiki, where arthropods accounted for an average of 57 % of their stomach contents. In high elevation (1600-3000 m) sites in Hawaii, arthropods comprised 33–54 % of mouse diets (Amarasekare 1994; Cole et al. 2000). Relative to mice, arthropods were a much smaller component of black rat stomach contents in our study (14 %) and that of Cole et al. (2000; 16 %). In Hawaiian lowland wet forest, there were no arthropods in black rat stomachs and only trace amounts of caterpillars in Pacific rat stomachs (Beard and Pitt 2006). Few data are available for arthropods in Pacific rat stomachs in Hawaii because these rats were not captured (Amarasekare 1994; Cole et al. 2000), or because arthropods were not segregated from other invertebrates when stomach contents were analyzed (Sugihara 1997). Despite the presence at Kahanahaiki of native and non-native birds and snails (Meyer and Shiels 2009; Shiels 2010), and non-native slugs, earthworms, and reptiles (Joe and Daehler 2008; Shiels 2010), there was no evidence of any of these organisms in rodent stomachs. The species composition and relative abundances of plants and animals available in rodent-occupied environments represent additional factors that can directly affect rodent diets (Kotler and Brown 1988; Ruffino et al. 2011); native birds, for example, are uncommon relative to non-native birds at our study site.
Caterpillars appear to be a highly attractive food item to all three rodents studied in Hawaii; some individuals of all species studied had caterpillars in their stomachs in high elevation environments (Amarasekare 1994; Sugihara 1997; Cole et al. 2000), and 100, 83 and 34 % of Pacific rats, mice, and black rats, respectively, had caterpillars in their stomachs in Kahanahaiki. The proportion of stomach contents comprised of caterpillars was greatest in mice (54 % in our study; 22 % in Cole et al. 2000) and least in black rats (3 % in our study; 4 % in Cole et al. 2000). On islands outside of Hawaii, caterpillars can also comprise the most common arthropod eaten by mice (Rowe-Rowe et al. 1989; Miller and Webb 2001; Ruscoe and Murphy 2005), and one of the most common groups eaten by Pacific rats (Bunn and Craig 1989; Atkinson and Towns 2005).
Although the three rodents in our study are omnivores, and thus appear to occupy the same general trophic level, they occupy different dietary niches. Stomach content analyses revealed that Pacific rats had an intermediate (short-term) diet between those of black rats and mice, yet the greater amount of caterpillars and unknown arthropods consumed by Pacific rats and mice relative to black rats may partially account for the slightly higher δ15N and δ13C for the two smaller rodents compared to black rats. Additionally, Pacific rats may be more similar to mice than to black rats in lifetime average diet (as indicated by δ15N and δ13C) as a result of isotopic incorporation rates of prey differing by rodent species (Gannes et al. 1997), or because Pacific rats shift their foraging microsites when the black rat is present (Lindsey et al. 1999; Atkinson and Towns 2005; Shiels 2010). The difference in δ15N among rodent species averaged <1.5 ‰, which does not typically justify assigning distinct trophic levels to different species (Peterson and Fry 1987; Lajtha and Michener 1994; Post 2002). Many isotope studies have examined the degree to which introduced rodents ate seabirds and the proportion of diet attributable to marine and terrestrial sources (Stapp 2002; Caut et al. 2008a; Quillfeldt et al. 2008; Ruffino et al. 2011). Marine inputs to rodent diets are unlikely at Kahanahaiki because the site is >3 km from the ocean, there are no seabirds, and home-ranges are typically <4 ha for each rodent (Shiels 2010). Therefore, δ13C differences in our study are more likely to involve unequal consumption of C3 and C4 plants (Gannes et al. 1997), which may help explain the slightly higher δ13C values for Pacific rats and mice relative to black rats. The majority of the C4 plants at Kahanahaiki are grasses (e.g., Paspalum conjugatum, Megathyrsus maximus), and many mice and some Pacific rats in our study were captured near grassy patches (A. Shiels, personal observation). In an inland forest on Stewart Island, New Zealand, Harper (2006) used δ15N and δ13C values to determine that diets of Pacific rats and black rats were similar. The only isotopic study available with wild house mice was by Quillfeldt et al. (2008) in the Falkland Islands where δ13C were similar to those in our study (−21 and −24 ‰); yet the δ15N for mice ranged from 12 to 31 ‰ and were indistinguishable from potential food items, including terrestrial plants (8–35 ‰), terrestrial invertebrates (14 ‰), and upland birds (16–19 ‰). Therefore, using isotopes to estimate trophic levels and determine the types of species which consumers feed upon may be challenging without supplemental dietary analysis such as stomach contents (Stapp 2002; Caut et al. 2008a; Quillfeldt et al. 2008; this study).
Many factors can affect the dietary niches of coexisting rodents. Much evidence points to the largest of the three rodents, the black rat, as the dominant competitor of the three species studied (Yom-tov et al. 1999; Russell and Clout 2004; Shiels 2010). Stokes et al. (2009) in Australia, and Harris and Macdonald (2007) in the Galápagos Islands, demonstrated that native rats (R. fuscipes and Nesoryzomys swarthi, respectively) suffered from interference competition rather than resource competition with the larger, non-native black rats. Furthermore, removal of black rats can result in population increases in coexisting rodents such as house mice (Harper and Cabrera 2010; Ruscoe et al. 2011). The average body masses of the three coexisting rodents in our study differed 4–10 fold. Size differences >2 fold allow sympatric congeners to occupy different niches but co-occur in the same trophic level (Hutchinson 1959; Eadie et al. 1987). Diet variation is perhaps just one of the niche differences among these three rodent species that enable their coexistence at Kahanahaiki and in many other ecosystems.
Differential resource uses, or dietary niches, of sympatric black rats, Pacific rats, and house mice at Kahanahaiki reflect unequal consumption of species that occupy different trophic levels. The ecological and conservation implications for island habitats containing these three introduced rodents are that (1) fruit appears to be a main component of the diet for black rats and Pacific rats, (2) mice and Pacific rats likely exhibit greater predation pressure per capita than black rats on arthropod communities, and (3) all three rodents typically chew, and probably destroy, most consumed seeds >2 mm in length. The degree to which species and trophic levels are exploited by each introduced rodent in Hawaii and elsewhere may largely depend upon the assortment of rodent species, and the available food items, that are present at a given site.
References
Amarasekare P (1994) Ecology of introduced small mammals on western Mauna Kea, Hawaii. J Mammal 75:24–38
Angel A, Wanless RM, Cooper J (2009) Review of impacts of the introduced house mouse on islands in the Southern Ocean: are mice equivalent to rats? Biol Invasions 11:1743–1754
Atkinson IAE, Towns DR (2005) Kiore. In: King CM (ed) The handbook of New Zealand mammals, 2nd edn. Oxford University Press, UK, pp 159–174
Beard KH, Pitt WC (2006) Potential predators of an invasive frog (Eleutherodactylus coqui) in Hawaiian forests. J Trop Ecol 22:345–347
Biró Z, Lanszki J, Szemethy L, Heltai M, Randi E (2005) Feeding habits of feral domestic cats (Felis catus), wild cats (Felis sylvestris) and their hybrids: trophic niche overlap among cat groups in Hungary. J Zool 266:187–196
Bonnaud E, Medina FM, Vidal E, Nogales M, Tershy B, Zavaleta E, Donlan CJ, Keitt B, Le Corre M, Horwath SV (2011) The diet of feral cats on islands: a review and call for more studies. Biol Invasions 13:581–603
Bunn TJ, Craig JL (1989) Population cycles of Rattus exulans: population changes, diet, and food availability. NZ J Zool 16:409–418
Caut S, Angulo E, Courchamp F (2008a) Dietary shifts of an invasive predator: rats, seabirds and turtles. J Appl Ecol 45:428–437
Caut S, Angulo E, Courchamp F (2008b) Discrimination factors (∆15N and ∆13C) in an omnivorous consumer: effect of diet isotopic ratio. Func Ecol 22:255–263
Caut S, Angulo E, Courchamp F (2009) Variation in discrimination factors (∆15N and ∆13C): the effect of diet isotopic values and applications for diet reconstruction. J Appl Ecol 46:443–453
Clark DA (1981) Foraging patterns of black rats across a desert-montane forest gradient in the Galapágos Islands. Biotropica 13:182–194
Clark DA (1982) Foraging behavior of a vertebrate omnivore (Rattus rattus): meal structure sampling, and diet breadth. Ecology 63:763–772
Cole FR, Loope LL, Medeiros AC, Howe CE, Anderson LJ (2000) Food habits of introduced rodents in high-elevation shrubland of Haleakala National Park, Maui, Hawaii. Pac Sci 54:313–329
Drake DR, Hunt TL (2009) Invasive rodents on islands: integrating historical and contemporary ecology. Biol Invasions 11:1483–1487
Drake DR, Bodey T, Russell J, Towns D, Nogales M, Ruffino L (2011) Direct impacts of seabird predators on island biota other than seabirds. In: Mulder CPH, Anderson WB, Towns DR, Bellingham PJ (eds) Seabird Islands: ecology, invasion and restoration. Oxford University Press, UK, pp 91–132
Eadie JM, Broekhoven L, Colgan P (1987) Size ratios and artifacts: Hutchinson’s rule revisited. Am Nat 129:1–17
Elton C (1927) Animal ecology. Sedgwick and Jackson, London
Fritts TH, Rodda GH (1998) The role of introduced species in the degradation of island ecosystems: a case history of Guam. Ann Rev Ecol Syst 29:113–140
Fukami T, Wardle DA, Bellingham PJ, Mulder CPH, Towns DR, Yeates GW, Bonner KI, Durrett MS, Grant-Hoffman MN, Williamson WM (2006) Above- and below-ground impacts of introduced predators in seabird-dominated island ecosystems. Ecol Lett 9:1299–1307
Gales RP (1982) Age- and sex-related differences in diet selection by Rattus rattus on Stewart Island, New Zealand. NZ J Zool 9:463–466
Gannes LZ, O’Brien DM, del Rio CM (1997) Stable isotopes in animal ecology: assumptions, caveats, and a call for more laboratory experiments. Ecology 78:1271–1276
Gause GF (1934) The struggle for existence. Hafner, New York
Giambelluca TW, Chen Q, Frazier AG, Price JP, Chen Y-L, Chu P-S, Eischeid J, Delparte D (2011) The Rainfall Atlas of Hawai‘i. http://rainfall.geography.hawaii.edu. Accessed May 9, 2012
Harper GA (2006) Habitat use by three rat species (Rattus spp.) on an island without other mammalian predators. NZ J Ecol 30:321–333
Harper GA, Cabrera LF (2010) Response of mice (Mus musculus) to the removal of black rats (Rattus rattus) in arid forest on Santa Cruz Island, Galápagos. Biol Invasions 12:1449–1452
Harris DB, Macdonald DW (2007) Interference competition between introduced black rats and endemic Galapagos rice rats. Ecology 88:2330–2344
Hutchinson GE (1957) Concluding remarks. Cold Spring Harbor Symposium Quant Biol 22:415–427
Hutchinson GE (1959) Homage to Santa Rosalia, or why are there so many kinds of animals? Am Nat 93:145–159
Innes J (2005) Ship rat. In: King CM (ed) The handbook of New Zealand mammals, 2nd edn. Oxford University Press, UK, pp 187–203
Joe SM, Daehler CC (2008) Invasive slugs as underappreciated obstacles to rare plant restoration: evidence from the Hawaiian Islands. Biol Invasions 10:245–255
Kami HT (1966) Foods of rodents in the Hamakua District, Hawaii. Pac Sci 20:367–373
Kotler BP, Brown JS (1988) Environmental heterogeneity and the coexistence of desert rodents. Ann Rev Ecol Syst 19:281–307
Lajtha K, Michener RH (1994) Stable isotopes in ecology and environmental science. Blackwell Scientific Publications, Oxford
Lindsey GD, Mosher SM, Fancy SG, Smucker TD (1999) Population structure and movements of introduced rats in an Hawaiian rainforest. Pac Conserv Biol 5:94–102
McConkey KR, Drake DR, Meehan HJ, Parsons N (2003) Husking stations provide evidence of seed predation by introduced rodents in Tongan rain forests. Biol Conserv 109:221–225
Meyer WM, Shiels AB (2009) Black rat (Rattus rattus) predation on non-indigenous snails in Hawai`i: complex management implications. Pac Sci 63:339–347
Miller AP, Webb PI (2001) Diet of house mice (Mus musculus L.) on coastal sand dunes, Otago, New Zealand. NZ J Ecol 28:49–55
Mosby J, Wodzicki K, Thompson HR (1973) Food of the kimoa (Rattus exulans) in the Tokelau islands and other habitats in the Pacific. NZ J Sci 16:799–810
Norman FI (1970) Food preferences of an insular population of Rattus rattus. J Zool London 162:493–503
Pender RJ, Shiels AB, Bialic-Murphy L, Mosher SM (in press) Large-scale rodent control reduces pre- and post-dispersal seed predation of the endangered Hawaiian lobeliad, Cyanea superba subsp. superba (Campanulaceae). Biol Invasions. doi:10.1007/s10530-012-0280-3
Pérez HE, Shiels AB, Zaleski HM, Drake DR (2008) Germination after simulated rat damage in seeds of two endemic Hawaiian palm species. J Trop Ecol 24:555–558
Peterson BJ, Fry B (1987) Stable isotopes in ecosystem studies. Ann Rev Ecol Syst 18:293–320
Phillips DL (2012) Converting isotope values to diet composition: the use of mixing models. J of Mammal 93:342–352
Pisanu B, Caut S, Gutjar S, Vernon P, Chapuis J-L (2011) Introduced black rats (Rattus rattus) on Ile de la Possession (Iles Crozet, Subantarctic): diet and trophic position in food webs. Polar Biol 34:169–180
Post DM (2002) Using stable isotopes to estimate trophic position: models, methods, and assumptions. Ecology 83:703–718
Quillfeldt P, Schenk I, McGill RAR, Strange IJ, Masello JF, Gladbach A, Roesch V, Furness RW (2008) Introduced mammals coexist with seabirds at New Island, Falkland Islands: abundance, habitat preferences, and stable isotope analysis of diet. Polar Biol 31:333–349
R Development Core Team (2010) R: a language and environment for statistical computing. The R Foundation for Statistical Computing, Vienna
Rowe-Rowe DT, Green B, Crafford JE (1989) Estimated impact of the feral house mouse on sub-Antarctic invertebrates at Marion Island. Polar Biol 9:457–460
Ruffino L, Russell JC, Pisanu B, Caut S, Vidal E (2011) Low individual-level dietary plasticity in an island-invasive generalist forager. Pop Ecol 53:535–548
Ruscoe WA, Murphy EC (2005) House mouse. In: King CA (ed) The handbook of New Zealand mammals. Oxford University Press, UK, pp 204–221
Ruscoe WA, Ramsey DSL, Pech RP, Sweetapple PJ, Yockney I, Barron MC, Perry M, Nugent G, Carran R, Warne R, Brausch C, Duncan RP (2011) Unexpected consequences of control: competitive vs. predator release in a four-species assemblage of invasive mammals. Ecol Lett 14:1035–1042
Russell JC, Clout MN (2004) Modeling the distribution and interaction of introduced rodents on New Zealand offshore islands. Global Ecol Biogeogr 13:497–507
Shiels AB (2010) Ecology and impacts of introduced rodents (Rattus spp. and Mus musculus) in the Hawaiian Islands. Dissertation, Department of Botany, University of Hawaii at Manoa
Shiels AB (2011) Frugivory by introduced black rats (Rattus rattus) promotes dispersal of invasive plant seeds. Biol Invasions 13:781–792
Shiels AB, Drake DR (2011) Are introduced rats (Rattus rattus) both seed predators and dispersers in Hawaii? Biol Invasions 13:883–894
St Clair JJH (2011) The impacts of invasive rodents on island invertebrates. Biol Conserv 144:68–81
Stapp P (2002) Stable isotopes reveal evidence of predation by ship rats on seabirds on the Shiant Islands, Scotland. J Appl Ecol 39:831–840
Stokes VL, Banks PB, Pech RP, Spratt DM (2009) Competition in an invaded rodent community reveals black rats as threats to native bush rats in littoral rainforest of south-eastern Australia. J Appl Ecol 46:1239–1247
Sugihara RT (1997) Abundance and diets of rats in two Hawaiian forests. Pac Sci 51:189–198
Sweetapple PJ, Nugent G (2007) Ship rat demography and diet following possum control in a mixed podocarp-hardwood forest. N Z J Ecol 31:186–201
Towns DR, Atkinson IAE, Daugherty CH (2006) Have the harmful effects of rats on islands been exaggerated? Biol Invasions 8:863–891
Williams PA, Karl BJ, Bannister P, Lee WG (2000) Small mammals as potential seed dispersers in New Zealand. Austral Ecol 25:523–532
Wilmshurst JM, Hunt TL, Lipo CP, Anderson AJ (2011) High precision radiocarbon dating shows recent and rapid initial human colonization of East Polynesia. Proc Natl Acad Sci 108:1815–1820
Wirtz WO (1972) Population ecology of the Polynesian rat, Rattus exulans, on Kure Atoll, Hawaii. Pac Sci 26:443–463
Yom-Tov Y, Yom-Tov S, Moller H (1999) Competition, coexistence, and adaptation amongst rodent invaders to Pacific and New Zealand islands. J Biogeogr 26:947–958
Acknowledgments
Primary funding was provided by the Oahu Army Natural Resources Program; additional funding to A.B.S. was from the Achievement Rewards for College Scientists (Maybell Roth Scholarship in Conservation Biology and Sarah Martin Award in Botany), the Charles Lamoureux Plant Conservation Fellowship, and the Watson Yoshimoto Wildlife Conservation Scholarship. Thanks to K. Kawelo, D. Peters, J. Rohrer, and L. Wilson for assistance capturing rodents, J. Liebherr for the B. epicurus identification, and C. Daehler, T. Hunt, and two anonymous reviewers for helpful comments on an earlier draft of this manuscript. This research was approved by the University of Hawaii Animal Use and Care Committee.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shiels, A.B., Flores, C.A., Khamsing, A. et al. Dietary niche differentiation among three species of invasive rodents (Rattus rattus, R. exulans, Mus musculus). Biol Invasions 15, 1037–1048 (2013). https://doi.org/10.1007/s10530-012-0348-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-012-0348-0