Abstract
Non-native species are recognized as important components of change to food web structure. Non-native prey may increase native predator populations by providing an additional food source and simultaneously decrease native prey populations by outcompeting them for a limited resource. This pattern of apparent competition may be important for plants and sessile marine invertebrate suspension feeders as they often compete for space and their immobile state make them readily accessible to predators. Reported studies on apparent competition have rarely been examined in biological invasions and no study has linked seasonal patterns of native and non-native prey abundance to increasing native predator populations. Here, we evaluate the effects of non-native colonial ascidians (Diplosoma listerianum and Didemnum vexillum) on population growth of a native predator (bloodstar, Henricia sanguinolenta) and native sponges through long-term surveys of abundance, prey choice and growth experiments. We show non-native species facilitate native predator population growth by providing a novel temporal resource that prevents loss of predator biomass when its native prey species are rare. We expect that by incorporating native and non-native prey seasonal abundance patterns, ecologists will gain a more comprehensive understanding of the mechanisms underlying the effects of non-native prey species on native predator and prey population dynamics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A positive correlation between non-native prey and native predator populations suggests that non-native prey provide an additional food source that may lead to elevated native consumer and to reduced native prey populations (Roemer et al. 2002; Inger et al. 2010). This pattern is referred to as apparent competition, where abundance or distribution of consumers is changed by a prey species hence altering the population dynamics of the other prey species (Holt and Kotler 1987). Direct competition between invasive and native species has received much attention (e.g., Hamilton et al. 1999; Grosholz 2002). Apparent competition between non-native prey and native consumer and prey populations has received less attention. Given that non-native species are a potent force of local, regional and global ecological change (Ruiz et al. 1997; Baiser et al. 2010), it is critical to evaluate their effect on population dynamics of native consumers and their prey.
We assessed the effect of non-native prey species on growth of a native predator in a benthic marine system using pre- and post- invasion field surveys and laboratory experiments. Food web studies in benthic marine systems are rare (see Rilov 2009 for review), but they are critical because they generate the strongest trophic cascades in nature (Shurin et al. 2002). This is particularly true for the Gulf of Maine, a region of relatively low species diversity and a high degree of seasonality in species composition (e.g., Dijkstra and Harris 2009). Because species richness is low, any additional prey to the Gulf of Maine may have a larger impact on predator abundance compared to systems with high species richness that have a larger number of prey species from which to choose.
We examined pre- and post- invasion population structure in native predator (bloodstar, Henricia sanguinolenta) by surveying two sites in the Gulf of Maine where there is a long-term record of species composition and timing of introduced species (e.g., Harris and Tyrrell 2001). In the Gulf of Maine, non-native colonial ascidians (tunicates, sea-squirts) are recent and common members of hard natural and artificial substrates (Dijkstra et al. 2007). In particular, Diplosoma listerianum and Didemnum vexillum are recognized as prey by the native bloodstar (Dijkstra et al. 2007), a predator of native sponges (Witman and Sebens 1990; Shield and Witman 1993). We assessed predator growth when fed a traditional native sponge diet (Haliclona oculata) and a diet of the two most common non-native species at the two sites (D. listerianum and D. vexillum). Finally, we examined annual abundance patterns of non-native colonial ascidians and native/established sponges using a panel study spanning about 2 years. The addition of abundant competitively superior prey may result in direct competition between native and non-native prey (e.g., Wethey and Walters 1986; Bak et al. 1996), yet may also result in apparent competition between the species. The growing interest in addition of non-native prey species to community food webs indicates a need to evaluate potential mechanisms of population growth of their native consumers.
Materials and methods
Study site and species
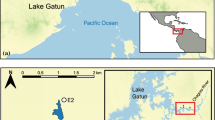
Pre- and post- invasion surveys documenting abundance and feeding behavior of H. sanguinolenta were conducted at the Isles of Shoals and Cape Neddick, ME. The Isles of Shoals is a cluster of islands located ~12 km off the coast of New Hampshire, USA and the Shoals Marine Laboratory is located on Appledore Island (42°59′19.35″N, 70°36′58.65″W; Fig. 1). Cape Neddick (43°09′57.31″N, 70°35′31.23″W) is a coastal site located in southern Maine (Fig. 1). Subtidal benthic and rocky intertidal communities at the Isles of Shoals and Cape Neddick are dominated by seaweeds and marine invertebrate filter feeders that compete for open substrate via overgrowth and chemical defense. While species compositions and densities of individual species are site specific, the composition of all hard bottom near shore benthic, fouling (communities on man-made structures) and intertidal communities studied to date in the Gulf of Maine are similar. Subtidal and intertidal rocky shores are dominated by seaweeds [e.g., Ascophyllum nodosum (rockweed) in the intertidal and Saccharina latissima (kelp) and the non-native Codium fragile spp. fragile (green fleece algae) in the subtidal]; fouling communities are dominated by sessile suspension feeding invertebrates. Within these habitats, non-native (e.g., D. listerianum and D. vexillum) and native (Didemnum albidum) encrusting colonial tunicates, and native sponges (H. oculata, Chalinula loosanoffi and Halichondria panicea) compete for open space.
Survey sites of H. sanguinolenta at the Isles of Shoals. 2005 to 2007 sites are marked with light gray circles. Dark circle is the survey site at Star Island between 1977 and 1978 (Hurlbert 1980)
Long-term abundance of the sponge H. panicea
To determine if sponge abundance is declining in rocky intertidal habitats similar to the observed decline in subtidal habitats (Dijkstra and Harris 2009, Harris, L.G. unpub. data), long-term sponge abundance (H. panicea) in the rocky intertidal was documented by students in the Shoals Marine Laboratory Field Marine Biology and Ecology course between 1982 and 2006 (data presented here were vetted by H. Weeks at the Shoals Marine Laboratory). Twenty-eight permanent transects were established by markers in 1982 at 4.1 m above mean low water. Transects ran along fixed bearings and students sampled in areas that represented the typical slope and exposure of each transect. Students identified and recorded the abundance of organisms. Percent cover of H. panicea was assessed using a 20 cm2 quadrat with a 16 square grid placed haphazardly along each transect. While abundance of H. panicea was documented at several of these rocky intertidal transects, only one had consistent long-term sponge data.
Survey of the bloodstar H. sanguinolenta and colonial ascidians
To document pre- and post-invasion densities of H. sanguinolenta, we compared its abundance at two sites [Star Island, NH (Isles of Shoals) and Cape Neddick, ME]. Surveys were conducted between June and August, 1977–1978 (reported in Hurlbert 1980) and between June and August, 2005 and 2007 at a site 8 m below mean low tide off the western shore of Star Island (Fig. 1). At Cape Neddick, surveys were taken at ~6 m below mean low tide in April 1980 and 2011. H. sanguinolenta densities at Cape Neddick and Star Island were documented by either a 0.25 m2 quadrat or by a 2.5 cm wand placed in the middle of each photograph. To establish that densities of bloodstars observed at Star Island reflected densities around the Isles of Shoals, we sampled 5 other sites between June and August, 2007 using a 0.25 m2 quadrat (n = 10/site; Fig. 1).
To examine dominance of non-native colonial ascidians during times of their peak abundance, we recorded abundances of all non-native colonial ascidians (D. listerianum, D. vexillum, Botrylloides violaceus and Botryllus schlosseri) at 3 sites between June and August, 2007 at the Isles of Shoals (Lunging Island, Gosport Harbor and Old pier) using a 0.25 m2 quadrat (n = 10/site).
Feeding behavior and growth studies of the bloodstar H. sanguinolenta
We surveyed active feeding (stomach everted) events and recorded choice of prey of 190 H. sanguinolenta at two locations [Cape Neddick, ME (n = 114) and the Isles of Shoals (n = 76)] between July and August 2007 and 2010, using SCUBA. At the Isles of Shoals, 4 sites were sampled which included Lunging Island, Gosport Harbor, Old Pier and Star Island (Fig. 1).
To compare growth of the bloodstar on a diet of native and non-native prey, we carried out a feeding trial using D. listerianum, D. vexillum as our non-native diet treatments, H. oculata as our native diet treatment, and a no food treatment. Ascidian treatments were selected to represent the two most common non-native colonial ascidians found at the Isles of Shoals and at Cape Neddick. Using SCUBA, we collected small sized (0.15–1.40 g) H. sanguinolenta from Cape Neddick, ME in May 2010 and individually placed them in mesh-covered (7.2 cm × 6.5 cm) treatment mesocosms. Mesocosms were placed in a flow through system at the University of New Hampshire’s Coastal Marine Laboratory. Initial star weights between treatments were not significantly different (F 3 = 0.540, P = 0.66). Treatments were checked weekly for food availability and food in containers was replaced as needed to ensure treatment individuals had an unlimited food supply. We measured the weight of the bloodstars underwater by placing the individuals in a pre-weighed container half-filled with seawater (Lambert et al. 2000). Bloodstars were weighed at 2 week intervals without blotting or other handling between May 20 and August 31. Any comparisons of growth include only those individuals that survived the entire observation period (D. vexillum n = 8; D. listerianum n = 9; H. oculata n = 7; No food, n = 8).
Temporal patterns of abundance of colonial ascidians and sponges
We assessed temporal patterns of percent cover of non-native and native prey using a panel study. Plexiglas panels (0.1 m2) were deployed in April 2008 and were photographed through December 2010. We determined percent cover of colonial ascidians and sponges using a 100 point grid placed over each photo.
Statistical analysis
All data were analyzed using JMP 8.0©. To homogenize the variances, abundance data were square-root transformed and percent cover data were square-root arcsine transformed prior to analysis. One-way ANOVAs were then used to test for differences in pre- and post-invasion densities of bloodstars at the Isles of Shoals and Cape Neddick, ME. Two-way ANOVA (site × species) was used to investigate non-native ascidian distribution across sites at the Isles of Shoals. On finding significant differences, a Tukey–Kramer test set to 0.05 significance was used to assess pair-wise differences between treatments. A t test was used on results from our laboratory experiment that examined differences in initial and end weights of bloodstar on the same diet.
Results
Long-term abundance of the sponge H. panicea
Abundance of H. panicea was variable from 1982 to 1998 at the Isles of Shoals (Fig. 2). Since 1998, H. panicea has not been observed in the rocky intertidal on Appledore Island. These data correspond to observed long-term declines in sponges in the subtidal zone around the Isles of Shoals (Harris and Dijkstra, unpub. obs.), and Portsmouth Harbor (Dijkstra and Harris 2009).
Survey of the bloodstar H. sanguinolenta and colonial ascidians
Post-invasion densities of H. sanguinolenta were significantly greater than pre-invasion densities at the Isles of Shoal and at Cape Neddick (F = 5.443, P < 0.025, F = 150.04, P < 0.01, Fig. 3). Elevated H. sanguinolenta densities at Star Island reflected an overall increase at the Isles of Shoals (P = 0.966; Fig. 4).
a Mean abundance (+SE) of H. sanguinolenta between 1977–1978 pre-invasion (Hurlbert 1980) and 2005–2007 (post-invasion). Mean abundances are combined average abundances of H. sanguinolenta between 1977–1978 and 2005–2007. One-way ANOVA determined a significance difference (*P < 0.025) in abundance of H. sanguinolenta between the two time periods. b Occurrence of H. sanguinolenta between 1979–1980 and 2011 pre and post-invasion of invasive colonial ascidians at Cape Neddick, ME. (*P < 0.01)
In our 2007 surveys, D. vexillum and D. listerianum were the most common ascidians at the Isles of Shoals (Fig. 5). There was a significant main effect of species, site and an interaction between site and species (Table 1; P < 0.05, Tukey–Kramer). We observed very few native colonial ascidians and sponges at all sites, and none were observed in our quadrats.
Feeding behavior and growth studies of the bloodstar H. sanguinolenta
Of the 76 H. sanguinolenta we observed actively feeding (demonstrated by an everted stomach) during dives at the Isles of Shoals, 50 % preyed on D. listerianum, 12 % on B. violaceus, 10 % on B. schlosseri, 9 % on D. vexillum and 15 % of bloodstars fed on debris composed of crushed barnacles and bryozoans (bryozoan complex; Fig. 6). Of the 114 H. sanguinolenta we observed during dives actively feeding at Cape Neddick, 64 % preyed on D. listerianum, 1 % on B. violaceus, 2 % on B. schlosseri, approximately 10 % on D. albidum, and 10 % on a bryozoan complex (Fig. 6). Sponges were not observed in our quadrats and were rarely observed at the Isles of Shoals and Cape Neddick. We did not witness H. sanguinolenta feeding on sponges.
Growth rates of H. sanguinolenta showed considerable variation among diets (Fig. 7). Bloodstars fed H. oculata increased weight over the first two months and then plateaued; overall, they grew significantly more than individuals in the other diet treatments (t = 2.29, P < 0.041). Bloodstars on either non-native diet did not significantly lose or gain weight [(P < 0.98 (D. listerianum t = 0.02, and D. vexillum t = 0.02)] compared to their initial weights, while bloodstars lacking food exhibited a steady, but not significant (t = −0.61, P < 0.27), decline in weight.
Temporal patterns of abundance of colonial ascidians and sponges
Non-native colonial ascidians and native sponges showed opposing seasonal dominance patterns with high percent cover of non-native prey observed in summer and fall months. Percent cover of native prey was highest during the winter and late spring (Fig. 8).
Discussion
Our results indicate that introduced prey can indirectly increase a native predator population by providing resources that are seasonally opposed to its native prey. Here, we show that consumption of non-natives will not directly result in greater predator biomass, but will sustain it, and limit the loss of biomass, during periods of absence of its native prey. Therefore, the addition of non-native prey may lead to increased native consumer pressure on native species (a process known as apparent competition), similar to the effects of non-native dune plants on rodents and native plants (Dangremond et al. 2010). However, direct competition between competitively superior non-native colonial ascidians and sponges also likely contributed to the observed decline in native sponge prey, particularly in the intertidal zone (Wethey and Walters 1986; Bak et al. 1996).
Other factors that may favor native predator population growth are temperature and rising plankton concentrations. Water temperature has increased over the last 30 years in the Gulf of Maine (Fogarty et al. 2008; Dijkstra et al. 2011). Though increasing sea surface temperature may positively influence population growth of many non-native species (Westerman et al. 2009), warmer waters may limit reproduction and growth of native species (Sorte et al. 2010; Moore et al. 2011). H. sanguinolenta is a cold water northern temperate species (Mah and Hansson 2011), and warming waters may likely restrict its growth and reproductive output. Another factor that may lead to elevated bloodstar densities is greater plankton concentrations. Although plankton concentrations have increased in the Gulf of Maine since the early 1980s (Pershing et al. 2005), and Anderson (1960) suggests that H. sanguinolenta filters particles from the water column, our results, along with others (Vasserot 1961), indicate positive growth can only occur when preying on larger food items. Though we did not witness sponge predation by the bloodstar during our field surveys, most likely because they were carried-out during a season when sponges are scarce, predation by bloodstars on sponges has been well documented (Hurlbert 1980; Witman and Sebens 1990; Shield and Witman 1993). Given that warmer temperatures and greater plankton concentrations are unlikely to enhance populations of H. sanguinolenta, the temporal mismatch in peak abundance of native and non-native prey likely resulted in the observed greater post-invasion bloodstar populations. Optimal foraging theory predicts that predators will switch their diet to include temporally abundant species (Hughes 1979).
Although our laboratory studies did not show significant weight gain of bloodstars on a non-native diet, they maintained weight on the non-native diet ((D. listerianum +1 % mean weight gain, D. vexillum −9.1 % mean weight loss), relative to the no food treatment (−21.4 % mean weight loss)). The offset seasonal distribution patterns of non-native and native prey, coupled with the results from the growth experiment suggests the native predator began its “growing” season at a higher biomass than it would in the absence of non-native prey. More biomass at the beginning of the season when the native prey is available leads to larger individuals that can directly (e.g., produce more offspring) or indirectly (e.g., higher nutrient reserves that can be transferred to juveniles) support reproductive efforts that increase population size.
Many terrestrial, freshwater and marine species undergo seasonal cycles of appearance and disappearance (Tonn and Magnuson 1982; Lechowicz and Koike 1995; Stachowicz and Byrnes 2006), yet studies that include seasonal distribution of native and non-native prey species are few. We show that non-native prey enhanced growth of the native predator population by providing a source of food during periods of rarity or absence of its native prey. Therefore, temporal native and non-native prey distribution patterns should be considered in future studies and be incorporated in models of the effects of non-native species on native consumer and prey populations.
References
Anderson JM (1960) Histological studies on the digestive system of a starfish, Henricia, with notes on Tiedemann’s pouches in starfishes. Biol Bull 119:371–398
Baiser B, Russell GJ, Lockwood JL (2010) Connectance determines invasion success via trophic interactions in model food webs. Oikos 119:1970–1976
Bak R, Lambrechts D, Joenje M, Nieuwland G, Van Veghel M (1996) Long-term changes on coral reefs in booming populations of a competitive colonial ascidian. Mar Ecol Prog Ser 133:303–306
Dangremond EM, Pardini EA, Knight TM (2010) Apparent competition with an invasive plant hastens extinction of an endangered lupine. Ecology 91:2261–2271
Dijkstra JA, Harris LG (2009) Maintenance of diversity altered by a shift in dominant species: implications for species coexistence. Mar Ecol Prog Ser 387:71–80
Dijkstra J, Harris LG, Westerman E (2007) The distribution and long-term temporal patterns of four invasive colonial ascidians in the Gulf of Maine. J Exp Mar Biol Ecol 342:61–68
Dijkstra JA, Westerman EL, Harris LG (2011) The effects of climate change on species composition, succession and phenology: a case study. Glob Change Biol 17:2360–2369
Fogarty M, Incze L, Hayhoe K, Mountain D, Manning J (2008) Potential climate change impacts on Atlantic cod (Gadus morhua) off the northeastern USA. Mitig Adapt Strat Glob Change 13:453–466
Grosholz ED (2002) Ecological consequences of coastal invasions. Trends Ecol Evol 17:22–27
Hamilton J, Halzapfel C, Mahall BE (1999) Coexistence and interference between a native perennial grass and non-native annual grasses in California. Oecologia 121:518–526
Harris LG, Tyrrell MC (2001) Changing community states in the gulf of maine: synergisms between invaders, overfishing and climate change. Biol Invasions 3:9–21
Holt RD, Kotler BP (1987) Short-term apparent competition. Am Nat 130:412–430
Hughes RN (1979) Optimal diets under the energy maximization premise: the effects of recognition time and learning. Am Nat 113:209–221
Hurlbert AW (1980) The functional role of asterias vulgaris (Verrill 1866) in three subtidal communities. Dissertation, University of New Hampshire, Durham
Inger R, McDonald R, Rogowski D, Jackson A, Parnell A, Preston S, Harrod C, Goodwin C, Griffiths D, Dick J, Elwood R, Newton J, Bearhop S (2010) Do non-native invasive fish support elevated lamprey populations? J Appl Ecol 47:121–129
Lambert WJ, Todd CD, Thorpe JP (2000) Variation in growth rate and reproductive output in British populations of the dorid nudibranch Adalaria proxima: consequences of restricted larval dispersal? Mar Biol 137:149–159
Lechowicz MJ, Koike T (1995) Phenology and seaonality of woody plants: an unappreciated element in global change research? Can J Bot 73:147–148
Mah C, Hansson H (2011) Henricia sanguinolenta (O.F. Müller, 1776). In: Mah CL (ed) World register of marine species. Available at http://www.marinespecies.org/aphia.php?p=taxdetails&id=123974
Moore PJ, Thompson RC, Hawkins SJ (2011) Phenological changes in intertidal con-specific gastropods in response to climate warming. Glob Change Biol 17:709–717
Pershing AJ, Greene CH, Jossi JW, O’Brien L, Broziak JKT, Bailey BA (2005) Interdecadal variability in the Gulf of Maine zooplankton community, with potential impacts on fish recruitment. J Mar Sci 62:1511–1523
Rilov G (2009) Predator-prey interactions in marine bioinvasions. In: Rilov G, Crooks JA (eds) Biological invasions in marine ecosystems: ecological management and geographic perspectives. Springer, Heidelberg, pp 261–285
Roemer GW, Donlan CJ, Courchamp F (2002) Golden eagles, feral pigs, and insular carnivores: how exotic species turn native predators into prey. Proc Nat Acad Sci 99:791–796
Ruiz GM, Carlton JT, Grosholz ED, Hines AH (1997) Global invasions of marine and estuarine habitats by non-indigenous species: mechanisms, extent, and consequences. Am Zool 37:621–632
Shield C, Witman J (1993) The impact of Henricia sanguinolenta (O.F. Muller) (Echinodermata: Asteroidea) predation on the finger sponges, Isodictya spp. J Exp Mar Biol Ecol 66:107–133
Shurin JB, Borer ET, Seabloom EW (2002) A cross ecosystem comparison of the strength of trophic cascades. Ecol Lett 5:785–791
Sorte CJB, Williams SL, Zerebecki RA (2010) Ocean warming increases threat of invasive species in a marine fouling community. Ecology 91:2198–2204
Stachowicz JJ, Byrnes JE (2006) Species diversity, invasion success, and ecosystem functioning: disentangling the influence of resource competition, facilitation, and extrinsic factors. Mar Ecol Prog Ser 311:251–262
Tonn WM, Magnuson JJ (1982) Patterns in the species composition and richness of fish assemblages in northern Wisconsin lakes. Ecology 63:1149–1166
Vasserot J (1961) Caractere hautement specialise du regime alimentaire chez les asterides Echinaster sepositus et Henricia sanguinolenta, predateurs de spongiares. Bulletin de la Societe Zoologique de France 86:796–809
Westerman EL, Whitlatch RB, Dijkstra JA, Harris LG (2009) Variation in brooding period masks similarities in response to changing temperatures. Mar Ecol Prog Ser 391:13–19
Wethey DS, Walters LJ (1986) Quantifying spatial patterns of overgrowth in epibenthic communities. Mar Ecol Prog Ser 29:271–278
Witman J, Sebens K (1990) Distribution and ecology of sponges at a subtidal rock in central Gulf of Maine. In: Rutzler K (ed) New perspectives in sponge biology. Smithsonian Institution, Washington
Acknowledgments
We would like to thank the Shoals Marine Laboratory and the class of Field Marine Biology and Ecology (instructors: C. Siddon and K. A. Miller) for collecting the intertidal data from the Isles of Shoals. The Shoals Marine Laboratory provided J.A. Dijkstra with housing and space. We thank H. Weeks for his critical comments on earlier drafts of the manuscript. We would also like to thank Dr. Jim Carlton and an anonymous reviewer for their helpful and constructive criticism. Finally, we would like to thank our dive partners: J. Friedman, C. Brooks, J. Mercer, O. Rhoades and C. Keough and O. Lambert for lab assistance. We thank S. J. Dijkstra and E. L.Westerman for their comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dijkstra, J.A., Lambert, W.J. & Harris, L.G. Introduced species provide a novel temporal resource that facilitates native predator population growth. Biol Invasions 15, 911–919 (2013). https://doi.org/10.1007/s10530-012-0339-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-012-0339-1