Abstract
Ants are the dominant soil faunal group in many if not most terrestrial ecosystems, and play a key role in soil structure and function. This study documents the impacts of invasion by the exotic cat’s claw creeper vine, Macfadyena unguis-cati (L.) Gentry (Bignoniaceae) on surface-situated (epigaeic) and subterranean (hypogaeic) ant communities in subtropical SE Queensland Australia where it is a major environmental weed of riparian areas, rainforest communities and remnant natural vegetation, smothering standing vegetation and causing canopy collapse. Soil ants were sampled in infested and uninfested areas at eight sites spanning both riparian and non-riparian habitats in subtropical SE Queensland. Patterns of ant species composition and functional grouping in response to patch invasion status, landscape type and habitat stratum were investigated using ANOVA and non-metric multidimensional scaling ordination. The epigaeic and subterranean strata supported markedly different ant assemblages, and ant communities also differed between riparian and non-riparian habitats. However, M. unguis-cati invasion had a surprisingly limited impact. There was a tendency for ant abundance and species richness to be lower in infested patches, and overall species composition was different between infested and uninfested patches, but these differences were relatively small, and did not occur consistently across sites. There were changes in functional group composition that conformed to known functional group responses to environmental change, but these were similarly limited and inconsistent across sites. Our study has shown that ant communities are surprisingly resilient to invasion by M. unguis-cati, and serves as a warning against making assumptions about invasion impacts based on visual appearances.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Invasive plant species often occur in near-monospecific stands, resulting in marked landscape transformation of habitat structure and complexity. This habitat modification can be expected to have a major impact on animal assemblages, and in particular lead to reduced faunal diversity because plant communities with greater diversity and structural complexity tend to support a richer fauna (e.g., Southwood 1978; Hunter and Price 1992; Wenninger and Inouye 2008; Wolkovich et al. 2009). While this trend has been documented in much plant invasion work comparing invaded versus non-invaded sites (e.g., Herrera and Dudley 2003; Standish 2004; Ernst and Cappuccino 2005; Gerber et al. 2008), some studies of faunal diversity have shown smaller than expected impact or even an effect opposite to the expected trend (e.g., Harris et al. 2004; Lindsay and French 2006; De Groot et al. 2007; Heleno et al. 2008; Pearson 2009; Parr et al. 2010). These inconsistent results suggest that the extent of impact of invasion is highly context dependent, being contingent upon landscape scenario, the characteristics of the invader/s, and the faunal group assessed. There have consequently been calls for studies of invasion impacts to assess their generality across a variety of situations (Ehrenfeld 2003; Vanderhoeven et al. 2006; Hejda et al. 2009; Wolkovich et al. 2009).
Ants are a dominant faunal group in most terrestrial habitats, particularly in the tropics (Hölldobler and Wilson 1990), where they play key roles as ecosystem engineers, mediators of energy and nutrient flow, and mutualists with plants and other insects (Folgarait 1998; Dunham and Mikheyev 2010; Sanders and van Veen 2011). Ants are also widely used as indicators of ecological change in environmental monitoring and assessment (Andersen and Majer 2004). Here we assess the impacts of near-monoculture stands of the invasive cat’s claw creeper, Macfadyena unguis-cati (L.) Gentry (Bignoniaceae) on soil ant biodiversity in eight sites representing a range of landscape settings in subtropical southeastern Queensland, Australia. Macfadyena unguis-cati is a perennial woody climbing vine native to tropical America (Downey et al. 2007), and has invaded much of the Indo-Pacific region (King and Dhileepan 2009). In Australia, M. unguis-cati is a major environmental weed in Queensland and New South Wales (NSW), where it poses a significant threat to biodiversity in riparian and forest communities (Batianoff and Butler 2003; Vivian-Smith and Panetta 2004; Osunkoya et al. 2009; Dhileepan et al. 2010).
Given the extent of habitat transformation caused by M. unguis-cati invasion, we expect it to have a marked impact on ant biodiversity. However, our focus is on the extent of context-dependency of ant responses, and we examine this by comparing responses in contrasting habitat types, as well as responses of ants from contrasting habitat strata. Our first hypothesis is that invasion impacts will differ between riparian and non-riparian habitats because of major differences in soil moisture and physico-chemical properties between these habitats. Invasion impacts can also be expected to be habitat-dependent because (a) levels of invasion might vary between habitats, (b) the effects of the invasion on vegetation structure might vary between habitats, and (c) the associated ant communities might be differentially sensitive to invasion. Our second hypothesis is that within a given site, invasion impacts will vary with the habitat stratum occupied by ants. Given that M. unguis-cati infestation transforms habitat structure above ground, we would expect greater impacts on surface-active (epigaeic) compared with the below ground (subterranean; hypogaeic) ants.
Methods
Study species
Macfadyenaunguis-cati is a high-climbing woody vine with twining stems (up to 6 cm in diameter) that produce horizontal runners and/or adventitious roots. It can grow successfully in a wide variety of light and soil conditions (Raghu et al. 2006; Osunkoya et al. 2010a). Some leaflets of its compound leaves are modified to form pronged, claw-like tendrils with deciduous horny hooks, which enable the plant to climb almost any structure (Raghu et al. 2006; Downey and Turnbull 2007). In densely infested areas, M. unguis-cati covers standing vegetation, including large trees and shrubs, eventually causing canopy collapse (Osunkoya et al. 2009; Dhileepan et al. 2010). In areas without standing vegetation or other structures (e.g. fences) the vines grow along the forest floor and form dense mats that preclude the recruitment and growth of native vegetation. In most infested sites, M. unguis-cati roots produce large numbers (~1,000 per m3) of subterranean, golf ball-size (25 mm × 7 mm) tubers that promote its persistence and aid its vegetative propagation (Osunkoya et al. 2009).
Study sites
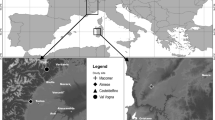
The study was conducted at eight sites (Oxley, Bardon, Carindale, Ipswich, Nerang, Boonah, Moogerah and Canungra) in the Brisbane-Gold coast region of south-eastern Queensland Australia, chosen on the basis of known M. unguis-cati infestations. The region experiences an average yearly rainfall of 900–2,000 mm (depending on topography and distance from the coast), approximately half of which occurs in the summer months of December to February. Temperatures range from an average daily minimum of 6°C in July to a maximum of 29.6°C in December. Predominant vegetation types in the area include tall open eucalypt (dry sclerophyll) forest and subtropical (microphyll vine) rainforest. Distances between study sites ranged from 10 to 150 km.
Macfadyenaunguis-cati infestation sites in Oxley (27°60′ S, 152°59′ E), Bardon (27°30′ S, 152°60′ E), Carindale (27°30′ S, 152°59′ E) and Ipswich (27°32′ S, 152°42′ E) were all in remnant natural open forest vegetation in non-riparian landscapes with gentle undulating topography, with each approximately 5–10 ha in size. The first three were within the Brisbane City Council forest parks, and the last was at Pine Mountain, which is managed by the Ipswich City Council. The other four sites [Nerang (27°60′ S, 153°20′ E), Canungra (28°10′ S, 153°10′ E), Moogerah (28°03′ S, 152°54′ E) and Boonah (27°60′ S, 152°41′ E)] represented riparian habitats dissecting larger open forest areas. The first two riparian sites are located in the Gold Coast hinterland, approximately 80 km south of Brisbane, while the latter two are in the Esk Shire, about 120 km west of Brisbane. In all eight sites, M. unguis-cati completely dominates the landscape, covering many of the trees (mainly species of Eucalyptus, Araucaria and Alphitonia) and much of the ground. Anecdotal evidence suggests M. unguis-cati has been a major weed in all of the chosen sites for at least 30 years. Occasionally other exotic, but less invasive vines such as Passiflorasuberosa and Aristilochiaelegans and native vines including Smilaxaustralis, Parsonsiastraminea and Pandoreajasminoides also occur, especially at the riparian sites. At these infested sites, subterranean tubers and seedlings of M. unguis-cati occur at extremely high densities, averaging 1,000/m3 and 1,280/m2, respectively (Osunkoya et al. 2009). The soils at these eight sites are slightly acidic (pH range: 5.9–6.3), with low clay (25%), total carbon (3–5%) and nitrogen (0.28–0.35%) contents (Osunkoya OO, unpublished data).
Ant sampling and processing
Epigaeic ants were sampled using standard pitfall traps, consisting of 4-cm diameter plastic containers dug into the soil with their rims flush with the soil surface. They were partly filled with 70% ethylene–glycol as a preservative. Hypogaeic species were sampled using subterranean traps following Andersen and Brault (2010). These were created from 1.5 ml eppendorf tubes (4 cm high, 1 cm diameter) with four holes (each 3 mm in diameter) drilled close to the top of the tube to allow access by ants. The upper part of the tube, just above the access holes, was coated with a mixture of peanut butter and honey to attract ants, and tubes were partly filled with 70% ethylene–glycol as a preservative. These were then buried into a hole of 10 cm depth dug with a hand-held augur, with a length of string attached to the lid to facilitate relocation and retrieval. Australian sclerophyll (open forest) habitats have a very depauperate fauna of specialist arboreal ants, with the vast majority of species foraging in trees but nesting on the ground (Andersen 2000). We therefore made no attempt to sample the arboreal fauna.
At each of the eight sites, sampling was conducted in and adjacent to 15 randomly located M. unguis-cati patches, each with an infestation radius of at least 18 m (i.e. ≥745 m2 in area). At each patch, one pitfall and one subterranean trap were set inside (infested), as well as at 5–10 m outside (i.e., in native vegetation; uninfested control). For the riparian sites, traps were located at least 5 m from the stream bank. Each trap was opened for 6 days during September (early spring) 2009. There was no substantial rain during the sampling period.
Ant samples were sorted to species level in the laboratory, and where possible named, with species authorities following Bolton (1995). Species that could not be confidently named were identified to species-group following Andersen (2000), and assigned letter codes (sp. A, sp. B, etc.) that apply only to this study. Voucher specimens of all species are held at the CSIRO Tropical Ecosystems Research Centre in Darwin, Northern Territory, Australia.
Data analysis
Data from pitfall and subterranean traps were analysed separately, unless otherwise indicated. Rarefaction curves, plotting the cumulative number of species recorded as a function of sampling effort (Gotelli and Colwell 2001), were used to assess sampling completeness and to compare ant species richness in relation to invasion status (infested vs. uninfested patches), landscape type (riparian vs. non-riparian) and habitat stratum (epigaeic vs. subterranean). Mean species richness, species diversity using the Simpson index and abundance per trap and per site were compared between invasion status and landscape type using nested ANOVA with abundance data square-root transformed to meet the assumption of normality.
Patterns of ant species composition were investigated using non-metric multidimensional scaling (NMDS) in two dimensions on both species abundance and presence/absence data, based on a Bray-Curtis dissimilarity matrix. The extent of clustering according to invasion status, landscape type and habitat stratum was assessed by analysis of similarity (ANOSIM), which compares the mean difference of ranks between and within groups, generating the statistic R (Clarke and Warwick 2001). Values of R range from −1 to +1, with values approaching R = 1 indicating a strong dissimilarity among samples. Both abundance and presence/absence data gave very similar results and so only those from presence/absence are presented. All multivariate analyses were performed using the software Primer vs 6.0 (Clarke and Gorley 2006).
The effects of M. unguis-cati invasion were also examined in terms of ant functional composition. Ant species were assigned to one of nine functional groups (Table 1) based on global responses of their species-groups to environmental stress and disturbance (Andersen 1995, 1997). Functional group abundances were compared between invasion status using univariate (χ2 analysis, t test or nested ANOVA) as well as multivariate techniques (NMDS).
Results
A total of 100 ant species from 41 genera were recorded during the study (Appendix), with the richest genera being Monomorium (8 species), Pheidole (8), Polyrhachis (8) and Camponotus (5). The fauna included two introduced species, one of which (Paratrechina longicornis) was the fourth most common species recorded in pitfall traps, especially in infested patches (overall catch across trap types: 153 vs. 42 in infested and uninfested patches; probability of a difference using t test: P < 0.001). The other was Tetramorium simillimum, represented by a single individual in a subterranean trap (Appendix). Many more individuals (2849 cf. 414) and species (91 cf. 36) were recorded from pitfall traps compared with subterranean traps (Appendix). The most common species in pitfall traps were Nylanderia sp. A (obscura gp.; 11.2% total individuals), Pheidole sp. G (ampla gp.; 8.0%), Pheidole sp. C (variabilis gp.; 7.5%), Paratrechina longicornis (6.6%), Iridomyrmex suchieri (6.1%) and I. purpureus (6.1%). The most common species in subterranean traps were Solenopsis sp. B (22.7%), Carebara sp. A (16.9%), Solenopsis sp. A (13.0%) and Solenopsis sp. C (7.5%). These last three species were rarely, and in one case (Solenopsis sp. C) never, recorded in pitfall traps. The fauna included an unidentified and apparently undescribed genus from the Tribe Pheidologetonini that was recorded almost exclusively in subterranean traps.
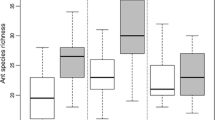
In pitfall traps, mean ant abundance was significantly higher in control (uninfested) compared with infested patches overall (Table 2). A total of 76 species were recorded in uninfested patches compared with 56 in infested patches (Appendix), and mean species richness per trap was significantly higher in uninfested patches (Table 2). However, these trends of higher values in control patches occur only in a minority of the sites sampled: Boonah, Bardon and Ipswich (Table 2). Simpson’s diversity (which simultaneously takes into account trap catch richness and abundance of individual species) did not differ between infested and uninfested patches (Table 2). In subterranean traps, patch invasion status had no effect on ant abundance, species richness or species diversity (Appendix; Table 2). The above described trends did not change when analyses were limited to the most common species with contribution >6% in each of the trap types used (data not shown). The higher species richness in control patches for pitfall but not subterranean catches is illustrated by the rarefaction curves (Fig. 1). The rarefaction curves also indicate that sampling of the regional subterranean fauna was reasonably complete, but that many epigaeic species were not collected.
Multivariate analysis (using NMDS) showed no overlap in species composition in samples from pitfall and subterranean traps (ANOSIM: R = 0.615, P < 0.001; Fig. 2a, b). Species composition also varied significantly with landscape type (riparian vs. non-riparian), for both pitfall (ANOSIM: R = 0.498, P = 0.001) and subterranean samples (ANOSIM: R = 0.158, P = 0.002). ANOSIM revealed statistically significant but modest differences in overall species composition between infested and uninfested patches for both pitfall and subterranean samples (Table 2). In both trap types there were statistically significant ANOSIM differences at four individual sites, two each from riparian and non-riparian habitats. The differences were twice as high for pitfall (4.7%) compared with subterranean traps (2.3%). This trend of minimal invasion effect was observed irrespective of landscape condition or trap type used (Fig. 2).
MDS ordination of Macfadyena unguis-cati infested (closed-symbols) and non-infested (open-symbols) vegetation patches based on ants presence/absence data collected in pitfall and subterranean traps across eight sites in Brisbane-Gold coast greater region, SE QLD, Australia. See Table 2 for tests of invasion effect within and across sites using ANOVA and ANOSIM. The dash-loops separate the pitfall from the subterranean traps, within which each site is represented with two points (infested and non-infested patches). Stress value is 0.18. ANOSIM values for pairwise tests are: surface/subterranean ants: R = 0.615; P < 0.001; riparian/non-riparian habitats, R = 0.359, P = 0.01
Ant functional group composition varied substantially with invasion status, especially more so for surface active (pitfall) ants (χ2 = 7.49; P < 0.005; Table 3, Fig. 3). For pitfall traps only, all indices of ant functional composition (abundance, number per trap, richness and diversity) were higher in uninfested (control) relative to M. unguis-cati infested patches (Table 3). Overall, Opportunists were more abundant in infested (60% total ants) compared with uninfested (34%) patches, whereas the reverse was true for Dominant Dolichoderinae (6.1% cf. 23.6%), Generalised Myrmicinae (22.5% cf. 32.5%), Hot-climate Specialists (0.0% cf. 2.4%) and Subordinate Camponotini (1.6% cf. 3.7%; Fig. 3). Multivariate analysis across all eight sites showed no significant effect of invasion status on functional group composition of the pitfall trap ants (ANOSIM: R = 0.162, P = 0.08) or for subterranean ants (ANOSIM: R = −0.17, P = 0.72; Fig. 4). However at the individual site level, significant effects of invasion status on functional group composition was seen at two of the eight sites for pitfall traps (Boonah and Bardon, ANOSIM: P < 0.05 in both cases; Table 3) and at four of the eight sites for subterranean traps (Boonah, Canungra, Carindale and Ipswich [ANOSIM: 0.01 < P < 0.05; Table 3])—suggesting a possible site specific pattern.
Functional group profiles of ants sampled from Macfadyena unguis-cati infested and non-infested (control) patches. Data have been pooled for all eight sites. Data are proportion of total species represented by each functional group. Using observed (raw) data, the functional group distribution differs significantly between infested and non-infested patches for surface active ants (χ2 = 7.49; P < 0.005) but not for subterranean ants (χ2 = 0.49; P < 0.55). Within each functional group, the significant level of the χ2 test is also indicated as follows: †P < 0.10; *P < 0.05. Abbreviations are: CCS cold climate specialists, CS cryptic species, DD dominant dolichoderinae, GM generalised myrmicinae, HCS hot climate specialists, OPP Opportunists, SC subordinate camponotini, SP specialist predators, TCS Tropical climate specialists
MDS ordination of Macfadyena unguis-cati infested (closed-symbols) and non-infested (open-symbols) patches across eight sites based on ant functional groups in pitfall (circles) and subterranean traps (triangles). The dash-loop separates the pitfall trap catch from the subterranean ones, within which each site is represented with two points (infested and non-infested patches). Stress value is 0.07. ANOSIM values for pairwise tests are: pitfall traps- infested/control patches R = 0.162; P = 0.08; subterranean traps- infested/control patches, R = 0.017, P = 0.72
Discussion
Invasion by M. unguis-cati results in a dramatic change in vegetation structure in infested areas, and this would be expected to have a very marked impact on invertebrate biodiversity (Crisp et al. 1998; Belnap and Phillips 2001; Wenninger and Inouye 2008; Heleno et al. 2008; Ostoja et al. 2009). However, our study revealed relatively weak impacts on ant communities. There was a tendency for ant abundance and species richness to be lower in infested patches, and overall species composition was different between infested and uninfested patches. However, these differences were relatively small, and did not occur consistently across sites. Variation in time since M. unguis-cati invasion of the study sites could affect level of impact of the weed and thus trends observed (Carpenter and Cappuccino 2005); however, we lack data on the history of our investigated sites, and hence cannot explore this line of argument further. Invasion by M. unguis-cati did have a greater impact on epigaeic compared with subterranean ants as we hypothesized, and perhaps as reflected in the greater habitat transformation above- compared with below-ground. Riparian and non-riparian habitats supported different ant communities—confirming the greater role of moisture and perhaps soil texture in the distribution and abundance of arthropods (Wenninger and Inouye 2008), but M. unguis-cati invasion did not have different impacts within these habitats.
Macfadyenaunguis-cati invasion had a significant impact on functional group composition, with several functional groups having reduced abundance in infested patches. Largest reductions occurred in Dominant Dolichoderinae, which are strongly associated with open habitats (Andersen 1995; Hoffmann and Andersen 2003). Hot-Climate Specialists are even more strongly thermophilic than the Dominant Dolichoderinae, and they were completely absent from infested patches. In contrast, the abundance of Opportunists increased markedly in infested patches. This can be attributed to the broad habitat tolerances of these species, and their release from competition with Dominant Dolichoderinae (Andersen 1995; Hoffmann and Andersen 2003). However, as was the case for species-level responses, impacts of M. unguis-cati invasion on ant functional groups were minimal and inconsistent among sites.
We only sampled during one time of the year (spring), and greater impacts of M. unguis-cati invasion might have been detected if we had sampled at other times. However, seasonal rates of ant activity are high during spring in this subtropical region (Vanderwoude et al. 1997), so most of the species would have been active during our sampling effort. Moreover, the Oxley site was intensively surveyed for surface-active invertebrates on three occasions during the summer prior to our study, and again only a weak difference (P = 0.09) in species composition could be detected between M. unguis-cati invaded and non-invaded patches (Anita Kusumaningsih and OO Osunkoya unpublished data, 2008). We therefore believe that our results are robust.
A relatively small impact of M. unguis-cati invasion on ant diversity would not have been so surprising if it were just a case of species characteristic of uninfested patches being replaced by species characteristic of denser vegetation types (cf. Sax 2002), but this was not the case in our study. It is quite conceivable that M. unguis-cati monocultures provide sufficient habitat complexity and productivity to support a diverse ant fauna. For example, the network of fleshy subterranean tubers (see Osunkoya et al. 2009) with their accompanying soil surface-running and inter-twined stems and leaf tendrils of several layers (at times up to 20 cm thick), coupled with accumulated decomposing litter, potentially creates a variety of micro habitats suitable for ants. The leaves of M. unguis-cati are also higher in nitrogen content compared to those of native species (Osunkoya et al. 2010b), a nutritional trait that is known to promote insect abundance (Bowdish and Stiling 1998; Wenninger and Inouye 2008) and thereby food supplies for ants. Nonetheless, it is very surprising that M. unguis-cati invasion had such a limited impact on ant species composition, given that ant taxa are known to be highly sensitive to environmental change (Andersen and Majer 2004).
Our findings are similar to those from a study of the impacts of the invasive Gamba grass (Andropogon gayanus) on ant biodiversity in tropical Australia (Parr et al. 2010). Gamba grass can transform grass-layer structure in invaded savannas, but Parr et al. (2010) could detect no impacts on epigaeic invertebrate assemblages, including none on ant abundance, species richness or species composition. This lack of response was attributed to the impact of Gamba grass invasion on habitat structure being primarily on vertical rather than horizontal complexity, so that conditions for ground-active invertebrates are not as affected as might appear. Invasion by M. unguis-cati clearly has an impact on horizontal habitat structure by reducing open spaces, and this was reflected by some changes in ant epigaeic functional group composition. However, it is possible that induced changes in horizontal habitat structure in M. unguis-cati invaded landscape are far more limited, as explained above.
Our study has shown that ant communities are surprisingly resilient to invasion by M. unguis-cati, at least for ground foraging and hypogaeic groups. It is very possible that many other faunal groups are not so resilient, especially those that are affected more strongly by vertical habitat structure (e.g. birds). However, our results serve as a warning against making assumptions about invasion impacts based on visual appearances, and highlight the need for studies that directly assess the effects of invasive species as a foundation for prioritising conservation management (see also Sax 2002).
References
Andersen AN (1995) A classification of Australian ant communities, based on functional groups which parallel plant life-forms in relation to stress and disturbance. J Biogeogr 22:15–29
Andersen AN (1997) Functional groups and patterns of organization in North American ant communities: a comparison with Australia. J Biogeogr 24:433–460
Andersen ANN (2000) The ants of Northern Australia: a guide to the Monsoonal Fauna. Melbourne, CSIRO Publishing
Andersen AN, Brault B (2010) Exploring a new biodiversity frontier: subterranean ants in northern Australia. Biodivers Conserv 19:2741–2750
Andersen AN, Majer JD (2004) Ants show the way down under: invertebrates as bioindicators in land management. Front Ecol Environ 2:291–298
Batianoff GN, Butler DW (2003) Impact assessment and analysis of sixty-six priority invasive weeds in south-east Queensland. Plant Protect Q 18:11–15
Belnap J, Phillips SL (2001) Soil biota in an ungrazed grassland: response to annual grass (Bromus tectorum) invasion. Ecol Appl 11:1261–1275
Bolton B (1995) A new general catalogue of the ants of the world. Harvard University Press, Cambridge
Bowdish TI, Stiling P (1998) The influence of salt and nitrogen on herbivore abundance: direct and indirect effects. Oecologia 113:400–405
Carpenter D, Cappuccino N (2005) Herbivory, time since introduction and the invasiveness of exotic plants. J Ecol 93:215–321
Clarke KR, Gorley RN (2006) Primer v 6: User manual/Tutorial. PRIMER-E, Plymouth
Clarke KR, Warwick RM (2001) Change in marine communities: an approach to statistical analyses and interpretation, 2nd edn. PRIMER-E, Plymouth
Crisp PN, Dickson KJM, Gibbs GW (1998) Does native invertebrate diversity reflect native plant diversity? A case study from New Zealand and implications for conservation. Biol Conserv 83:209–220
De Groot M, Kleijn D, Jogan N (2007) Species groups occupying different trophic levels respond differently to the invasion of semi-natural vegetation by Solidago canadensis. Biol Conserv 136:612–617
Dhileepan K, Treviño M, Bayliss D, Saunders M, Shortus M, McCarthy J, Snow EL, Walter GH (2010) Introduction and establishment of Carvalhotingis visenda (Hemiptera: Tingidae) as a biological control agent for cat’s claw creeper Macfadyena unguis-cati (Bignoniaceae) in Australia. Biol Control 55:58–62
Downey PO, Turnbull I (2007) The biology of Australia weeds 48. Macfadyena unguis-cati (L.) A.H.Gentry. Plant Protect Q 23:82–91
Dunham AE, Mikheyev AS (2010) Influence of an invasive ant on grazing and detrital communities and nutrient fluxes in a tropical forest. Divers Distrib 16:33–42
Ehrenfeld J (2003) Effects of exotic plant invasions on soil nutrient cycling processes. Ecosystems 6:503–523
Ernst CM, Cappuccino N (2005) The effect of an invasive alien vine, Vincetoxicum rossicum (Asclepidaceae), on arthropod populations in Ontario old fields. Biol Invasions 7:417–425
Folgarait PJ (1998) Ant biodiversity and its relationship to ecosystem functioning: a review. Biodivers Conserv 7:1221–1244
Gerber E, Krebs C, Murrell C, Moretti M, Rocklin R, Schaffner U (2008) Exotic invasive knotweeds (Fallopia spp.) negatively affect native plant and invertebrate assemblages in European riparian habitats. Biol Conserv 141:646–654
Gotelli NJ, Colwell RK (2001) Quantifying biodiversity: procedures and pitfalls in the measurement and comparison of species richness. Ecol Lett 4:379–391
Harris RJ, Toft RJ, Dugdale JS, Williams PA, Rees JS (2004) Insect assemblages in a native (Kanuka—Kunzea ericoides) and an invasive (gorse—Ulex europaeus) shrubland. N Z J Ecol 28:35–47
Hejda M, Pysek P, Vojtéch J (2009) Impact of invasive plants on the species richness, diversity and composition of invaded communities. J Ecol 97:393–403
Heleno RH, Ceia RS, Ramos JA, Memmott J (2008) Effects of alien plants on insect abundance and biomass: a food-web approach. Conserv Biol 23:410–419
Herrera AM, Dudley TL (2003) Reduction of riparian arthropod abundance and diversity as a consequence of giant reed (Arundo donax) invasion. Biol Invasions 5:167–177
Hoffmann BD, Andersen AN (2003) Responses of ants to disturbance in Australia, with particular reference to functional groups. Aust Ecol 2:444–464
Hölldobler B, Wilson EO (1990) The ants. The Belknap Press of Harvard University Press, Cambridge
Hunter MD, Price PW (1992) Playing chutes and ladders: heterogeneity and the relative roles of bottom–up and top–down forces in natural communities. Ecol 73:724–732
King A, Dhileepan K (2009) Clinging on: a review on the biological control of cat’s claw creeper. Biocontrol News Inf 30:53N–56N
Lindsay EA, French K (2006) The impact of the weed Chrysanthemoides monilifera ssp. rotundata on coastal leaf litter invertebrates. Biol Invasions 8:177–192
Ostoja SM, Schupp EW, Sivy K (2009) Ant assemblages in intact big sagebrush and converted cheatgrass-dominated habitats in Tooele County, Utah. West N Am Nat 69:223–234
Osunkoya OO, Pyle K, Scharaschkin T, Dhileepan K (2009) What lies beneath? A study on the pattern and abundance of subterranean tuber bank of the invasive liana cat’s claw creeper (Macfadyena unguis-cati (Bignoniaceae) (L) Gentry). Aust J Bot 57:132–138
Osunkoya OO, Bayliss D, Panetta FD, Vivian-Smith G (2010a) Variation in ecophysiology and carbon economy of invasive and native woody vines of riparian zones in south in south eastern Queensland. Aust Ecol 35:636–649
Osunkoya OO, Bayliss D, Panetta FD, Vivian-Smith G (2010b) Leaf trait co-ordination in relation to construction cost, carbon gain and resource-use efficiency in exotic invasive and native woody vine species. Ann Bot 106:371–380
Parr CL, Ryan BJ, Setterfield SA (2010) Habitat complexity and invasive species: the impacts of Gamba grass (Andropogon gayanus) on invertebrates in an Australian tropical savanna. Biotropica 42:688–696
Pearson D (2009) Invasive plant architecture alters trophic interactions by changing predator abundance and behavior. Oecologia 159:549–558
Raghu S, Dhileepan K, Trevino M (2006) Response of an invasive liana to simulated herbivory: implications for its biological control. Acta Oecol 29:335–345
Sanders D, van Veen FJF (2011) Ecosystem engineering and predation: the multi-trophic impact of two ant species. J Anim Ecol 80:569–576
Sax DF (2002) Equal diversity in disparate species assemblages: a comparison of native and exotic woodlands in California. Global Ecol Biogeogr Lett 11:49–57
Southwood TRE (1978) Ecological methods. Chapman and Hall, New York
Standish RJ (2004) Impact of an invasive clonal herb on epigaeic invertebrates in forest remnants in New Zealand. Biol Conserv 116:49–58
Vanderhoeven S, Dassonville N, Chapuis-Lardy L, Hayez M, Meerts P (2006) Impact of the invasive alien plant Solidago gigantean on primary productivity, plant nutrient content and soil mineral nutrient concentrations. Plant Soil 286:259–268
Vanderwoude C, Andersen AN, House APN (1997) Community organization, biogeography and seasonality of ants in an open forest of southeastern Queensland. Aust J Zool 45:523–537
Vivian-Smith G, Panetta FD (2004) Seedbank ecology of the invasive vine, cat’s claw creeper (Macfadyena unguis-cati (L.) Gentry). In: Sindel BM, Johnson SB (eds) Proceedings of the fourteenth Australian weeds conference, Wagga Wagga, New South Wales. Weed Society of New South Wales: Wagga Wagga, NSW, pp 531–537
Wenninger EJ, Inouye RS (2008) Insect community response to plant diversity and productivity in a sagebrush-steppe ecosystem. J Arid Environ 72:24–33
Wolkovich EM, Bolger DT, Holway DA (2009) Complex responses to invasive grass litter by ground arthropods in a Mediterranean scrub ecosystem. Oecologia 161:697–708
Acknowledgments
We thank Claire Tailly for much needed assistance during the field work and Magen Pettit for assistance with specimen processing. Travel, field work and accommodation funds were provided by CSIRO to Claire Polo and Claire Tailly and by the Queensland Land Protection Trust Fund to OO Osunkoya. Thanks also to Dane Panetta for critical reading of the manuscripts and comments.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
See Table 4 below.
Rights and permissions
About this article
Cite this article
Osunkoya, O.O., Polo, C. & Andersen, A.N. Invasion impacts on biodiversity: responses of ant communities to infestation by cat’s claw creeper vine, Macfadyena unguis-cati (Bignoniaceae) in subtropical Australia. Biol Invasions 13, 2289–2302 (2011). https://doi.org/10.1007/s10530-011-0040-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-011-0040-9