Abstract
The ragweed beetle, Ophraella communa, is a potential biological control agent of common ragweed that appeared in Nanjing City in 2001 and has since dispersed throughout southeast China. We compared the cold hardiness of five different O. communa populations by measuring the supercooling point (SCP), water and glycerol contents of adult beetles. All indices of cold hardiness varied significantly among the sampled populations. Male beetles from the most northerly population (Nanjing) had the lowest water content of any sampled and, although female beetles from Nanchang and Miluo had water content similar to those from Nanjing, they were still lower than those of females from Fuzhou and Wuchang. Beetles from the most southerly population (Fuzhou) had the highest SCP, although Nanchang males were not significantly different from Fuzhou males. Glycerol content yielded resolution of populations as follows: Nanjing > Wuchang = Miluo = Nanchang > Fuzhou, with beetles from Nanjing yielding twice the glycerol content of Fuzhou beetles. These results suggest that overwintering O. communa use freeze avoidance to survive winter cold and that geographically separated populations of O. communa are diverging with respect to their baseline cold hardiness in accordance with the severity of low temperatures experienced during the coldest winter months in each locality. The apparent ability of O. communa to rapidly adapt to different climatic conditions is predicted to facilitate its continued range expansion across mainland China, with consequent benefits in terms of fortuitous biological control of common ragweed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Because insects are ectothermic, the low temperatures experienced during winter months can impede the establishment and persistence of perennial populations in temperate regions. However, insects have evolved various physiological mechanisms to improve their cold-hardiness, and hence their survival, under winter conditions (Bale 2002; Chen and Kang 2002a; Danks 1996; Lee 1991; Sinclair 1999; Wang and Kang 2005). In general, insects that survive subzero temperatures in winter are classified as being either freeze tolerant or freeze intolerant (Salt 1961; Asahina 1969). The latter employ mechanisms that prevent ice nucleation within cells, whereas the former control ice crystal nucleation and growth in various ways to prevent cellular damage. Many insects improve their physiological tolerance of cold by increasing the concentrations of antifreeze-like compounds in their bodies (Salt 1961; Goto et al. 2001; Holden and Storey 1994; Montiel 1998; Palmer and Siebke 2008; Worland and Convey 2008). Furthermore, different geographic populations of an insect species may vary significantly in cold tolerance, largely because of adaptation or acclimation to different climatic conditions (Chen and Kang 2002b; Palmer and Siebke 2008; Régnière and Bentz 2007; Worland et al. 2004).
Glycerol is perhaps the most common of various ‘polyols’ that insects may use to resist freezing at subzero temperatures (Goto et al. 2001; Ishiguro et al. 2007; Liu et al. 2007). Prior to the onset of winter, many insect species accumulate reserves of glycogen that are subsequently broken down into glycerol during the coldest months of winter in order to improve cold-hardiness (Ishiguro et al. 2007; Li et al. 2002; Muise and Storey 1999; Tsumuki 1990; Tsumuki and Kanehisa 1978). Glycerol concentrations can be seen to increase in both intracellular and extracellular compartments in all body parts and, in some cases, reserves may approach 20% of body mass (Pfister and Storey 2006). Since glycerol formation consumes both water and glycogen, it not only increases the viscosity of body fluids at low temperatures, but simultaneously reduces the amount of water available to participate in ice formation within and between cells. Thus, glycerol accumulation is typically associated with both reduced supercooling points (SCPs) and lower water content (Holmstrup et al. 1999; Nedvěd et al. 1998; Neven 1999; Rivers et al. 2000). With regard to insects, the SCP refers to the temperature at which spontaneous nucleation of body water occurs and lethal ice crystals form in the insect tissue (Zachariassen 1985; Régnière and Bentz 2007). The SCP is often >20°C below the freezing point of body fluids, and is thus often considered a measure of maximum cold hardiness (Lee 1991). In some species, e.g., Sarcophaga crassipalpis (Lee and Denlinger 1985), Dendroctonus frontalis (Tran et al. 2007) and Pyrrhocoris apterus (Hodkova and Hodek 1994), the SCP also represents the lower lethal temperature. For these reasons, the SCP is an important ecological index of cold-hardiness in insects (Carrillo et al. 2005).
The ragweed beetle, Ophraella communa LeSage (Coleoptera: Chrysomelidae), is native to North America (Futuyma 1990; Palmer and Goeden 1991), and is a potential biological control agent of invasive annual ragweed, Ambrosia artemisiifolia L. (Asterales: Asteraceae) that is now widely distributed across Australia, Japan, Korea and China (Zhou et al. 2010a). In Japan, O. communa was first discovered in Chiba Prefecture in 1996 (Takizawa et al. 1999; Yamazaki et al. 2000) and, by 2004, had spread to all prefectures of Japan (except Hokaido and Okinawa) and the islands of Kyushu and Shikoku (Shiyake and Moriya 2005; Tamura et al. 2004; Watanabe and Hirai 2004). It fortuitously appeared on common ragweed in the suburb of Nanjing City, Jiangsu province, China in 2001 (Meng and Li 2005). It is now distributed in more than seven provinces in South China and has significantly impeded the spread of common ragweed in some areas (Zhou et al. 2010b). Earlier studies indicated that O. communa was adapted to subtropical conditions, the optimum temperature range for development falling within the average temperature range of subtropical regions of China where most invasive ragweed is present (Zhou et al. 2010a). However, the current distribution of O. communa includes more temperate regions of China at higher latitudes where temperatures during the coldest months of the year are significantly lower. The purpose of the present study was to compare the baseline cold hardiness of O. communa populations from different geographical regions in order to gain insight into the potential for further range expansion in China. To this end, we used laboratory measurements to compare the SCP, water and glycerol content of adult O. communa from various populations.
Materials and methods
Host plants
Seeds of ragweed were germinated in soil in an unheated greenhouse and the resulting seedlings were transplanted individually into 15 cm diameter plastic pots, watered every four days, and fertilized with a solution of 13:7:15 (N:P:K) twice a month. The plants were maintained in an unheated greenhouse and used in experiments when they reached 50 cm in height.
Insects
Ophraella communa pupae were collected in mid-August, 2009, from five different provinces in southeast China (Fig. 1) by picking ragweed leaves with pupae attached. More than 800 pupae were randomly collected from 40 common ragweed plants at each collection site, 20–25 pupae per plant. The geographic coordinates of the collection sites and their climatic characteristics are reported in Table 1. All pupae were transported to a laboratory at the Institute of Plant Protection, Hunan Academy of Agricultural Sciences, in Changsha City, Hunan Province where they were stored in transparent plastic boxes (19 × 12 × 6 cm) covered with organdy mesh fabric at 26–27°C, a relative humidity of 70 ± 5% and a photoperiod of 14:10 (L:D) until emergence of adults five to six days later. Newly emerged adults were sexed and males and females were held separately on potted ragweed plants, 20 adults per plant. Each plant was isolated in a ventilated, aluminum frame cage (40 × 40 × 60 cm) under the same laboratory conditions as above, and adults were used in experiments when they were two days old.
Measurement of water content
For assessment of water content, we chose adults of uniform size (i.e., similar weight). The fresh weight of adult beetles (n = 10 per population) was obtained by weighing them individually on an electronic balance (AB204-S, sensitivity ≤0.1 mg, Mettler Toledo, Switzerland). Beetles were then dryed in an oven at 70°C for 48 h and reweighed. The dry weight of each individual was then subtracted from its fresh weight to calculate the water content (WC), which was expressed as a percentage of fresh body weight.
Determination of supercooling points (SCPs)
Adult beetles (n = 30 of each gender from each population) were hand-picked from ragweed plants and then starved for 12 h. Each adult beetle was fixed to a thermocouple that was attached to an automatic data recorder (uR100, Model 4152, Yokogawa Electric Co., Seoul, Korea) via a bridge. The thermocouple with the adult was then lowered into a freezing chamber at −25°C and the body temperature of the adult beetle was monitored as it decreased at a rate of about 1°C per minute from 26°C (Liu et al. 2007). The SCP was taken to be the temperature recorded by the thermocouple just before the sudden increase in temperature caused by the emission of the latent heat of crystallization (Wang et al. 2006).
Glycerol assays
Adult beetles (n = 5 of each gender from each population) were hand-picked from ragweed plants and then starved for 12 h prior to analysis. The whole-body glycerol content of beetles was determined using an enzymatic assay (337-40A, Sigma Chemical Company). Individual adults (n = 5 per population) were homogenized in 25 mM sodium phosphate buffer (pH 7.4) and then centrifuged at 12,000 g for 10 min at 25°C. The supernatant was then deproteinized with 6% (w/v) perchloric acid, and the protein precipitate that formed was removed by centrifugation (12,000 g for 5 min). The supernatant was then neutralized with 5 M potassium carbonate to pH 7.0. Glycerol levels were determined spectrophotometrically by measuring sample absorbance at 540 nm (Yoder et al. 2006).
Data analysis
Data were checked for normality and homoscedasticity as appropriate and, if needed, were arcsine square-root or log-transformed before analysis by factorial ANOVA with location and gender as independent fixed factors, followed by one-way ANOVAS where the sexes were compared separately (SAS Institute 2004). Fisher’s protected LSD test (α = 0.05) was used to separate treatment means when more than two groups were compared. Linear regression was used to test for a relationship between body weight and SCP.
Results
Water content
Collection location had a significant effect on the water content of O. communa beetles (F 4,90 = 11.559, P < 0.001) but gender did not (F 1,90 = 1.520, P = 0.221), although there was a significant location*gender interaction (F 4,90 = 5.655, P < 0.001). Both male (F 4,45 = 9.008, P < 0.001) and female (F 4,45 = 7.994, P < 0.001) beetles varied among populations in water content. The water content of female beetles was significantly lower in the Nanchang, Miluo and Nanjing populations than in the Fuzhou and Wuchang ones, whereas male water content was lower in the Nanjing population than in all others (Fig. 2). Male water content was higher than that of females in the Nanchang population (F 1,18 = 8.23, P = 0.01), and lower than that of females in the Wuchang population (F 1,18 = 4.62, P < 0.05), with no significant effect of gender in the other three populations (Fuzhou: F 1,18 = 1.92, P = 0.18; Miluo: F 1,18 = 2.42, P = 0.14; Nanjing: F 1,18 = 3.93, P = 0.06).
Mean (+SE) water content (percent fresh weight) of O. communa adults from five geographic populations (n = 10). Columns bearing the same letters were not significantly different (LSD, α = 0.05) from others of the same gender (males: upper case letters, females: lower case letters). Asterisks indicate a significant difference (ANOVA, P < 0.05) between males and females within a population
Supercooling points
There was no effect of location on beetle weight (F 4,290 = 0.702, P = 0.591) but females were consistently heavier than males (11.1 ± 0.04 vs. 7.0 ± 0.02 mg, F 1,290 = 8876.302, P < 0.0001) and the location*gender interaction was not significant (F 4,290 = 0.175, P = 0.951). Location had a significant effect on SCP (F 4,290 = 18.542, P < 0.001), whereas gender did not (F 1,290 = 2.814, P = 0.095) and the location*gender interaction was not significant (F 4,290 = 0.531, P = 0.713). There was significant variation among populations in the SCPs of both males (F 4,145 = 7.179, P < 0.001) and females (F 4,145 = 12.631, P < 0.001). With the exception of the Nanchang males, male and female beetles of the Fuzhou population had significantly higher SCPs than beetles of the same gender in other populations, the latter being similar to one another (Fig. 3). There was no effect of gender on SCP in any population (Fuzhou: F 1,48 = 1.70, P = 0.201; Nanchang: F 1,48 = 0.07, P = 0.796; Miluo: F 1,48 = 0.42, P = 0.523; Wuchang: F 1,48 = 0.07, P = 0.792; Nanjing: F 1,48 = 0.41, P = 0.523). The frequency distribution of SCPs varied noticably among the five populations, lower SCPs tending to increase in frequency at higher latitudes (Fig. 4). Thus, the distribution of SCPs was skewed toward higher values in the Fuzhou beetles and lower values in the Wuchang and Nanjing beetles. Linear regression suggested no significant relationship between body weight and SCP for either males (F 148 = 0.52, P = 0.473) or females (F 148 = 0.08, P = 0.782).
Mean (+SE) supercooling points (°C) of O. communa adults from five geographic populations (n = 30). Columns bearing the same letters were not significantly different (LSD, α = 0.05) from others of the same gender (males: upper case letters, females: lower case letters). There were no significant differences (ANOVA, P > 0.05) between males and females within populations
Glycerol content
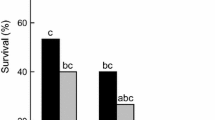
There were significant effects of location (F 4,37 = 34.522, P < 0.0001) and gender (F 1,37 = 5.976, P = 0.019) on O. communa glycerol content, but the location*gender interaction was not significant (F 4,37 = 1.494, P = 0.224). Both males (F 4,20 = 19.57, P < 0.001) and females (F 4,20 = 34.622, P < 0.0001) varied among populations in glycerol content (Fig. 5). The Fuzhou beetles had the lowest glycerol content, the Nanchang, Miluo and Wuchang beetles were intermediate, and the Nanjing beetles yielded values higher than all other populations. Glycerol content was not significantly affected by gender in any population (Fuzhou: F 1,8 = 0.03, P = 0.863; Nanchang: F 1,8 = 0.61, P = 0.479; Miluo: F 1,8 = 3.44, P = 0.137; Wuchang: F 1,8 = 0.17, P = 0.704; Nanjing: F 1,8 = 1.88, P = 0.247).
Mean (+SE) glycerol content (μg/mg) of O. communa adults from five geographic populations (n = 30). Columns bearing the same letters were not significantly different (LSD, α = 0.05) from others of the same gender (males: upper case letters, females: lower case letters). There were no significant differences (ANOVA, P > 0.05) between males and females within populations
Discussion
The physiological processes of cold tolerance in insects are mediated in complex ways and affect a variety of important life history traits (Régnière and Bentz 2007). Thus, geographic variation in insect cold hardiness is often of interest to entomologists and ecologists (Chen and Kang 2002b; Palmer and Siebke 2008; Worland et al. 2004). Of the climatic factors we compared among our sample locations, the most notable variation was in mean January temperature which declined sharply with increasing latitude (Table 1). Whereas many chrysomelid beetles have been reported to rely on freezing tolerance to cope with cold temperatures (Zachariassen et al. 2008), the adults of some species use ‘freeze avoidance’ (sensu Bale 1993) when overwintering (e.g., Hiiesaar et al. 2009) and our results indicate that O. communa uses the latter strategy. Previous work has shown that the cold hardiness of freeze-avoiding insects may be associated with increased glycerol content, reduced water content, and reduced SCP temperature (Yoder et al 2006; Ishiguro et al. 2007; Liu et al. 2007). For example, Sformo et al. (2010) examined the extremely cold tolerant beetle Cucujus clavipes puniceus (Coleoptera: Cucujidae) in Alaska and found that decreasing temperature during the onset of winter resulted in a five-fold reduction in body water content and concomitant increases in glycerol content. Generally, reduced water content serves to elevate the concentration of cryoprotectant substances such as glycerol and thus enhance cold hardiness (Danks 2000; Liu et al. 2007). Since our study examined newly emerged adults held under standardized summer conditions, the data reported here reflect inherent differences in the baseline cold hardiness of the sampled populations, rather than their ability to acclimate to the onset of cold weather.
Our results indicate that male beetles of the most northern O. communa population (Nanjing) had significantly lower water content than those of populations at lower latitudes, but the trend for females was inconsistent, those from Nanchang and Miluo being similar to those from Nanjing (Fig. 2). Since water content is inversely related to fat content, some variation in the former is likely attributable to variation in nutritional condition. Nanchang males had higher water content than their female counterparts, whereas the reverse was true for Wuchang males, suggesting that water content may be constrained by different factors in males and females. Furthermore, Wuchang lies in the Yangtze valley, a region with many lakes and moist conditions, a factor that may explain their relatively high water content in such a northern location. A study of cold tolerance in Chilo suppressalis populations in China revealed a similar pattern of geographic variation (Zhang et al. 2005).
SCPs have been used as an index of cold hardiness in many insects (Liu et al. 2007; Palmer and Siebke 2008; Worland and Convey 2008). High supercooling points are typically attributed to the absence of ice-nucleating agents or an accumulation of cryoprotectant elements, or some combination of both (Milonas and Savopoulou-Soultani 1999). Males and females of the Fuzhou population had significantly higher SCP values than their counterparts in other populations, although Nanchang males were not significantly different from Fuzhou males or those of any other population (Fig. 3). The frequency distributions of SCP values revealed some skew towards lower values in the three northern populations, an effect that appeared more pronounced among males, whereas values were more normally distributed in the two southern populations (Fig. 4). However, the data for glycerol content resolved populations into three distinct groups without any gender-based ambiguity. The northern population had the highest glycerol content and the southern the lowest, with the three other populations intermediate and not significantly different from one another (Fig. 5).
The observed differences among O. communa populations in baseline glycerol content suggest divergence among populations in baseline cold hardiness and this presumably correlates with their ability to acclimate to cold weather conditions. However, the biosynthesis of glycerol can be up- or down-regulated as a function of acclimation. Holden and Storey (1994) showed that feedback inhibition of aldolase regulated the biosynthesis of glycerol in Epiblema scudderiana (Lepidoptera: Tortricidae) such that an adequate glycerol pool was maintained during winter months, with cessation of synthesis at warmer temperatures. Thus, further studies are warranted to test for possible differences in the capacity of these populations to respond to cold weather with increased glycerol biosynthesis, and possibly seasonal changes in water content and SCP.
Common ragweed is an invasive weed in China with impacts on both agriculture and human health due to the allergenicity of its pollen. The present distribution of O. communa in China largely overlaps the range of common ragweed, especially throughout subtropical regions where the beetle is favored by warm temperatures (Zhou et al. 2010a) and high relative humidity (Zhou et al. 2010b). The biology of O. communa facilitates good phenological synchrony with its host plant in China, it is quite host specific to A. artemesiifolia (Meng and Li 2005), and there is potential for substantial impact on stand establishment and seed production in some areas (Meng et al. 2007). At present, the beetle occurs as far north as Tianchang (Anhui Province), Xuyi and Xuzhou (Jiangsu Province) (Meng et al. 2007). The results of the present study indicate that O. communa populations possess ecological plasticity with respect to cold hardiness that may facilitate further range expansion of this fortuitous biological control agent into more temperate regions of mainland China.
References
Asahina E (1969) Frost resistance in insects. Adv Insect Physiol 6:1–49
Bale JS (1993) Classes of insect cold hardiness. Funct Ecol 7:751–753
Bale JS (2002) Insects and low temperatures: from molecular biology to distributions and abundance. Philos T R Soc B 357:849–862
Carrillo MA, Heimpel GE, Moon RD, Cannon CA, Hutchison WD (2005) Cold hardiness of Habrobracon hebetor (Say) (Hymenoptera: Braconidae), a parasitoid of pyralid moths. J Insect Physiol 51:759–768
Chen B, Kang L (2002a) Cold hardiness and supercooling capacity in the pea leafminer, Liriomyza huidobrensis. Cryoletters 23:173–182
Chen B, Kang L (2002b) Analysis of trends of occurrence and geographic variation of pea leafminer, Liriomyza huidobrensis. Plant Quarant 16:138–140
Danks HV (1996) The wider integration of studies on insects cold-hardiness. Eur J Entomol 93:383–403
Danks HV (2000) Dehydration in dormant insect. J Insect Physiol 46:837–852
Futuyma DJ (1990) Observations on the taxonomy and natural history of Ophraella Wilcox (Coleoptera: Chrysomelidae), with a description of a new species. J New York Entomol S 98:163–186
Goto M, Li YP, Honma T (2001) Changes of diapause and cold hardiness in the Shonai ecotype larvae of the rice stem borer, Chilo suppressalis Walker (Lepidoptera: Pyralidae) during overwintering. Appl Entomol Zool 36:323–328
Hiiesaar K, Williams I, Luik A, Metspalu L, Muljar R, Jogar K, Karise R, Mand M, Svilponis E, Ploomi A (2009) Factors affecting cold hardiness in the small striped flea beetle, Phyllotreta undulata. Entomol Exp Appl 131:278–285
Hodkova M, Hodek I (1994) Control of diapause and supercooling by the retrocerebral complex in Pyrrhocoris apterus. Entomol Exp Appl 70:237–245
Holden CP, Storey KB (1994) Purification and characterization of aldolase from the cold hardy insect Epiblema scudderiana: enzyme role in glycerol biosynthesis. Insect Biochem Molec 24:265–270
Holmstrup M, Costanzo J, Lee RE Jr (1999) Cryoprotective and osmotic responses to cold acclimation and freezing in freeze-tolerant and freeze-intolerant earthworms. J Comp Physiol B 169:207–214
Ishiguro S, Li YP, Nakano K, Tsumuki H, Goto M (2007) Seasonal changes in glycerol content and cold hardiness in two ecotypes of the rice stem borer, Chilo suppressalis, exposed to the environment in the Shonai district, Japan. J Insect Physiol 53:392–397
Lee RE Jr (1991) Principles of insect low temperature tolerance. In: Lee RE Jr, Denlinger DL (eds) Insects at low temperature. Chapman & Hall, New York, pp 17–46
Lee RE Jr, Denlinger DL (1985) Cold tolerance in diapausing and non-diapausing stages of the flesh fly, Sarcophaga crassipalpis. Physiol Entomol 10:309–315
Li YP, Ding L, Goto M (2002) Seasonal changes in glycerol content and enzyme activities in overwintering larvae of the Shonai ecotype of the rice stem borer, Chilo suppressalis Walker. Arch Insect Biochem 50:53–61
Liu ZD, Gong PY, Wu KJ, Wei W, Sun JH, Li DM (2007) Effects of larval host plants on over-wintering preparedness and survival of the cotton bollworm, Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae). J Insect Physiol 53:1016–1026
Meng L, Li BP (2005) Advances on biology and host specificity of the newly introduced beetle, Ophraella communa Lesage (Coleoptera :Chrysomelidae), attacking Ambrosia artemisiifolia (Compositae) in continent of China. Chin J Biol Control 21:65–69
Meng L, Xu J, Li HB (2007) Dispersal and bionomics of the alien Ophraella communa in China mainland. Chin J Biol Control 2:5–10
Milonas P, Savopoulou-Soultani M (1999) Cold hardiness in diapause and non-diapause larvae of the summer fruit tortrix, Adoxophes orana (Lepidoptera: Tortricidae). Eur J Entomol 96:183–187
Montiel PO (1998) Profiles of soluble carbohydrates and their adaptive role in maritime Antarctic arthropods. Polar Biol 19:250–256
Muise AM, Storey KB (1999) Regulation of glycogen synthetase in a freeze-avoiding insect: role in cryoprotectant glycerol synthesis. CryoLetters 20:223–228
Nedvěd O, Lavy D, Verhoef HA (1998) Modelling the time-temperature relationship in cold injury and effect of high temperature interruptions on survival in a chill-sensitive collembolan. Funct Ecol 12:816–824
Neven LG (1999) Cold hardiness adaptations of codling moth, Cydia pomonella. Cryobiology 38:43–50
Palmer WA, Goeden RD (1991) The host range of Ophraella communa Lesage (Coleoptera: Chrysomelidae). Coleopts Bull 45:115–120
Palmer CM, Siebke K (2008) Cold hardiness of Apteropanorpa tasmanica Carpenter (Mecoptera: Apteropanorpidae). J Insect Physiol 54:1148–1156
Pfister TD, Storey KB (2006) Responses of protein phosphatases and cAMP-dependent protein kinase in a freeze-avoiding insect, Epiblema scudderiana. Arch Insect Biochem 62:43–54
Régnière J, Bentz B (2007) Modeling cold tolerance in the mountain pine beetle, Dendroctonus ponderosae. J Insect Physiol 53:559–572
Rivers DB, Lee RE Jr, Denlinger DL (2000) Cold hardiness of the fly pupal parasitoid Nasonia vitripennis is enhanced by its host Sarcophaga crassipalpis. J Insect Physiol 46:99–106
Salt RW (1961) Principles of insect cold-hardiness. Ann Rev Entomol 6:55–74
SAS Institute (2004) SAS user’s® guide: statistics. SAS Institute, Cary, NC
Sformo T, Walters K, Jeannet K, Wowk B, Fahy GM, Barnes BM, Duman JG (2010) Deep supercooling, vitrification and limited survival to −100°C in the Alaskan beetle Cucujus clavipes puniceus (Coleoptera: Cucujidae) larvae. J Exp Biol 213:502–509
Shiyake S, Moriya S (2005) Expansion of Ophraella communa LeSage in East Asia. Kontyu to Shizen 40:11–13
Sinclair BJ (1999) Insect cold tolerance: how many kinds of frozen? Eur J Entomol 96:157–164
Takizawa HA, Saito A, Sato K, Hirano Y, Ohno M (1999) Invading insect, Ophraella communa LeSage, 1986. Range expansion and life history in Kanto District, Japan. Gekkanlushi 338:26–31
Tamura Y, Hattori M, Konno K, Kono Y, Honda H, Ono H, Yoshida M (2004) Triterpenoid and caffeic acid derivatives in the leaves of ragweed, Ambrosia artemisiifolia L. (Asterales: Asteraceae), as feeding stimulants of Ophraella communa LeSage (Coleoptera: Chrysomelidae). Chemoecology 14:113–118
Tran JK, Ylioja T, Billings RF, Régnière J, Ayres MP (2007) Testing a climatic model to predict population dynamics of a forest pest, Dendroctonus frontalis (Coleoptera: Scolytidae). Ecol Appl 17:882–899
Tsumuki H (1990) Environmental adaptations of the rice stem borer, Chilo suppressalis, and the blue alfalfa aphid, Acyrthosiphon kondoi, to seasonal fluctuations. In: Hoshi M, Yamashita O (eds) Advances in invertebrate reproduction, vol 5. Elsevier Science Publishers, Amsterdam, pp 273–278
Tsumuki H, Kanehisa K (1978) Carbohydrate content and oxygen uptake in larvae of rice stem borer, Chilo suppressalis Walker. Ber Ohara Inst Landwirt Biol Okayama Univ 17:98–110
Wang HS, Kang L (2005) Effect of cooling rates on the cold hardiness and cryoprotectant profiles of locust eggs. Cryobiology 51:220–229
Wang HS, Zhou CS, Guo W, Kang L (2006) Thermoperiodic acclimations enhance cold hardiness of the eggs of the migratory locust. Cryobiology 53:206–217
Watanabe M, Hirai Y (2004) Host-use pattern of the ragweed beetle Ophraella communa LeSage (Coleoptera: Chrysomelidae) for overwintering and reproduction in Tsukuba. Appl Entomol Zool 39:249–254
Worland MR, Convey P (2008) The significance of the moult cycle to cold tolerance in the Antarctic collembolan Cryptopygus antarcticus. J Insect Physiol 54:1281–1285
Worland MR, Wharton DA, Byars SG (2004) Intracellular freezing and survival in the freeze tolerant alpine cockroach Celatoblatta quinquemaculata. J Insect Physiol 50:225–232
Yamazaki K, Imai C, Natuhara Y (2000) Rapid population growth and food-plant exploitation pattern in an exotic leaf beetle, Ophraella communa LeSage (Coleoptera: Chrysomelidae), in western Japan. Appl Entomol Zool 35:215–223
Yoder JA, Benoit JB, Denlinger DL, Rivers DB (2006) Stress-induced accumulation of glycerol in the flesh fly, Sarcophaga bullata: Evidence indicating anti-desiccant and cryoprotectant functions of this polyol and a role for the brain in coordinating the response. J Insect Physiol 52:202–214
Zachariassen KE (1985) Physiology of cold tolerance in insects. Physiol Rev 65:799–832
Zachariassen KE, Li NG, Laugsand AE, Kristiansen E, Pedersen SA (2008) Is the strategy for cold hardiness in insects determined by their water balance? A study on two closely related families of beetles: Cerambycidae and Chrysomelidae. J Comp Physiol B 178:977–984
Zhang J, Wu KM, Lin KJ, Li HG, Guo YY (2005) Diapause characteristics and cold-hardiness of temperate and subtropical populations in Chilo suppressalis. Sci Agric Sin 38:2451–2456
Zhou ZS, Guo JY, Chen HS, Wan FH (2010a) Effects of temperature on survival, development, longevity and fecundity of Ophraella communa (Coleoptera: Chrysomelidae), a biological control agent against invasive ragweed, Ambrosia artemisiifolia L. (Asterales: Asteraceae). Environ Entomol 39:1021–1027
Zhou ZS, Guo JY, Chen HS, Wan FH (2010b) Effects of humidity on the development and fecundity of Ophraella communa (Coleoptera: Chrysomelidae). Biocontrol 50:313–319
Acknowledgments
We thank Dr. Rui Wang of our Laboratory, Mr. Hong-Song Chen, Mr. Min Luo, Ms. Wei Guo and Dr. You-Zhi Li (Hunan Agricultural University), Mr. Xing-Wen Zheng, Mr. Yong-Xiang Fang and Ms. Hai-Yan Zheng (Jiangxi Agricultural University), Prof. Yuan-Hua Luo (Institute of Plant Protection, Hunan Academy of Agricultural Sciences) for their assistance with experiments. This work was funded by the National Basic Research and Development Program of China, Grant No. 2009CB119200, and the National Key Technologies Research and Development Program of China No. 2006BAD08A18. We are thankful to two anonymous reviewers whose commentary strengthened the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Drs. Zhou and Guo are joint first authors of this manuscript (equal contribution).
Rights and permissions
About this article
Cite this article
Zhou, ZS., Guo, JY., Michaud, J.P. et al. Variation in cold hardiness among geographic populations of the ragweed beetle, Ophraella communa LeSage (Coleoptera: Chrysomelidae), a biological control agent of Ambrosia artemisiifolia L. (Asterales: Asteraceae), in China. Biol Invasions 13, 659–667 (2011). https://doi.org/10.1007/s10530-010-9857-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-010-9857-x