Abstract
The strategy for cold-hardiness and water balance features of two closely related families of Coleoptera, Cerambycidae and Chrysomelidae, were investigated. Cerambycids were freeze-avoiding with low supercooling points, whereas chrysomelids froze at high temperatures and were tolerant to freezing. Hence, the two families have adopted different strategies for cold-hardiness. Due to their low trans-cuticular water permeability, the cerambycids have low rates of evaporative water loss. Chrysomelids have much higher trans-cuticular water permeability, but freezing brings their body fluids in vapour pressure equilibrium with ice and prevents evaporative water loss. The differences in cold-hardiness strategies and rates of water loss are likely to reflect the water content of the diets of the two families. Cerambycids feed on dry wood with low water content, causing a restrictive water balance. Chrysomelids feed on leaves with high water content and may use evaporation through the cuticle as a route of water excretion. Haemolymph ice nucleators help chrysomelids to freeze at a high temperature and thus to maximize the period they spend in the water saving frozen state. The diet-related differences in water balance may be the reason why the two families have developed different strategies for cold-hardiness.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Insects use two main strategies for cold-hardiness. They may seek to avoid freezing, in many cases by allowing their body fluids to stay supercooled at low temperatures, or they may develop tolerance to freezing (Salt 1961; Sinclair 1999).
Many freeze-avoiding insects remove from their body fluids or inactivate all particles that may trigger freezing. This process brings the temperature of spontaneous nucleation (the supercooling point) down to about −20°C (Zachariassen and Kristiansen 2000; Zachariassen et al. 2004a). A further depression of the supercooling points can be achieved by the accumulation of polyols, which in the middle of the winter may reach multi-molal concentrations. Freeze-avoiding insects also produce antifreeze proteins (AFPs), which in various ways stabilize the insects in the supercooled state (Olsen and Duman 1997a, b; Zachariassen and Kristiansen 2000; Ramløv 2000).
Insects tolerant to freezing establish a protective extracellular freezing at a high subzero temperature, often by the aid of potent extracellular ice nucleating agents (INAs), produced specifically for this function (Zachariassen and Hammel 1976). Also hibernating freeze-tolerant insects may produce high levels of polyols, which increase their cold-hardiness by reducing the amount of body water that freezes in a colligative manner (Zachariassen 1979; van der Laak 1982).
The factors causing some insects to develop freeze-avoidance and others to become tolerant to freezing are not fully understood, but as outlined in the following paragraphs, the topic has been addressed by several authors.
The cold hardening effect of the polyols appears to be considerably stronger in freeze-tolerant insects than in freeze-avoiding ones (Zachariassen 1980), and freeze-tolerance seems to be the preferred strategy for cold-hardiness among insects hibernating in extremely cold continental regions such as Canada and inner Alaska (Miller 1982; Ring 1982).
Storey and Storey (1986) contended that there is a limit to how long freeze-tolerant organisms can stay frozen before anaerobic metabolites become accumulated to harmful levels. This would counteract freezing tolerance among organisms exposed to freezing conditions for long periods.
Freezing tolerance seems to offer advantages with regard to water balance. As pointed out by Lundheim and Zachariassen (1993), the vapour pressure of ice is lower than that of supercooled water at the same temperature, and hibernating supercooled insects may lose substantial amounts of body water (Ring 1982). However, they limit their water loss by reducing the water permeability of the body wall to a minimum. In the frozen state the body fluids of freeze-tolerant insects are in vapour pressure equilibrium with ice. Frozen insects will neither lose nor gain water during hibernation, and they seem generally to have leaky body walls.
In alpine areas in the Arctic and temperate regions the temperatures fluctuate considerably on a daily basis, and temperatures well below 0°C may frequently occur at night even in the summer. Insects living in such areas are active during the day, but exposed to subfreezing temperatures at night. They feed during day and have food particles with a nucleating capacity in the intestine even at night. This makes it impossible to meet the cold with a freeze-avoidance strategy. Instead they have developed freezing tolerance and produce potent extracellular ice nucleators, which trigger freezing at a high subzero temperature (van der Laak 1982). The same freezing tolerance strategy is developed among insects which live under similar conditions in tropical alpine regions (Sømme and Zachariassen 1981).
Voituron et al. (2002) approached the problem by using a mathematical fitness model, based on pre-hibernation energy stores and energy consumption during hibernation as limiting factors for survival. Supercooled insects have a low but still significant energy consumption, whereas insects in the frozen state consume very little energy. Hence, at the end of the winter, frozen insects may be expected to have greater energy stores than freeze-avoiding ones. The authors did not include water balance in their model, because they assumed that both freeze-avoiding and freeze-tolerant organisms bring their body fluid into vapour pressure equilibrium with ice by the production of polyols.
The strategy for cold-hardiness seems also to be related to the ionic composition of the body fluids. Many groups of insects differ from other animals in having low extracellular concentrations of sodium, but among organisms with the normal high extracellular concentration of sodium freeze-tolerance seems to be the preferred strategy for cold-hardiness (Zachariassen et al. 2004b). The adaptive value of this is not known.
The beetle families Cerambycidae (Longhorn beetles) and the Chrysomelidae (leaf beetles) are closely related, in that they both belong to the suborder Phytophaga. As the name of the suborder indicates, the beetles of both families feed on plant material, and the beetles of both families are likely to have the low extracellular sodium concentrations characteristic of phytophagous insects (Sutcliffe 1963; Jeuniaux 1971). This contention is supported by the few measurements carried out on these beetles. The haemolymph of the cerambycid Rhagium inquisitor has about 40 mM Na+, 40 mM K+ and 55 mM Mg++ (Dissanayake and Zachariassen 1980), whereas the haemolymph of the chrysomelid Leptinotarsa decemlineata has about 20 mM Na+, 25 mM K+ and 80 mM Mg++ (Sutcliffe 1963). However, the few species of cerambycids investigated so far are all freeze-avoiding, whereas the chrysomelids are tolerant to freezing (Lundheim and Zachariassen 1993; Ødegaard et al. 2003; van der Laak 1982). The adaptive importance of this is not understood.
The purpose of the present study has been to make a more comprehensive investigation of the cold-hardiness strategies of species of the two families and more precisely to see if the evolution of the strategies can be explained by the water balance features of the beetles.
Materials and methods
Adult beetles used for the present investigation were collected in the winter and summer from their natural sites in various locations in Norway. Winter beetles were collected from their hibernation sites and kept in the laboratory at 0 to +5°C for up to 1 month before they were used in the experiments, whereas summer beetles were held at room temperature (20°C) for up to 2 weeks.
Some species of beetles are hard to find in their natural habitat in the winter. This is the case for cerambycid species such as Molorchus minor, Semanotus undatus and Acanthocinus aedilis. Adult beetles of the latter species leave their pupal chamber under the bark of pine logs in the early autumn and move to an unknown hibernation site. For this reason the measurements were made on laboratory acclimated specimens collected from timber logs in early September. Except for the Phyllodecta species, all chrysomelids are difficult to find in the winter. For this reason the number of specimens was low for a number of species used in the present investigation.
The supercooling point of the beetles was measured by cooling the beetles in close contact with a copper-constantan thermocouple probe. The beetles and the probe were wrapped in Scotch tape and placed inside a sample holder consisting of three plastic tubes with different diameters, one outside the other, to reduce the rate of cooling. The sample holder was placed inside a deep freezer (−25°C) or a Binder temperature regulated chamber (−40°C). A reference thermocouple probe was placed at 0°C in a thermos with an ice–water mixture. The thermocouples generate an output of 40 μV per degree temperature difference, and they were connected to a voltage line recorder with a span of 2mV. The freezing of the beetles was indicated by a sudden increase in temperature due to the release of heat of fusion of water. The lowest temperature recorded prior to the temperature increase was taken as the supercooling point.
The effect of freezing was evaluated after allowing the beetles to freeze out until the temperature had returned a second time to the supercooling point. The evaluation of survival was based on behavioural criteria, in that only beetles which were able to move in a normal co-ordinated manner were classified as survivors (Zachariassen 1979). In the present study there was no doubt about the effects, because the beetles either survived freezing in a very good shape or they were seemingly dead.
The polyol accumulation of the beetles was evaluated from the haemolymph osmolality. Polyol-free insects have a body fluid osmolality of 300–500 mOsm, whereas accumulation of polyols may bring the osmolality up to several thousand mOsm.
Samples of haemolymph were taken from the beetles and handled as described by Zachariassen et al. (1982). The cuticle on the ventral side of the thorax was pierced with a needle, and the exuding haemolymph was drawn into the attenuated end of a flame drawn glass capillary tube by means of the capillary force. To avoid evaporation from the sample a droplet of paraffin oil was added to the capillary tube, which was then closed in the wide end by melting in a flame. The capillary tube was centrifuged for about 15 s in a Compur microcentrifuge, bringing the body fluid sample down to the closed end of the tube, isolated from air by a column of paraffin oil.
The haemolymph osmolality was determined from the melting point (MP) on a Clifton nanolitre osmometer. This instrument allows tiny (20 nL) samples of haemolymph to be observed through a binocular microscope while the temperature is controlled within ±0.01°C. The samples were frozen by rapid cooling to −40°C and then allowed to warm up slowly. As the frozen sample is warmed, the amount of ice is gradually reduced, and the temperature at which the last tiny ice crystal disappears is taken as the MP. The instrument displays the osmolality values, which have been calculated from the MPs via the osmolal melting point depression (1.86°C/Osm).
The presence of antifreeze proteins (AFPs) in the body fluid was indicated by measuring the thermal hysteresis activity in the haemolymph. AFPs have the capacity to prevent the growth of ice crystals normally seen when partly frozen fluid samples undergo cooling. Failure of the crystal to grow when the sample is slowly cooled reveals the presence of AFPs. The antifreeze effect of the AFPs is limited in that following sufficient cooling there is a sudden rapid growth of the crystal. The separation of the MP and the temperature of ice growth is referred to as thermal hysteresis, and the temperature of ice growth is referred to as the hysteresis freezing point (HFP). The difference between the MP and the HFP is referred to as the hysteresis activity. Freeze-avoiding insects may have a hysteresis activity of up to about 5°C. The measurements of thermal hysteresis were carried out on the same samples as were used for MP measurements by refreezing the sample and allowing a small ice crystal to remain.
The total evaporative water loss (TWL) was determined as loss of body mass while the beetles were kept inside a desiccator with silica gel (relative air humidity ≈5%) at 20°C. The body mass was measured on a Mettler analytical balance (exact within ±0.1mg) or on a Cahn electronic balance (exact within ±0.1μg).
The metabolic rate (M) was measured as rate of oxygen consumption by the use of Engelmann constant pressure respirometers (Engelmann 1963) as described by Røskaft et al. (1986). All values were recalculated to 760 mmHg and 20°C.
The cuticular water permeability of the beetles was expressed as the fraction that cuticular water loss makes up of the total water loss. These fractions were estimated by relating the water loss data of the present species to a curve obtained for tropical desert beetles by Zachariassen et al. (1987). The rate of water loss and the metabolic rate were measured, and the values were plotted in a double logarithmic diagram. Zachariassen et al. (1987) found that when plotted in this way, the rates of water loss of various species of tenebrionids and carabids from East African dry savannah are linearly related to their metabolic rate. Other results, obtained by Zachariassen and Maloiy (1989) and Zachariassen (1991), indicate that the body wall of these beetles is almost completely water-proof, implying that the line relating their rates of water to their metabolic rates provides a good approximate measure of the rate of respiratory water loss.
Lundheim and Zachariassen (1993) found that the values of water loss and metabolic rate of freeze-avoiding beetles were situated along the line obtained for the desert beetles, i.e. the beetles have reduced their cuticular water permeability so much that their water loss is predominantly respiratory. The freeze-tolerant species had rates of water loss well above this line, indicating that these beetles have a substantial trans-cuticular water loss.
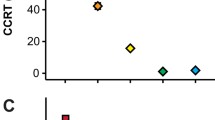
In accordance with the method described earlier, the logarithmic values of total water loss (log TWL) of the species of the present study were plotted against the respective logarithms of the metabolic rates (log M), together with the line from the African desert beetles as shown in Fig. 1. The respiratory water loss (log RWL) of a species can be estimated as the ordinate value of the line corresponding to the log M value of the same species. This value can be found by inserting the log M value into the formula for the line shown in Fig. 1 (log RWL = 0.902 log M – 3.48). Log RWL − log TWL represents the logarithm of the quotient between RWL and TWL, and the fraction of respiratory rate of water loss (FRWL) of the total rate of water loss can be found as the antilogarithm of this difference
Logarithmic values of rates of water loss of cerambycids (squares) and chrysomelids (triangles) as a function of the logarithm of their metabolic rate. Open symbols represent values measured on summer beetles, whereas closed symbols are measured on winter hardened beetles. Values are taken from Table 2. The solid line represents corresponding data for East African desert beetles obtained by Zachariassen et al. (1987)
The fraction of trans-cuticular water loss of the total water loss (FTCWL) can be estimated by subtracting FRWL from the fraction of rate of total water loss (=1) as shown in formula 2
The statistical significance of the difference between groups was tested by the use of Student’s t test (Bailey 1974).
Results
The cold-hardiness data of seven species of winter hardened cerambycids and five species of winter hardened chrysomelids are shown in Table 1. The results in Table 1 show that all the seven species of cerambycids are freeze-avoiding. Most of the species have supercooling points around or below −20°C, and they have a substantial thermal hysteresis activity in their body fluids. Some of the species also accumulate solutes to multi-molal levels.
All the five species of investigated chrysomelids had supercooling points above −10°C, and all were tolerant to freezing. They have potent INAs in their haemolymph, and with one exception they accumulate polyols to multi-molal levels. None of the chrysomelids were found to have thermal hysteresis activity in their body fluids.
The metabolic rates and rates of water loss of beetles of the two families are shown in Table 2 together with their average body mass. The logarithm of the rates of water loss and the metabolic rates are plotted in Fig. 1 together with the curve of Zachariassen et al. (1987), relating respiratory water loss of East African desert tenebrionids and carabids to their metabolic rate.
Figure 1 shows that the points representing metabolism and water loss of cerambycids are distributed around the line representing African desert beetles, whereas the points representing chrysomelids are situated above the line, in most cases far above. Summer and winter individuals of the same species are situated clearly apart. The summer beetles have generally a higher metabolic rate and hence also a higher rate of water loss, which can be attributed mainly to the high respiratory water loss.
The quotients between trans-cuticular and total water loss can be estimated from the data in Fig. 1 by using Eq. 2. The calculated quotients of the cerambycids and chrysomelids in the present study are shown in Table 3. The quotients in Table 3 appear to differ substantially between the two families. For cerambycid beetles the mean quotient is 0.0278 ± 0.443, whereas for chrysomelids the mean quotient is 0.727 ± 0.175. The quotients are significantly different on level P < 0.002 (Student’s t test, t = 4.098, n–2 = 12). This implies that for cerambycids the average trans-cuticular water loss is only about 3% of the total water loss, whereas for the chrysomelids the corresponding value is 73%.
Discussion
The results indicate that the winter acclimated cerambycid beetles are generally freeze-avoiding, with supercooling points of −20°C or lower, and with substantial thermal hysteresis in the haemolymph. An exception is the timberman beetle Acanthocinus aedilis, which froze just below −10°C and displayed no hysteresis activity in the haemolymph. These beetles appear to hibernate at protected sites near the ground and are probably not exposed to the low temperatures that prevail above the snow level in the winter.
The chrysomelids on the other hand, seem generally to be freeze-tolerant. Most species accumulate polyols to multimolal levels in the winter, suggesting that they are highly cold-tolerant. One species, Hydrothassa marginella, did not show elevated haemolymph osmolality in the winter. This is probably related to the fact that these beetles live in damp places (Linssen 1959) such as marsh areas and riverbanks, where they hibernate in humid soil or under stones. In such locations they are likely to be protected against exposure to very low temperatures.
The two families are closely related, and the beetles of both families are herbivorous. They are then also likely to have similar ionic compositions in the body fluids (Sutcliffe 1963; Jeuniaux 1971). This raises the question as to which factors have caused the beetles of the two families to evolve different strategies for cold-hardiness.
Lundheim and Zachariassen (1993) contended that freeze-avoiding insects must limit their evaporative water loss by having a body wall with low water permeability, whereas freeze-tolerant insects, which are in vapour pressure equilibrium with ice as long as they are frozen, may avoid an intolerable water loss in spite of having a leaky body wall.
The results of the present study seem to be in accordance with this contention. Figure 1 shows that the freeze-avoiding cerambycids have rates of water loss that are situated close to the curve for African dry habitat beetles, i.e. they have a very low cuticular water permeability. As shown in Table 3, the mean quotient between the trans-cuticular water loss and the total water loss of the cerambycids is 0.028, implying that only about 3% of the total water loss goes through the cuticle. The chrysomelids have in most cases rates of water loss that are far above the line for African desert beetles. The mean quotient between the trans-cuticular water loss and total water loss for the chrysomelids is 0.727, i.e. 73% of the water loss is trans-cuticular. The chrysomelids Chrysomela populi and Linaeidea aenea seem to be exceptions, in that in the summer their fraction of cuticular water loss was of the same magnitude as that of the cerambycids with the highest rates of water loss. Nevertheless, the water balance features of the beetles of two families seem largely to be related to their strategies for cold-hardiness as observed by Lundheim and Zachariassen (1993).
Voituron et al. (2002) did not include the water balance aspect in their fitness model based on pre-hibernation energy stores and energy consumption during hibernation. The authors contended that vapour pressure equilibrium is achieved by organisms using either of the two strategies; freeze-tolerant organisms by being in vapour pressure equilibrium with ice as long as they stay frozen, and freeze-avoiding organisms by accumulating polyols to reach vapour pressure equilibrium with external ice. The latter contention is not correct because the vapour pressure of ice is lower than that of supercooled water at the same temperature. The freeze-avoidance strategy involves hibernation in a supercooled state, and hibernating supercooled insects are thus per definition not in vapour pressure equilibrium with ice. Furthermore, the solute concentrations required to reach vapour pressure equilibrium with ice are considerably higher than those normally seen in hibernating insects. To be in vapour pressure equilibrium at −15°C they must accumulate polyols to give the body fluids a melting point of −15°C, which corresponds to an osmolality of 8 Osm. At lower temperatures, even higher concentrations are required to attain vapour pressure equilibrium. This contention is supported by data presented by Ring (1982), who found that larvae of the freeze-avoiding beetle Pytho deplanatus hibernating in inner Canada use dehydration to increase their body fluid concentration. When larvae with a body fluid concentration of about 3.4 Osm were kept at −15°C for 2 weeks, their relative body water content dropped from 41 to 30%. This body fluid concentration is about the highest seen in ordinary hibernating insects, but it still did not bring the body fluid in vapour pressure equilibrium at −15°C. This implies that freeze-avoiding insects without the dehydration tolerance of P. deplanatus must rely on a nearly water impermeable body wall to preserve their body water during hibernation. Consequently the water balance and the body wall permeability may be an important factor to determine the choice of cold-hardiness strategy.
Lundheim and Zachariassen (1993) concluded that the water balance features of beetles have developed as a result of their strategy for cold-hardiness, but this does not explain why the beetles of the two families investigated here consistently follow different strategies of cold-hardiness. Hence, one may speculate whether it is the other way around, i.e. that the difference in cold-hardiness strategy may reflect the differences in the water balance situation of the beetles.
The reason for the differences in the water balance of the two beetle families is likely to be the different water contents of their diet. With few exceptions cerambycids feed on wood with low-relative water content. According to data for firewood, fresh wood may have a water content of about 50% (water weight/dry weight), whereas dry dead wood may have a water content of only about 10%. The cerambycids in the present study all develop in dead and relatively dry wood. Hence, due to their dry diet cerambycids must have a restrictive water budget, reflected in their low cuticular water permeability. The chrysomelids on the other hand feed on leaves with water content up to above 80% (Evans and Ting 1974), and they probably have a dietary surplus of water. In addition to urinary water loss, they may use evaporative water loss through the cuticle as a route of water excretion. This indicates that it is the dietary water content which determines the strategy of cold-hardiness of the two beetle families. The low cuticular water permeability of the cerambycids prevents a comprehensive water loss, even when they hibernate in a supercooled state. The water-rich diet causes most chrysomelids to have a highly water permeable cuticle, but by staying frozen most of the winter they may limit their water loss to a tolerable minimum.
It is important for the chrysomelids to stay frozen most of their hibernation period and thus reduce the dangerous period in the supercooled state to a minimum. By adding potent ice nucleators to their haemolymph, the freeze-tolerant beetles will enter the frozen state at a high subzero temperature and extend the water saving frozen period. In addition to the freeze-protective function of the extracellular ice nucleators, the need to maintain water balance may be a major factor behind the evolution of ice nucleating agents.
Two species of chrysomelids, C. populi and L. aenea, had relatively low rates of water loss, i.e. in the range of the most leaky cerambycids. C. populi is still tolerant to freezing. This indicates that the development of freezing tolerance may also have been promoted by other factors than water balance. As pointed out by van der Laak (1982), several of the chrysomelid species live in high altitude inland areas, where the temperature may drop well below 0 even in the summer. The beetles remain on the leaves throughout the night, and are therefore exposed to subfreezing temperatures. Since they are feeding during the day, they are likely to have ice nucleating food particles in their intestine even at night. The beetles prevent an injurious freezing of the intestine by producing potent extracellular ice nucleators, which trigger a protective freezing in the haemolymph at a high subzero temperature. Hence, the freezing tolerance in at least some chrysomelids may also have been promoted by cold exposure during their activity period in the summer.
The results for the three species that were measured both summer and winter (C. populi, P. viminalis and R. mordax) may suggest that there are seasonal differences in the rates of water loss, in that all three species display higher rates of the evaporative water loss in summer than in winter (see Fig. 1). They also display elevated respiratory rates in the summer, and the increased rates of water loss in the summer can to a great extent be ascribed to an increased respiratory water loss. The seasonal variations in rates of water loss should be the object of further studies.
Abbreviations
- AFP:
-
Antifreeze protein
- FRWL:
-
Fraction of respiratory water loss.
- FTCWL:
-
Fraction of trans-cuticular water loss
- HFP:
-
Hysteresis freezing point
- INA:
-
Ice nucleating agent
- M:
-
Metabolic rate
- MP:
-
Melting point
- RWL:
-
Rate of respiratory water loss
- TWL:
-
Rate of total water loss
References
Bailey NTJ (1974) Statistical Methods in Biology. Unibooks, English Universities Press, London, 200 pp
Dissanayake P, Zachariassen KE (1980) Effect of warm acclimation on the cationic concentrations in the extracellular and intracellular body fluid of hibernating Rhagium inquisitor beetles. Comp Biochem Physiol A 65:347–350
Engelmann MD (1963) A constant pressure respirometer for small arthropods. Entomol News 74:181–187
Evans LS, Ting IP (1974) Ozone sensitivity of leaves: relationship to leaf water content, gas transfer resistance and anatomical characteristics. Am J Bot 61:592–597
Jeuniaux C (1971) Hemolymph—Arthropoda. In: Florkin H, Scheer BT (eds) Chemical Zoology, vol VI. Academic Press, New York, pp 63–118
van der Laak S (1982) Physiological adaptations to low temperature in freezing tolerant Phyllodecta laticollis beetles. Comp Biochem Physiol A 73:613–620
Linssen EF (1959) Beetles of the British Isles II. Frederick Warne & Co. Ltd., London, 295 p
Lundheim R, Zachariassen KE (1993) Water balance of over-wintering beetles in relation to strategies for cold tolerance. J Comp Physiol B 163:1–4
Miller LK (1982) Cold-hardiness strategies of some adult and immature insects overwintering in interior Alaska. Comp Biochem Physiol A 73:595–604
Ødegaard F, Ramløv H, Zachariassen KE (2003) Thermal hysteresis, cold stress and species distribution. In: Ivanov B, Maximov T (eds) Influence of climatic and ecological changes on permafrost ecosystems. Publishing House of the Siberian Division of the Russian Academy of Sciences, Yakutsk, pp 190–193
Olsen TM, Duman JG (1997a) Maintenance of the supercooled state in the overwintering pyrochroid beetle larvae, Dendroides canadensis: role of hemolymph ice nucleators and antifreeze proteins. J Comp Physiol B 167:105–113
Olsen TM, Duman JG (1997b) Maintenance of the supercooled state in the gut fluid of overwintering pyrochroid beetle larvae, Dendroides canadensis: role of ice nucleators and antifreeze proteins. J Comp Physiol B 167:114–122
Ramløv H (2000) Aspects of natural cold tolerance in ectothermic animals. Hum Rep 15:26–46
Ring R (1982) Freezing-tolerant insects with low supercooling points. Comp Biochem Physiol A 73:605–612
Røskaft E, Zachariassen KE, Maloiy GMO, Kamau JMZ (1986) Temperature regulation and water balance of day-active Zophosis congesta beetles in East Africa. J Trop Ecol 2:139–146
Salt RW (1961) Principles of insect cold hardiness. Ann Rev Entomol 6:55–74
Sinclair BJ (1999) Insect cold tolerance: how many kinds of frozen? Eur J Entomol 96:157–164
Sømme L, Conradi-Larsen EM (1979) Frost resistance in alpine, adult Melasoma collaris (Coleoptera). Oikos 33:80–84
Sømme L, Zachariassen KE (1981) Adaptations to low temperatures in high altitude insects from Mount Kenya. Ecol Entomol 6:199–204
Storey KB, Storey JM (1986) Freeze-tolerance and intolerance as strategies of winter survival of terrestrially-hibernating amphibians. Comp Biochem Physiol A 83:613–617
Sutcliffe DW (1963) The chemical composition of haemolymph in insects and some other arthropods in relation to their phylogeny. Comp Biochem Physiol 9:121–135
Voituron Y, Mouguet N, de Mazancourt C, Claubert J (2002) To freeze or not to freeze? An evolutionary perspective on the hold-hardiness strategies of overwintering ectotherms. Am Nat 160:255–270
Zachariassen KE (1979) The mechanism of the cryoprotective effect of glycerol in beetles tolerant to freezing. J Insect Physiol 25:29–32
Zachariassen KE (1980) The role of polyols and nucleating agents in cold-hardy beetles. J Comp Physiol 140:227–234
Zachariassen KE (1991) Routes of transpiratory water loss in a dry habitat tenebrionid beetle. J Exp Biol 157:425–437
Zachariassen KE, Hammel HT (1976) Nucleating agents in the haemolymph of insects tolerant to freezing. Nature 262:285–287
Zachariassen KE, Kristiansen E (2000) Ice nucleation and antinucleation in nature. Cryobiology 41:257–279
Zachariassen KE, Maloiy GMO (1989) Water balance of beetles as an indicator of environmental humidity. Fauna Norvegica B 36:27–31
Zachariassen KE, Baust JG, Lee RE Jr (1982) A method for quantitative determination of ice nucleating agents in insect hemolymph. Cryobiology 19:180–184
Zachariassen KE, Andersen J, Maloiy GMO, Kamau GMZ (1987) Transpiratory water loss and metabolism of beetles from arid areas in East Africa. Comp Biochem Physiol A 68:403–408
Zachariassen KE, Kristiansen E, Pedersen SA, Hammel HT (2004a) Ice nucleation in solutions and freeze-avoiding insects–homogeneous or heterogeneous? Cryobiology 48:309–321
Zachariassen KE, Kristiansen E, Pedersen SA (2004b) Inorganic ions in cold-hardiness. Cryobiology 48:126–133
Acknowledgments
The present study was supported by the Norwegian Research Council (NRF) over grant no. 1167106/V40 to Karl Erik Zachariassen. The authors would like to thank Christer Reiråskag for help with collecting the beetles. The present experiments comply with the “Principles of Animal Care”, publication No. 86-23, revised 1985 of the National Institute of Health and also with the current laws of Norway.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by H.V. Carey.
Rights and permissions
About this article
Cite this article
Zachariassen, K.E., Li, N.G., Laugsand, A.E. et al. Is the strategy for cold hardiness in insects determined by their water balance? A study on two closely related families of beetles: Cerambycidae and Chrysomelidae. J Comp Physiol B 178, 977–984 (2008). https://doi.org/10.1007/s00360-008-0284-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-008-0284-6