Abstract
The little fire ant, Wasmannia auropunctata, probably arrived in Israel in ca. 1998 and was identified in 2005; this is the first record of this species from open areas outside the tropics and subtropics. It survives harsher conditions than in its native habitats, with minimal annual temperatures as low as 6°C, and 5–12 consecutive rainless months (under 15 mm rainfall per month). It is now known from 26 localities in Israel, mostly in irrigated gardens. As in other regions where they have invaded, these ants pose a serious threat to local biodiversity. At high densities they displaced almost all the local ant species sampled, affecting population abundances, species richness, and community structure. W. auropunctata seems to have a detrimental effect also on other ground arthropods, judging from the observed decline in spider and beetle abundances. We show here that this tropical species can pose a critical threat to local arthropods at a wider range of climatic conditions than was previously known.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Invasive species are regarded as one of the greatest threats to biodiversity worldwide, second only to habitat destruction (Schmitz and Simberloff 1997). Among these, social insects, and in particular invasive ants, are especially destructive (Holway et al. 2002). Invasive ants have a complex negative impact on their new ecosystems, affecting mainly but not only other ant species; they are agricultural pests and also pose a danger to human health (Passera 1994; Williams 1994). In addition they were found to affect soil properties and mutualistic interactions of other ant species with plants and other arthropods (Holway et al. 2002).
The most devastating impact of invasive ants on their new environment is their ability to displace local ant species (Holway et al. 2002), in some cases not only by reducing their numbers, but also by changing community organization (Sanders et al. 2003). Ants are an important component of natural ecosystems; they are numerically dominant and represent a large proportion of the animal biomass in almost every terrestrial habitat worldwide (Alonso and Agosti 2000). Therefore, the decline in ant diversity may have substantial consequences for many other organisms (e.g. Christian 2001; Ness and Bronstein 2004).
The little fire ant, Wasmannia auropunctata, is a widespread and abundant invasive ant (Holway et al. 2002). Native to South and Central America, it has been introduced to other localities in these regions as well as to West Africa, Australia, and several Caribbean and Pacific islands (Department of Primary Industries and Fisheries [DPI&F] 2006; McGlynn 1999; Holway et al. 2002; Wetterer and Porter 2003). It is listed among the “one hundred of the world’s worst invasive alien species” tabulated by the Invasive Species Specialist Group (ISSG) of the International Union for Conservation of Nature (IUCN) (Lowe et al. 2000) owing to its particularly severe environmental impact. It devastates local ant species, which it outcompetes and displaces, mainly on oceanic islands (Clark et al. 1982; Jourdan 1997; Holway et al. 2002; Armbrecht and Ulloa-Chacon 2003; Wetterer and Porter 2003). It also causes population declines of local invertebrates through extensive predation (Clark et al. 1982; Lubin 1984; Romanski 2001) and is renowned for harming domestic and possibly also wild vertebrates (Jourdan et al. 2001; Romanski 2001; Holway et al. 2002; Wetterer and Porter 2003). It is an agricultural pest in 35 countries. W. auropunctata is also infamous for its unpleasant sting (Wetterer and Porter 2003).
This species reproduces mostly or entirely by nest budding rather than nuptial flights (Hölldobler and Wilson 1977), and its natural long-range dispersal is limited (Lubin 1984). Therefore, W. auropunctata spreads in its non-native range primarily through human activities (Holway et al. 2002), such as transfer of plants, soil, food packaging, logs, and wood products (Lubin 1984; Roque-Albelo and Causton 1999; Romanski 2001; Wetterer and Porter 2003). W. auropunctata’s success as an invader is attributed to its ability to form supercolonies, its polyphagous habits, and its ability to use a wide range of habitats (Ulloa-Chacon and Cherix 1990; Le Breton et al. 2004; Errard et al. 2005). Insight into this species’ social behavior, ecology, and modes of spread can ultimately help control or at least contain it.
Wasmannia auropunctata was first identified in Israel in 2005. The Israeli population reflects a single introduction of one queen and one male genotypes, that reproduce clonally (Vonshak et al. 2009). In Israel W. auropunctata forms one supercolony with no intraspecific aggression in its entire invasive range; however, the ants exhibit high interspecific aggression in experimental laboratory settings (Vonshak et al. 2009).
Invasion trajectory models, based on bioclimatic envelopes of species’ known distribution range, are commonly used in order to predict invasive species’ potential spread (e.g. Welk et al. 2002). These as well as empirical studies have suggested that the climate in invaded ranges of invasive ants approximately matches that of their native range (e.g. Linepithema humile in Mediterranean climate [Holway 1998] and Pheidole megacephala in tropical climate [Hoffmann et al. 1999]). It was therefore surprising to find a successful invasion of the tropical ant W. auropunctata in Mediterranean and even desert regions of Israel.
Our objectives were: (a) to assess the introduction pathways and invasion trajectories of W. auropunctata in Israel; (b) to characterize the climatic conditions of the W. auropunctata population in Israel and to assess whether it is confined to irrigated gardens inside villages or found in native habitats as well; (c) to study the impact of W. auropunctata on the local arthropod fauna. Specifically we addressed its effect on population abundances, species richness, and community structure of ants, spiders, and beetles; and we test whether W. auropunctata’s impact on the local fauna in a Mediterranean climate conforms to the impact known from tropical habitats.
Methods
The preliminary survey
We conducted a preliminary survey of W. auropunctata’s distribution in Israel between January and March 2006 in several villages and their surroundings in the Jordan Valley, where the ant was first detected. These included gardens in villages, open and agricultural lands, and undisturbed river banks.
Three main methods were used for the preliminary survey: peanut butter baits, active search, and pitfall traps: (a) flat wooden sticks smeared with peanut butter were positioned as baits in every sampling point for at least 1 h. The 20–40 (proportional to sampled area) evenly distributed sampling points were selected by setting grids on aerial photos of each investigated area; (b) active search for ants under stones, logs, potted plants, etc.; (c) In order to study the ants’ distribution in cultivated lands, we set 47 pitfall traps (7.5 cm diameter, filled with detergent) and 47 peanut butter baits placed at 20 m intervals along the outskirts of an infested village (Kibbutz Afiqim; Fig. 1; Table 1). Another transect of 47 baits was set 5 m into the cultivated lands. The peanut butter baits were checked twice: in the afternoon, 2 h after they were set, and on the following morning; the pitfall traps were collected after 24 h and sorted immediately. W. auropunctata was identified in situ whereas other ant species were preserved in 80% ethanol for later identification. All specimens collected were deposited in the National Entomological Collections (Museum of Natural History) at Tel-Aviv University. Each collecting site was recorded either by GPS or directly on the aerial photo and digitized using ArcView 8.2.
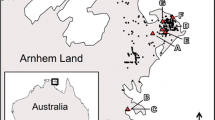
A map of Israel (excluding the south) showing the villages infested with Wasmannia auropunctata so far (circles) and meteorological stations, corresponding to Table 2 (asterisks)
Impact on the arthropod fauna
We studied W. auropunctata’s impact on three major local arthropod groups: ants, beetles, and spiders. The study was conducted during February–March 2006. The impact on species richness and abundance of these groups was investigated using pitfall traps. Ant populations were studied by using both baits and pitfall traps. The study area included three adjacent villages in the Jordan Valley: Afiqim, Bet Zera, and Sha’ar HaGolan (Fig. 1; Table 1), that share a similar climate (Jaffe 1988), soil (Dan 1988) and irrigation. All plots were located in disturbed habitats comprising irrigated public gardens comprising mainly lawns and perennial plants. Prior to setting the plots, we conducted preliminary sampling using active search to assess W. auropunctata densities. High density plots (“high”) were characterized by very high foraging activity of W. auropunctata and abundant nests. Low density plots (“low”) were typified by low foraging activity and scarce nests. Plots in which we could not find any W. auropunctata were considered clear of this species (“none”). There were a total of six plots per village, two plots for each density, except for Sha’ar HaGolan where only one low density plot was located. Eight 7.5 cm diameter pitfall traps containing 85% propylene glycol and 15% ethanol as preservatives were set in every plot for 5 days. The traps were placed in two rows set at least 10 m apart, each row comprised four traps placed at 10 m intervals. The contents of all pitfall traps were sorted in the laboratory, and ants, spiders, and beetles were identified to the species level.
In addition, four baits containing honey, peanut butter, and cracked cookies on a Petri dish were placed at both ends of each row of traps on the last day, several hours before the pitfall traps were collected. The baits were checked after 30 and 60 min and after about 4 h, just before the pitfall traps were collected. Ants on the baits were identified to species on the spot, except for Monomorium, Pheidole and Tetramorium, which were identified only to the genus level. Owing to unresolved taxonomic status, Plagiolepis ancyrensis was combined with Plagiolepis sp., and Tetramorium simillimum was combined with Tetramorium sp. of the simillimum group for all analyses.
Statistical analyses
All analyses were made excluding W. auropunctata from the data set. Because few ants were attracted to the baits, the three time intervals were pooled and analyzed similarly to the pitfall traps. Because ants are spatially clumped (Longino 2000) we analyzed the pitfall traps and baits data as follows: (a) we pooled the number of individuals and species by site in order to asses the impact of W. auropunctata on overall ant abundance (number of individuals) and species richness (number of species). These analyses were conducted by ANOVA (on square root transformed counts) for factor density (high, low, none) followed by Tukey HSD post-hoc test; (b) we counted the number of traps occupied by each species in areas of differing W. auropunctata density (“none” and “low”) in order to asses differences in relative species abundance. We compared each species frequency between “none” and “low” densities to that expected by chance using a G-test for homogeneity of replicates followed by a post-hoc test for heterogeneous groups (Sokal and Rohlf 1981, p. 728). Since most species were represented by few individuals, we analyzed only species that were trapped in at least ten traps and within three or more plots.
For beetles and spiders overall comparisons of number of individuals per trap (abundances) and number of species per trap (species richness) were conducted by ANOVA (on square root transformed counts) for factor density (high, low, none), separately for Afiqim, Bet Zera, and Sha’ar HaGolan (species richness was studied only for Bet Zera and Sha’ar HaGolan owing to a problem with the preservative used for specimens collected in Afiqim). The probabilities of the individual ANOVA results were combined following Sokal and Rohlf (1981, p. 779). Redundancy analysis (RDA) was used to test the relationship between the communities of spiders and beetles and the explanatory variables: W. auropunctata density (high, low, none), and locality (Bet Zera and Sha’ar HaGolan), with data pooled by site. The significance of each variable was tested using a Monte-Carlo permutation test (4999 permutations, main effects: design-based permutation; interactions: model-based permutation [ter Braak and Šmilauer 2002]). For spiders, a partial analysis was carried out in order to study the partial effects of W. auropunctata as the explanatory variable, with locality as a covariable (similarly to the effects of partial regression coefficients in a multiple regression; Leps and Šmilauer 1999).
All ordination analyses were performed using CANOCO FOR WINDOWS 4.5 (ter Braak and Šmilauer 2002). Other statistical analyses were performed using Statistica 7.1 (StatSoft Inc., Oklahoma, USA).
Results
The preliminary survey
In the 2006 preliminary survey we found W. auropunctata in eight localities, and since then in 18 additional localities (Fig. 1; Table 1). This is the first record of W. auropunctata in the Middle East; it is the northernmost point (33°13′) where this ant nests outdoors. Table 2 presents climatic data of W. auropunctata’s native range in Brazil, and introduced range worldwide, as well as of five sampling sites in Israel adjacent to infested localities (asterisks in Fig. 1). In Israel the ant survives in Mediterranean and even desert climatic conditions with temperature extremes in winter and in summer that are considerably greater than those of their native range, and of other invaded regions. More importantly, in Israel there are several consecutive dry months in summer (5–12 months with less than 15 mm rainfall compared to 0–2 months in the rest of the invasive range; Table 2), creating a significantly drier habitat than in the native range or other regions invaded by this species.
Of the 94 pitfall traps set in the preliminary survey at the edges of cultivated crops and around abandoned greenhouses W. auropunctata was found in 11 traps. Only a few workers were found at all sites (1.7 ± 6.14 per trap), except for the greenhouse site, in which there were 20.3 ± 16.7 workers per trap. Manual search for this species’ nests and activity revealed their presence in low densities in additional sites, all with frequent human activity (fishponds, cemeteries, etc.). Finally, these ants were also found in an undisturbed habitat along the Jordan River.
Impact on the arthropod fauna
Results obtained from the pitfall traps corroborated our a priori assessments of W. auropunctata densities in the different plots (Table 3). Ant abundance, species richness, and community composition were all significantly affected by W. auropunctata density (Fig. 2; statistical analyses in Table 3). We found a significant impact of W. auropunctata density on other ant abundance and species richness per plot, with significant differences between “high” and “none”, and between “high” and “low” in both pitfall traps and baits, but no differences between the “none” and “low” plots. A total of 42 ant species were recorded from the Jordan Valley region during the research period, 25 of which were represented in pitfall trap catches; ten taxa were represented at baits (Table 4). Although there were no significant differences in ant species diversity between the “none” and “low” plots (Fig. 2), a more detailed inspection revealed nonetheless important differences. The proportion of traps occupied by ants in “none” vs. “low” sites differed significantly from homogeneity (for the ant species found in more than ten traps in at least 3 plots. Table 5, G H = 18.57, df = 7, P < 0.05), with two heterogeneous groups (critical value X 2.05[7] = 14.07), Paratrechina jaegerskioeldi and Cardiocondyla emeryi (G = 12.33) were found in fewer traps in “low” compared to “none” sites, and Tetramorium davidi (G = 7.46) was found in more traps in “low” sites. As only few taxa were identified from the baits, and even fewer were represented in three on more sites, we did not perform a G-test analysis for the bait data.
The impact of Wasmannia auropunctata density on other ant abundance (light columns) and species richness (dark columns). The data represent mean (± SE) number of ants per site (eight pitfall traps) excluding W. auropunctata (The average number of W. auropunctata per site is indicated in parenthesis)
Population density of W. auropunctata also affected the two other ground arthropod groups, spiders, and beetles. Spider abundances were significantly lower at high densities of W. auropunctata, but the reduction in species richness was only marginally significant (Fig. 3; Table 6). A partial redundancy analysis showed that 48% of the variance was explained by high densities of W. auropunctata when locality served as covariable (F = 11.78, P = 0.006), and locality explained 23% of the variance when W. auropunctata’s density served as a covariable (F = 4.95, P = 0.048). In addition, the RDA analysis also showed that high densities of W. auropunctata had different impacts on different spider families in that the dominant spider family, Linyphiidae (67.85% of the individuals), was highly affected by high densities of the ant, compared to other families. Similar significant reductions in beetle abundances and species richness (Fig. 4; Table 7) were noted at high W. auropunctata densities, but the redundancy analysis (RDA) did not reveal any significant factors.
Discussion
Wasmannia auropunctata is a globally known invasive species that generally occupies tropical and subtropical habitats (McGlynn 1999) climatically similar to those of its native range in South and Central America. In contrast to the prediction that invasive ants will establish only in habitats similar to those in their native range (Holway 1998; Hoffmann et al. 1999), in Israel W. auropunctata is established in climatic conditions that are very different from those found in their native and other introduced habitats worldwide (see below). Israel’s climate is typified by dry summers and greater temperature extremes. It is possible that in Israel W. auropunctata was first established in irrigated gardens in the warm climate of the Jordan Valley, and only afterwards spread into less favorable habitats (the Golan Heights, Arava Valley, etc.). Bytinski-Salz (1966) claimed that irrigation in the Mediterranean climate of Israel simulates a subtropical or tropical climate, facilitating the dispersal of pests of tropical origin. Indeed, the ants seem to be confined almost entirely to irrigated habitats, most frequently inside villages, or at most in open and undisturbed areas adjacent to irrigated land or natural water sources. It is likely that the ants enter houses during summer in search of moisture, since most stinging appears to have occurred in kitchens and bathrooms during that season. The significance of wet conditions to the ant’s spread was emphasized by Meier (1994), who demonstrated that following an extraordinary El Ninõ year, W. auropunctata expanded its distribution in the Galapagos Islands to arid habitats. A similar trend was shown in studies on the distribution of the red imported fire ant (Solenopsis invicta) in the US, where at first the ants were established in habitats with similar climate to their native range, but later they expanded their distribution into climatically dissimilar habitats (Fitzpatrick et al. 2007).
Despite the climatic differences between Israel and the ant’s tropical and subtropical range, its ecology seems to be quite uniform throughout its range. W. auropunctata was found to nest in superficial nests in the ground under stones and inside hollow parts of plant material such as tree bark, similar to the species behavior in tropical habitats (Wetterer and Porter 2003). Nest size is diverse–from dozens of workers to thousands, with or without brood, and with 0–100 queens per nest (Vonshak, unpublished data). The studied colonies probably comprise satellite nests of larger colonies, as no aggression was found between them (Vonshak et al. 2009).
Introduction pathways and spread inside Israel
Wasmannia auropunctata was introduced into Israel as early as 1998, judging from reports of stinging made by residents of the infested areas. Definite identification of the species was made in 2005 after specimens were received through the Ministry of Agriculture and Rural Development.
Genetic analysis at 12 microsatellite loci points to a single introduction of one queen and one male genotype in Israel (Vonshak et al. 2009). Most alleles observed in Israel were not found in any of the invasive populations, including a population from Gabon (J. Foucaud and A. Estoup, personal communication), refuting an early hypothesis based on historical records of wood imports that the ant was introduced from Gabon. We therefore assume that W. auropunctata may have arrived from sites located in its native range. Since wood was also imported from Brazil to the plywood factory in Afiqim, this country may have been the origin of the Israeli population. W. auropunctata’s distribution in Israel indicates that human activities account for its dispersal in this area, as it was found outside villages only in places visited regularly by people (e.g., cemeteries). In addition we found at least two human-mediated dispersal pathways–potted plants and logs.
Impact on the arthropod fauna
We found a remarkable impact of the little fire ant on abundance, species richness, and community composition of the local ant species. Most ant species sampled were highly affected by W. auropunctata, but only at high densities of this invasive ant. However, a closer inspection of species diversity revealed that a few tramp species, living near human residences, were also found in lower frequencies in low-density plots compared to control plots, whereas other species were either unaffected or even found at higher frequencies in low-density plots. Most species nevertheless were absent at high densities. For example, while Tetramorium davidi is a native species that might get an advantage from the decrease in the incidence of other species, Paratrechina longicornis is a tramp species that could be more tolerant of low densities of W. auropunctata compared to other species as it is opportunistic; opportunist ants can continue foraging in the presence of dominant species (Hölldobler and Wilson 1990). Another species, Monomorium clavicorne, seemed to be unaffected by W. auropunctata’s presence, even at high densities. This tiny ant (1.2 mm) is distributed in tropical Africa and the Middle East (Collingwood 1985), and it is widespread in Israel. It is ecologically similar to W. auropunctata, nests under stones and recruits to bait in large numbers. It is possible that this species can tolerate W. auropunctata’s presence to some extent, possibly owing to the passivity of the workers, which were observed feeding from the same bait as W. auropunctata (similar to the reports of Grangier et al. 2007, dealing with ants of the genus Cyphomyrmex). The endemic species sampled in this study are widely distributed in Israel, and therefore are not threatened by W. auropunctata to date. Nevertheless, as W. auropunctata has also been established in natural habitats, its future spread may threaten other species, especially those with very localized distribution.
King and Tschinkel (2006) suggested that Solenopsis invicta ants do not affect ant community structure in their invaded habitats, and that the observed reduction in local ant species is a result of habitat disturbance rather than competition. This is definitely not the case for W. auropunctata in Israel. As all research plots with different W. auropunctata densities were located at similarly disturbed habitats (irrigated public gardens in three adjacent villages), the significant impact found on local ant species cannot be explained by habitat disturbance but rather by W. auropunctata presence and density.
We found a negative impact of the little fire ant on spider abundance, richness, and community composition and structure. Spiders could be affected either directly through predation by W. auropunctata or indirectly, because of the reduction in general arthropod abundance. Different spider families were affected differently, with the strongest impact on the most abundant family, the sheet-web spider family (Linyphiidae). Members of this family have agricultural importance as they reduce agricultural crop pest abundances (Symondson et al. 2002). Thus their reduction could lead to an indirect negative impact of W. auropunctata on agriculture. Beetle abundances and species richness were also negatively affected by W. auropunctata, but not their community structure. The observed decline in ground arthropod populations conforms to observations in previous studies from this ant species’ invasive range in the tropics (Clark et al. 1982; Lubin 1984; Le Breton et al. 2003). Conversely, in its native range, W. auropunctata is not a dominant species (Tennant 1994), except in disturbed habitats (e.g. Armbrecht and Ulloa-Chacon 2003; Orivel et al. 2009). Wetterer and Porter (2003) suggested that W. auropunctata is a less serious pest in more temperate areas such as Florida, the Bahamas, and Bermuda, compared to its serious impact in the Solomon Islands, Vanuatu, and Gabon. However, based upon our study we conclude that W. auropunctata also has a dramatic impact on the local arthropod fauna in a more temperate area.
In sum, it appears that W. auropunctata arrived at Kibbutz Afiqim in northern Israel on logs imported from Brazil to the local plywood factory and later dispersed in Israel through commercial transport of chopped wood, logs, and potted plants. The ants have a substantial negative impact on the local myrmecofauna in heavily infested villages as well as on the abundance of other arthropods. This study revealed the possible consequences of the continued spread of W. auropunctata in a Mediterranean climate. So far there is no indication of impact on agriculture, although we found the ants near fish ponds and on the border of agricultural fields. Although ants were found in open landscapes and in natural habitats, their distribution appears to be limited at present by their need for water. Water availability could be key to the spread of W. auropunctata in our region. The ability of W. auropunctata to thrive in a Mediterranean climate suggests that we should re-evaluate its potential for spread in broader climatic regions than considered so far.
References
Alonso LE, Agosti D (2000) Biodiversity studies, monitoring, and ants: an overview. In: Agosti D, Majer JD, Alonso LE, Schultz TRE (eds) Ants. Standard methods for measuring and monitoring biodiversity. Smithsonian Institution Press, Washington, pp 1–8
Armbrecht I, Ulloa-Chacon P (2003) The little fire ant Wasmannia auropunctata (Roger) (Hymenoptera: Formicidae) as a diversity indicator of ants in tropical dry forest fragments of Colombia. Environ Entomol 32:542–547
Bytinski-Salz H (1966) An annotated list of insects and mites introduced into Israel. Isr J Entomol 1:15–48
Christian CE (2001) Consequences of a biological invasion reveal the importance of mutualism for plant communities. Nature 413:635–639
Clark DB, Guayasamin C, Pazmino O, Donoso C, YPd Villacis (1982) The tramp ant Wasmannia auropunctata: Autecology and effects on ant diversity and distribution on Santa Cruz Island, Galapagos. Biotropica 14:196–207
Collingwood CA (1985) Hymenoptera: Fam. Formicidae of Saudi Arabia. Fauna of Saudi Arabia 7:230–302
Dan J (1988) The soils of the land of Israel. In: Yom-Tov Y, Tchernov E (eds) The zoogeography of Israel. Dr. W. Junk Publishers, Dordrecht, pp 95–128
Department of Primary Industries and Fisheries (DPI&F) (2006) Country or multicountry feature for distribution records for Wasmannia auropunctata. In: Invasive Species Specialist Group (ISSG) database. Available via DIALOG. http://www.issg.org/database/species/ecology.asp?fr=1&si=58. Accessed 10 Oct 2008
Errard C, Delabie J, Jourdan H, Hefetz A (2005) Intercontinental chemical variation in the invasive ant Wasmannia auropunctata (Roger) (Hymenoptera Formicidae): a key to the invasive success of a tramp species. Naturwissenschaften 92:319–323
Fitzpatrick MC, Weltzin JF, Sanders NJ, Dunn RR (2007) The biogeography of prediction error: why does the introduced range of the fire ant over-predict its native range? Global Ecol Biogeogr 16:24–33
Grangier J, Le Breton J, Dejean A, Orivel J (2007) Coexistence between cyphomyrmex ants and dominant populations of Wasmannia auropunctata. Behav Process 74:93–96
Hoffmann BD, Andersen AN, Hill GJE (1999) Impact of an introduced ant on native rain forest invertebrates: Pheidole megacephala in monsoonal Australia. Oecologia 120:595–604
Hölldobler B, Wilson EO (1977) The number of queens: an important trait in ant evolution. Naturwissenschaften 64:8–15
Hölldobler B, Wilson EO (1990) The Ants. The Belknap Press of Harvard University Press, Cambridge
Holway DA (1998) Effect of argentine ant invasions on ground-dwelling arthropods in northern california riparian woodlands. Oecologia 116:252–258
Holway DA, Lach L, Suarez AV, Tsutsui ND, Case TJ (2002) The causes and consequences of ant invasions. Annu Rev Ecol Syst 33:181–234
Israel Meteorological Service, climate information. Available via DIALOG. http://www.ims.gov.il/IMSEng/CLIMATE. Accessed 24 Jun 2009
Jaffe S (1988) Climate of Israel. In: Yom-Tov Y, Tchernov E (eds) The zoogeography of Israel. Dr. W. Junk Publishers, Dordrecht, pp 79–94
Jourdan H (1997) Threats on pacific islands: the spread of the tramp ant Wasmannia auropunctata (Hymenoptera: Formicidae). Pac Conserv Biol 3:61–64
Jourdan H, Sadlier RA, Bauer AM (2001) Little fire ant invasion (Wasmannia auropunctata) as a threat to New Caledonian lizards: evidences from a sclerophyll forest (Hymenoptera: Formicidae). Sociobiology 38:283–301
King JR, Tschinkel WR (2006) Experimental evidence that the introduced fire ant, Solenopsis invicta, does not competitively suppress co-occurring ants in a disturbed habitat. J Anim Ecol 75:1370–1378
Kugler J (1988) The zoogeography of social insects of Israel and Sinai. In: Yom-Tov Y, Tchernov E (eds) The zoogeography of Israel. Dr. W. Junk Publishers, Dordrecht, pp 251–275
Le Breton J, Chazeau J, Jourdan H (2003) Immediate Impacts of Invasion by Wasmannia auropunctata (Hymenoptera: Formicidae) on Native Litter Ant Fauna in a New Caledonian Rainforest. Austral Ecol 28:204–209
Le Breton J, Delabie JHC, Chazeau J, Dejean A, Jourdan H (2004) Experimental evidence of large-scale unicoloniality in the tramp ant Wasmannia auropunctata (Roger). J Insect Behav 17:263–271
Leps J, Šmilauer P (1999) Multivariate analysis of ecological data. Faculty of Biological Sciences. University of South Bohemia, Ceske Bodejovice
Longino JT (2000) What to do with the data. In: Agosti D, Majer JD, Alonso LE, Schultz TRE (eds) Ants. Standard methods for measuring and monitoring biodiversity. Smithsonian Institution Press, Washington, pp 186–203
Lowe S, Browne M, Boudjelas S (2000) 100 of the world’s worst invasive alien species. Aliens 12:S1–S12
Lubin YD (1984) Changes in the native fauna of the Galapagos Islands following invasion by the little red fire ant, Wasmannia auropunctata. Biol J Linn Soc 21:229–242
McGlynn TP (1999) The worldwide transfer of ants: geographical distribution and ecological invasions. J Biogeogr 26:535–548
Meier RE (1994) Coexisting patterns and foraging behavior of introduced and native ants (Hymenoptera Formicidae) in the Galapagos Islands (Ecuador). In: Williams DF (ed) Exotic ants: biology, impact and control of introduced species. Westview Press, Boulder, pp 174–180
Ness JH, Bronstein JL (2004) The effects of invasive ants on prospective ant mutualists. Biol Invasions 6:445–461
Orivel J, Grangier J, Foucaud J, Le Breton J, Andrès FX, Jourdan H, Delabie JHC, Fournier D, Cerdan P, Facon B, Estoup A, Dejean A (2009) Ecologically heterogeneous populations of the invasive ant Wasmannia auropunctata within its native and introduced ranges. Ecol Entomol 34:504–512
Passera L (1994) Characteristics of tramp species. In: Williams DF (ed) Exotic ants: biology, impact and control of introduced species. Westview Press, Boulder, pp 23–43
Romanski A (2001) Introduced species summary project: little fire ant (Wasmannia auropunctata). Columbia University, New York
Roque-Albelo L, Causton C (1999) El Nino and introduced insects in the Galapagos Islands: different dispersal strategies, similar effects. Noticias de Galapagos 60:36–60
Sanders NJ, Gotelli NJ, Heller NE, Gordon DM (2003) Community disassembly by an invasive species. Proc Natl Acad Sci USA 100:2474–2477
Schmitz DC, Simberloff D (1997) Biological invasions: a growing threat. Issues Sci Technol 13:33–40
Sokal RR, Rohlf FJ (1981) Biometry: the principles and practice of statistics in biological research. W. H. Freeman, San Francisco
Symondson WOC, Sunderland KD, Greenstone MH (2002) Can generalist predators be effective biocontrol agents? Annu Rev Entomol 47:561–594
Tennant LE (1994) The ecology of Wasmannia auropunctata in primary tropical rainforest in Costa Rica and Panama. In: Williams DF (ed) Exotic ants: biology, impact and control of introduced species. Westview Press, Boulder, pp 80–90
ter Braak CJF , Šmilauer P (2002) CANOCO reference manual and CanoDraw for Windows user's guide: software for canonical community ordination (version 4.5). Microcomputer power, Ithaca, New York, USA
Ulloa-Chacon P, Cherix D (1990) The little fire ant Wasmannia auropunctata R. (Hymenoptera: Formicidae). In: Vander Meer RK, Jaffe K, Cedeno A (eds) Applied myrmecology: a world perspective. Westview Press, Boulder, pp 281–289
Vonshak M, Dayan T, Foucaud J, Estoup A, Hefetz A (2009) The interplay between genetic and environmental effects on colony insularity in the clonal invasive little fire ant Wasmannia auropunctata. Behav Ecol Sociobiol. doi:10.1007/s00265-009-0775-9
Welk E, Schubert K, Hoffmann MH (2002) Present and potential distribution of invasive garlic mustard (Alliaria petiolata) in North America. Divers Distrib 8:219–233
Wetterer JK, Porter SD (2003) The little fire ant, Wasmannia auropunctata: Distribution, impact, and control. Sociobiology 42:1–41
Williams DF (1994) Exotic ants: biology, impact and control of introduced species. Westview Press, Boulder
World Meteorological Organization (WMO) web site on World City Forecasts, Climatology and Weather Observations. Available via DIALOG. http://www.worldweather.org/webapps/search.jsp. Accessed 7 Dec 2008
Acknowledgments
Special thanks to Uri Roll for the field and GIS assistance; to Matan Ben-Ari for the field and laboratory assistance and to David Meir, Ariella Gotlieb and Erez Maza for field assistance. We thank Prof. Jacques H. C. Delabie and Dr. Bernhard Seifert for helping to identify the ant species, the late Dr. Gershom Levi for spider identifications and Prof. Vladimir Chikatunov for beetle identifications. We also wish to thank Prof. David Wool, Tal Levanony and Efrat Gavish-Regev for statistical advice, and Dr. Shai Meiri and Prof. Dan Simberloff for their useful comments to early drafts of the manuscript. We thank the Ministry of Environmental Protection, Israel Nature and Parks Authority, the Israeli Ministry of Science, Culture and Sport for supporting the National Collections of Natural History at Tel Aviv University as a biodiversity, environment, and agriculture research knowledge center, and the Jordan Valley Regional Council for their support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vonshak, M., Dayan, T., Ionescu-Hirsh, A. et al. The little fire ant Wasmannia auropunctata: a new invasive species in the Middle East and its impact on the local arthropod fauna. Biol Invasions 12, 1825–1837 (2010). https://doi.org/10.1007/s10530-009-9593-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-009-9593-2