Abstract
Although predictions of potential distributions of invasive species often assume niche conservatism, recent analyses suggest that niche shifts can also occur. Thus, further studies are necessary to provide a better understanding of niche dynamics and to predict geographic distribution in invaded areas. The present study investigated the niche shift hypothesis at a broad biogeographical scale, using the comprehensive distribution of the invasive species Zaprionus indianus in its native (Africa) and invaded (America and India) ranges. Z. indianus is a very successful invasive species that presents high adaptive flexibility and extreme physiological tolerance. To investigate whether Z. indianus changed its climatic niche from Africa to America and India, multivariate analyses, as well as ecological niche modeling procedures (GARP, MAXENT and Mahalanobis distances), were used. Multivariate analyses showed that the niche spaces of Z. indianus in Africa, India and the Americas were significantly different (Wilks’ λ from a Multivariate Analysis of Variance, MANOVA = 0.115; P < 0.0001). Out of 108 occurrences in America, only 11 (ca 10%) were classified, by Canonical Variate Analysis scores, as belonging to its original range in Africa, whereas only 5% of the 39 occurrences in India were classified as belonging to Z. indianus’ original range. Consensus results from MAXENT, GARP and Mahalanobis distances correctly predicted only 27% of the occurrences in India and 85% of occurrences in America. Thus, all analyses showed that Zaprionus indianus quickly expanded ranges into different environments in the invaded areas, suggesting climatic niche shifts, primarily in India.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Niche conservatism is the tendency of a species to retain ancestral ecological characteristics (Wiens and Graham 2005). The idea that ecological niches evolve slowly can be derived theoretically and has been empirically supported since the classical paper by Peterson et al. (1999). However, the question remains as to whether or not niches are conserved throughout evolutionary time and to what extent species can quickly adapt to new environmental conditions, spreading into new habitats never used before through the modification of ecological niches (Dietz and Edwards 2006; Losos 2008). The balance between niche conservatism and niche shift may explain many biogeographical patterns, and it has important implications for ecology, evolution and conservation biology (Wiens and Graham 2005; Thuiller 2007).
It is well known that invasive species potentially affect agriculture, forestry, human health and natural ecosystems (GISP 2008). Nonetheless, invasive species also provide replicated experiments across large spatial and temporal scales that enable researchers to observe ecological and evolutionary processes in real time (Sax et al. 2007). Despite the ecological time scale of many such invasions, they may offer a good system to test the niche conservatism hypothesis. If niches are conserved during the invasion process, species would only be able to occupy regions with a climate similar to that of the native range, so it would be possible to accurately predict the range of an introduced species from its native range. Alternatively, if species adapt to the conditions in the new areas, they would expand their distribution beyond their original niche, and it would be difficult to predict the introduced range from its native range (Wiens and Graham 2005).
Areas at risk of successful introduction, spread and future distribution of invasive species are often estimated by bioclimatic envelope modeling, also known as ecological niche modeling or species distribution modeling (Peterson and Vieglais 2001; Peterson 2003; Thuiller et al. 2005; Chen et al. 2007; Giovanelli et al. 2008; Roura-Pascual et al. 2009). Ecological niche models assume equilibrium of species’ distribution with current climate (i.e., the range is determined by the current niche and not strongly affected by stochastic processes, especially dispersal and other historical processes—see Araújo and Pearson 2005) and, under these circumstances, they allow accurate predictions of future distributions following introduction to a new area or in response to altered environmental conditions under niche conservatism (Guisan and Zimmermann 2000). Thus, the degree of correspondence between the geographical distributions of invasive species, in their native and introduced ranges, can help in the investigation of the niche conservative hypothesis (Broennimann et al. 2007; Fitzpatrick et al. 2007; Steiner et al. 2008). However, it is important to consider that the variables used in modeling processes usually reflect only particular components of a species’ niche (Soberon 2007) and that methodological issues and idiosyncratic characteristics of niche models must also be ruled out (Araujo and Guisan 2006; Peterson 2007; Peterson and Nakazawa 2008).
Niche conservatism allows for predicting the potential distributions of invasive species, but some recent studies have shown that niche shifts can also occur. Broennimann et al. (2007) showed a shift in the climatic niche of the plant Centaurea maculosa after introduction from Europe to North America in the 1890s. Fitzpatrick et al. (2007) also found that fire ants (Solenopsis invicta), with their native ranges in South America, were capable of establishing in harsher environments (see also Peterson and Nakazawa 2008; Fitzpatrick et al. 2008). Thus, uncritical assumptions of niche conservatism appear to be questionable at best, as it is not ubiquitous (Pearman et al. 2008; Losos 2008). Investigations focused on this particular issue are still lacking, especially those considering natural conditions at broad biogeographical scales.
As emphasized by Pearman et al. (2008), a better understanding of niche dynamics may help identify whether niche shifts happen in biological invasions, and whether niche stasis predominates in response to climate change within a region. This procedure will improve the assessment of the invasion risk and the predictions of species’ responses to climate changes as well. This target is achievable with empirical data on the life-history characteristics, ecological circumstances and evolutionary histories associated with species niches. Therefore, investigations considering more organisms, particularly those showing different life-history strategies, are important not only to add new evidence for species niche shift, but also to shed light on the ways in which niches are changed. In this context, the present study represents a new initiative that investigates the niche shift hypothesis at a broad biogeographical scale, using the comprehensive distribution of the invasive species Zaprionus indianus in its native and introduced ranges.
Methodology
Data
Zaprionus indianus (Diptera: Drosophilidae) is an excellent model for ecological, evolutionary and biogeographical studies focused on biological invasions. This Afro-tropical species is one of the most successful colonizing species in the genus and has spread into many tropical continents (Throckmorton 1975; Chassagnard and Kraaijeveld 1991; Chassagnard and Tsacas 1993). Its introduction to India was probably more than three decades ago, where it is now both abundant and widespread (Parkash and Yadav 1993). Lately, it has been introduced to South America, where it was first recorded in 1998 (Vilela 1999) and has since increased its range throughout the Americas (Goñi et al. 2001; Tidon et al. 2003; Van Der Linde et al. 2006).
Native and introduced distribution datasets consisted of presence data only (occurrences). We obtained all global occurrence data available for Z. indianus from three distinct sources: (1) an online drosophilid database (Bächli 2008; http://taxodros.unizh.ch/); (2) scientific papers; and (3) field work from Laboratório de Biologia Evolutiva of Universidade de Brasília. We obtained 204 spatially unique records, of which 194 were located in Africa, India or the Americas.
Twelve variables were used to test niche conservatism during invasion. Ten of these were bioclimatic parameters derived from the WORLDCLIM datasets (Hijmans et al. 2005; http://www.worldclim.org): precipitation of coldest quarter, precipitation of warmest quarter, precipitation seasonality (coefficient of variation), annual precipitation, mean temperature of wettest quarter, mean temperature of driest quarter, maximum temperature of warmest month, minimum temperature of coldest month, temperature seasonality (the standard deviation of the monthly mean temperatures), and annual mean temperature. The remaining two were topographic variables (elevation and slope) derived from the US Geological Survey’s Hydro1 k database (http://edcdaac.usgs.gov/gtopo30/hydro/). We used a cell precision of 0.0417° (nearly 4 km in cells near the Equator).
Multivariate analyses
Niche models are frequently used to evaluate potential niche shifts and conservatism in niche space by projecting models built in the native range into the invaded area and comparing predictions with observed ranges (e.g., Fitzpatrick et al. 2007). When differences between predicted and observed ranges are found, one can conclude that niche shifts have occurred. However, using niche models may be complicated by many issues, including assumptions of equilibrium between current climate and species occurrence, both in native and invaded ranges (Araújo and Pearson 2005). In addition, alternative niche models currently available usually provide different predictions. It is critical that they provide both accurate and transferable models in geographic space for testing niche conservatism/shift, an issue that is still under debate (see Elith et al. 2006; Peterson et al. 2007).
It is important to note that if the idea is to evaluate differences in niche characteristics between native and invaded ranges (and not to project in which regions the species would potentially invade), species distribution models are not the only possibility—other approaches are available. It may be sufficient to use multivariate analyses to compare the centroids of niche space (avoiding the problems of niche modeling and its projection into geographical space). Here we defined these centroids by the occurrences of Z. indianus in the three regions, including Africa (n = 49 occurrences), the Americas (n = 108) and India (n = 37). The Wilks’ λ from a Multivariate Analysis of Variance (MANOVA) is given the ratio between pooled within-groups (W) and total (T) covariance matrices (so that among-group covariance A = T − W). Values close to 1.0 indicate equality of covariance matrices within and among regions and, thus, no statistical differences among their centroids. Statistical significance of Wilks’ λ was approximated by an F-distribution (Johnson and Wichern 1992). Thus, the null hypothesis in MANOVA ecologically equates to the niche conservatism hypothesis.
It is also possible to extend the results of MANOVA by a Canonical Variate Analysis (CVA), in which an eigenanalysis of the matrix H = W −1 A is performed. This is analogous to a principal component of the main directions of variation among groups (in this case, the regions of native and invaded ranges). Since we are comparing three regions, two axes were extracted, and the projections of original data on eigenvectors (the canonical variates) allow evaluating the relative position of groups in the multivariate space. The coefficients in the eigenvectors indicate which variables better discriminate among areas along each of the canonical dimensions. Finally, it is possible to use the canonical scores to obtain a probability of regional occurrence. This can be used both as a measure of “similarity” among regions and to evaluate the ability of CVA in discriminating the areas (see Johnson and Wichern 1992 and Legendre and Legendre 1998 for an overall review of these techniques). All these analyses were performed using both the entire set of 12 variables and based on a stepwise procedure to reduce the number of variables, selecting which variables were more effective in discriminating the regions. Multivariate analyses were performed in SYSTAT 12.0.
Niche models
Despite the problems mentioned above, studies dealing with niche shifts during invasion processes typically use niche models to project models built in native ranges to the invaded domain (e.g., Peterson 2003; Thuiller et al. 2005; Chen et al. 2007; Giovanelli et al. 2008; Roura-Pascual et al. 2009). To visualize whether species’ distribution models fitted from the native range correctly predict the extent of potential invasion in the new ranges, we used three different presence-only methods (see Tsoar et al. 2007). The methods used were Maximum Entropy (MAXENT; Phillips et al. 2006, 2008), Genetic Algorithm for Rule Set Production (GARP; Stockwell and Noble 1992) and Mahalanobis distances (Farber and Kadmon 2003). These methods have been widely used and evaluated, and details of implementation and comparative analyses of their statistical performance can be found elsewhere (Segurado and Araújo 2004; Elith et al. 2006; Phillips and Dudik 2008; Tsoar et al. 2007; Meynard and Quinn 2007; Allouche et al. 2008). In all cases, only occurrence data from continental Africa (n = 49) were used to estimate the potential global range of Z. indianus, and the ability of each model to predict invaded ranges was given by the proportion of occurrences in the invaded ranges that were predicted, or not, by the models based on native range.
The evaluation of all three models was based on Area under the Receiver Operating Characteristic curve (AUC from ROC curve), which measures the ability of a model to discriminate between sites where a species is present versus those where a species is absent. AUC ranges from 0 to 1, where a score of 1 indicates perfect discrimination, and a score of 0.5 implies that discrimination is no better than a random selection (Elith et al. 2006). For the different models, an environmental “suitability” map of the species’ predicted distribution was produced by estimating the species’ potential distribution as a function of the environmental variables for each cell within a specific area. However, the ROC curve was used to convert the cumulative probabilities of occurrence to a binary estimate of potential presence, which was in turn used to evaluate if occurrences in invaded ranges were correctly predicted or not (for each model individually and for an overlap, or ensemble, of the different models—see Araújo and New 2007; Marmion et al. 2009). Despite recent discussion on the validity of AUC and ROC curves (e.g., Peterson et al. 2008; Lobo et al. 2008), they were used here only as a description of a single model fit and not in a comparative fashion among species.
Results
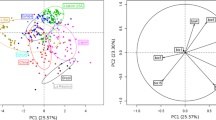
Multivariate analyses (MANOVA and CVA) showed that the niche spaces of Z. indianus in Africa, India and the Americas were significantly different (Wilks’ λ from MANOVA = 0.115; P < 0.0001). The first canonical variate, primarily associated with temperature seasonality, minimum temperature of the coldest month and elevation (Table 1), explained 88% of the variability among regions and clearly discriminated the niches of India from Africa (Fig. 1). The second canonical axis (12%) was primarily associated with minimum temperature of the coldest month, annual mean temperature and annual precipitation (Table 1), tended to separate America from Africa (Fig. 1). These results indicate a niche shift of Z. indianus across the invaded areas.
Canonical ordination of the environmental variables associated with the presence of Z. indianus in its native African range as well as in its invaded American and Indian ranges. Ellipses are the 95% confidence intervals along the two canonical axes. The first canonical axis explains 88% of the variation among groups
Although there was a clear difference between the modeled niches of native and introduced ranges, Indian climate envelope differed more from African climate envelope than did the American. Out of 108 occurrences in America, only 11 (ca 10%) were classified by CVA scores as belonging to its original range in Africa, whereas only 5% of the 39 occurrences in India were classified as belonging to Z. indianus’ original range. Stepwise analyses indicated that eight variables are more important to these patterns (Table 1), but there was not a qualitative change in any of the results presented above (for example, Wilks’ λ from MANOVA shifted from 0.115 to 0.121).
The AUC value from the three distribution models were all higher than 0.9, indicating that they have a “very good” ability to discriminate between sites where the species is present versus those where it is absent. However, in all cases, even with the high fit for the African occurrences, all models failed to predict the geographic distribution of Z. indianus in India, and they predicted only partially the distribution in the Americas (Fig. 2). Nonetheless, the model shows a high suitability of occurrence in the area of South American introduction (São Paulo State, Brazil; Vilela 1999) (Fig. 2). MAXENT produced a bit more restricted range that could suggest a false niche shift, as suggested by Peterson et al. (2007), but it is important to note that GARP and Mahalabonis distances also failed to predict invaded ranges (even considering that models in native range cover a much larger area). The real occurrences correctly predicted by the MAXENT corresponded to 8% in India, and this proportion increased to approximately 49% in America. GARP and Mahalanobis distances predicted 75% and 77% of occurrences in the Americas, but only 24% and 8% of the occurrences in India, respectively. When the predicted ranges from the three methods combined (ensemble), only 27% of the occurrences in India were correctly predicted based on the native range, and the number increased to 85% when occurrences in America were evaluated.
The potential predicted distribution of Z. indianus in the world, based on three different niche models built using African occurrences only, including MAXENT (a), Mahalanobis distances (b) and GARP (c). Shaded area indicated predicted range across the globe, truncating each model based on ROC curve. All models had very high fit (AUC > 0.9). Models based on African occurrences failed to predict the geographic distribution of this invasive species in India and predicted only partially correct the distribution in Americas
Discussion
Multivariate Analyses of Z. indianus distribution in environmental space in invaded and native areas provide support for a niche shift model. Niche modeling using three different methods reiterated results from the multivariate analyses, since they could not correctly predict the distribution of Z. indianus in Africa, and predicted, only partially, its distribution in America. In principle, this occurs because MAXENT, GARP and Mahalanobis distances (as any other species distribution model) assume strict niche conservatism when projecting distributions into unknown regions. Thus, results from multivariate comparison of niches and niche models clearly indicate that climate conditions occupied by Z. indianus in their introduced ranges (especially in India) are different from the original climate conditions of its native range in Africa. Because the multivariate analyses were used here to illustrate the Z. indianus niche shift rather than to retrospectively predict the phenomenon (i.e., modeling in the native range and comparing projected and observed occurrences in the invaded area), this study does not discuss the theoretical and methodological questions commonly addressed by the ecological niche modeling evaluations (e.g., Peterson et al. 2007; Peterson and Nakazawa 2008). Nonetheless, the modeling approach based on GARP, MAXENT and Mahalanobis distances improves the robustness of the present findings as it corroborated the climatic niche change, especially in India, evidenced for this invasive species.
It is possible to devise a hierarchical framework to explain differences between niche centroids in invaded and native species’ ranges, based on multiple ecological and evolutionary processes. It is difficult to disentangle all these alternative explanations, but in the case of Z. indianus, the double invasion allows us to contrast alternative scenarios for the observed changes based on the visual inspection of multivariate space expressed in the two CVA axes, comparing niches in native (Africa) and invaded regions (India and America).
It is important to rule out shifts that are due to methodological artifacts and that, in reality, are the same in the native and invaded areas. Peterson and Nakazawa (2008), for example, showed that models produce different predictions when using different environmental datasets (see also Fitzpatrick et al. 2008). Here we tested different combinations of the variables used, and in all cases, the Wilks’ λ is still highly significant (results not shown). Although this is not the only source of methodological problems, we can rule out that divergences observed are due to model overfitting or the selection of particular environmental variables or combination of variables. The same debate appears when dealing with species distribution models, especially MAXENT (see Peterson et al. 2007). Indeed, although in our results MAXENT has the smallest predictive power and generates the more restricted and conservative distributions in India and Americas, the two other models used, GARP and Mahalanobis distances, also failed to correct predict invaded ranges.
Lack of equilibrium in both native and invaded ranges would also cause problems for inferring niche shifts. For example, it is clear that, in the initial phases of invasion, niches in the invaded area must be smaller than in the native range and are actually found “inside” the niche space in the native range (see DeMarco et al. 2008). In this case, although multivariate analyses (and niche models) would allow distinguishing centroids, this would be due to lack of equilibrium between the current environment and occupation in the invaded range because there was not enough time for the species to achieve this equilibrium. An evaluation of distribution of occurrences in the space of CVA would reveal the nested pattern of invaded niche inside native niche. On the other hand, if the native range is strongly determined by biotic interactions, this may bias information from the original niche, and the records of occurrence may represent pos-interactive equilibrium of species distribution. Consequently, its expansion in invaded areas would be explained by competitive release, and, in this case, niche conservatism is still valid, since there was no evolution of the species niche. Therefore, it would be possible to see the pattern in the reduced multivariate space displayed by CVA.
Lack of equilibrium between occurrences and climate in native and invaded ranges discussed above would be classified as a “plastic” effect and does not really involve niche shifts in an evolutionary context (i.e., changes involving divergences in the species’ fundamental niche, in a Hutchinsonian sense). Again, at least two different evolutionary processes should be involved in this evolutionary shift. First, niche shifts can occur by adaptive responses to new environmental conditions in the invaded areas and be driven by natural selection for climatic tolerance. Alternatively, niche shifts would occur by stochastic processes following a neutral divergence due to isolation. Indeed, Losos (2008) recently pointed out that niche conservatism would be inferred only if divergence between species’ niche is smaller than expected by a purely stochastic process.
There is evidence that introduced populations can quickly adapt to local conditions (Sakai et al. 2001; Lambrinos 2004; Whitney and Gabler 2008). Such successful invaders often present a high genetic variability and a wide physiological tolerance (Ehrlich 1986), which allow them to spread and establish in different niches. Genetic variability allows invasive species to experience rapid changes linked to new selection pressures in the novel environment, resulting in the latitudinal clines shown by several other invasive Drosophila populations (Gilchrist et al. 2001; Jong and Bochdanovits 2003). These variations usually correlate with local climatic conditions, especially temperature, and always converge on the pattern showed by the ancestral populations. These patterns, in Drosophila species, are frequently interpreted as adaptations to the novel abiotic environmental pressures in the introduced range (Huey et al. 2005).
Multivariate analyses show that the divergence between India and Africa are much larger than between Africa and America. Moreover, the orientation of the ellipses throughout the multivariate space seems to be different in Fig. 1, corroborating Fitzpatrick et al.’s (2008) suggestions that adaptive processes can occur along different axes of climatic variation. Indeed, the invasive populations of Z. indianus in India establish themselves in a very different climate from that of the original African range, and they span environments whose temperatures are more variable and considerably lower in the coldest months, especially at high elevations. Temperature is a major determinant factor in explaining the distribution and abundance of many ectotherm species, and it is widely recognized that the activity of drosophilids occurs between 12 and 32°C (Grossfield 1978). Extreme temperature values can be tolerated by drosophilids only if applied for a period shorter than the total development time (David et al. 1983), favoring those that are long-developing species. At 18°C, the biological cycle of Z. indianus can last up to a month (Nava et al. 2007), which is considerably high for a colonizing drosophilid (Atkinson 1979). In addition, Z. indianus populations in India show clinal variations for several traits (Parkash and Yadav 1993; Karan et al. 1999, 2000; David et al. 2006), which probably reflects its adaptive process to a wider range of climatic conditions. Thus, the divergence of Indian Z. indianus populations might constitute a genetic strategy for this colonizing species in terms of its broader niche-width (Parkash and Yadav 1993).
On the other hand, the Z. indianus that invaded South America probably met climatic conditions more similar to those in its native distribution, especially in summer, and there is indeed a higher overlap of these two regions in the multivariate space. Since its unintentional recent introduction to Brazil (Vilela 1999), this invasive species has rapidly expanded its range into a wide latitudinal range throughout the whole continent, but the niches are not much larger than in Africa. However, studies at local scales show that the abundance of Z. indianus varies greatly among habitat types. It is more abundant in the summer season and open environments (Tidon et al. 2003; Silva et al. 2005). Such environments present climate conditions more similar to the African climate, and they appear to play important roles in the establishment success and spread of Z. indianus in the Americas. Additionally, the difference between niches in Africa and America is not strong, and they overlap more, such that it is not possible to rule out the simpler idea of competitive release or other plastic processes related to non-equilibrium in native and introduced ranges (and thus support niche conservatism).
The climatic niche shift found in Z. indianus is new empirical evidence that invasive species can occupy a distinct niche following their introduction into new areas. Z. indianus is a successful invasive species that has rapidly established and expanded in its new ranges, surviving and reproducing in environments with different climatic conditions than its original niche. These results are in agreement with the conclusions of Broennimann et al. (2007) and Fitzpatrick et al. (2007, 2008). We are not aware of any other empirical evidence that both explicitly tests the niche shift and presents comparably robust data for invasive animal species across more ranges, finding multiple patterns that can be interpreted in an hierarchical framework. Of course, the successful establishment and spread of a species into a new area is a complex biological phenomenon, driven by many factors. Invasion dynamics depends on the interaction between invasive species traits (e.g., genetic variability and phenotypic plasticity) and the characteristics of the invaded environment, for example, community saturation and disturbance level. Nevertheless, this framework may provide a step towards disentangling these multiple processes and allow a better understanding of the ecological and evolutionary mechanisms driving niches during biological invasions.
References
Allouche O, Steinitz O, Rotem D, Rosenfeld A, Kadmon R (2008) Incorporating distance constraints into species distribution models. J Appl Ecol 45:599–609
Araujo MB, Guisan A (2006) Five (or so) challenges for species distribution modelling. J Biogeogr 33:1677–1688
Araújo MB, New M (2007) Ensemble forecasting of species distributions. Trends Ecol Evol 22:42–47
Araújo MB, Pearson RG (2005) Equilibrium of species’ distributions with climate. Ecography 28:693–695
Atkinson WD (1979) A comparison of the reproductive strategies of domestic species of Drosophila. J Anim Ecol 48:53–64
Bächli G (2008) TaxoDros: The database on taxonomy of Drosophilidae. Available at http://taxodros.unizh.ch/. Accessed September 2008
Broennimann O, Treier UA, Muller-Scharer H et al (2007) Evidence of climatic niche shift during biological invasion. Ecol Lett 10:701–709
Chassagnard MT, Kraaijeveld AR (1991) The occurrence of Zaprionus sensu stricto in the Palearctic region (Diptera: Drosophilidae). Ann Soc Entomol Fr 27:495–496
Chassagnard MT, Tsacas L (1993) The subgenus Zaprionus s. str. Definition of species groups and revision of the Vittiger subgroup (Diptera, Drosophilidae). Ann Soc Entomol Fr 29:173–194
Chen P, Wiley EO, Menyset KM (2007) Ecological niche modelling as a predictive tool: silver and bighead carps in North America. Biol Invasions 9:43–51
David JR, Allemand R, van Herrewege J et al (1983) Ecophysiology: abiotic factors. In: Ashburner M, Carson HL, Thompson JN (eds) The genetics and biology of Drosophila. Academic Press, New York, pp 105–170
David JR, Araripe LO, Bitner-Mathe BC et al (2006) Quantitative trait analysis and geographic variability of natural populations of Zaprionus indianus, a recent invader in Brazil. Heredity 96:53–62
DeMarco P, Diniz-Filho JAF, Bini LM (2008) Spatial analysis improves species distribution modelling during range expansion. Biol Lett 4:577–580
Dietz H, Edwards PJ (2006) Recognition that causal processes change during plant invasion helps explain conflicts in evidence. Ecology 87:1359–1367
Ehrlich PR (1986) Which animal will invade? In: Mooney HA, Drake JA (eds) Ecology of biological invasions of North America and Hawaii. Springer, New York, pp 79–95
Elith J, Graham CH, Anderson RP et al (2006) Novel methods improve prediction of species’ distributions from occurrence data. Ecography 29:129–151
Farber O, Kadmon R (2003) Assessment of alternative approaches for bioclimatic modeling with special emphasis on the Mahalanobis distance. Ecol Model 160:115–130
Fitzpatrick MC, Weltzin JF, Sanders NJ et al (2007) The biogeography of prediction error: why does the introduced range of the fire ant over-predict its native range? Glob Ecol Biogeogr 16:24–33
Fitzpatrick MC, Dunn RR, Sanders NJ (2008) Data set matter, but so do evolution and ecology. Glob Ecol Biogeogr 17:562–565
Gilchrist GW, Huey RB, Serra L (2001) Rapid evolution of wing size clines in Drosophila subobscura. Genetica 112:273–286
Giovanelli JGR, Haddad CFB, Alexandrino J (2008) Predicting the potential distribution of the alien invasive American bullfrog (Lithobates catesbeianus) in Brazil. Biol Invasions 10:585–590
GISP (2008) The global invasive species programme. Available at http://www.gisp.org/. Accessed Sept 2008
Goñi B, Fresia P, Calvino M et al (2001) First record of Zaprionus indianus Gupta, 1970 (Diptera, Drosophilidae) in southern localities of Uruguay. Drosoph Inf Serv 84:61–65
Grossfield J (1978) Non sexual behaviour of Drosophila. In: Ashburner M, Wright TRF (eds) The genetics and biology of Drosophila. Academic Press, New York, pp 1–126
Guisan A, Zimmermann NE (2000) Predictive habitat distribution models in ecology. Ecol Model 135:147–186
Hijmans RJ, Cameron SE, Parra JL et al (2005) Very high resolution interpolated climate surfaces for global land areas. Int J Climatol 25:1965–1978
Huey R, Gilchrist G, Hendry A (2005) Using invasive species to study evolution. In: Sax D, Stachowicz J, Gaines D (eds) Species invasions: insights into ecology, evolution & biogeography. Sinauer Associates, Sunderland, pp 139–184
Johnson RA, Wichern DW (1992) Applied multivariate statistical analysis. Prentice Hall, New Jersey
Jong G, Bochdanovits Z (2003) Latitudinal clines in Drosophila melanogaster: body size, allozyme frequencies, inversion frequencies, and the insulin-signalling pathway. J Genet 82:207–223
Karan D, Moreteau B, David JR (1999) Growth temperature and reaction norms of morphometrical traits in a tropical drosophilid: Zaprionus indianus. Heredity 83:398–407
Karan D, Dubey S, Moreteau B et al (2000) Geographical clines for quantitative traits in natural populations of a tropical drosophilid: Zaprionus indianus. Genetica 108:91–100
Lambrinos JG (2004) How interactions between ecology and evolution influence contemporary invasion dynamics. Ecology 85:2061–2070
Legendre P, Legendre L (1998) Numerical ecology. Elsevier, Amsterdam
Lobo JM, Jimenez-Valverde A, Real R (2008) AUC: a misleading measure of the performance of predictive distribution models. Glob Ecol Biogeogr 17:145–151
Losos JB (2008) Phylogenetic niche conservatism, phylogenetic signal and the relationship between phylogenetic relatedness and ecological similarity among species. Ecol Lett 11:995–1007
Marmion M, Parviainen M, Luoto M et al (2009) Evaluation of consensus methods in predictive species distribution modeling. Diver Distr 15:59–69
Meynard CN, Quinn JF (2007) Predicting species distributions: a critical comparison of the most common statistical models using artificial species. J Biogeogr 34:1455–1469
Nava DE, Nascimento AM, Stein CP et al (2007) Biology, thermal requirements, and estimation of the number of generations of Zaprionus indianus (Diptera: Drosophilidae) for the main fig producing regions of Brazil. Fla Entomol 90:495–501
Parkash R, Yadav JP (1993) Geographical clinal variation at 7 esterase-coding loci in Indian populations of Zaprionus indianus. Hereditas 119:161–170
Pearman PB, Guisan A, Broennimann O et al (2008) Niche dynamics in space and time. Trends Ecol Evol 23:149–158
Peterson AT (2003) Predicting the geography of species’ invasions via ecological niche modeling. Q Rev Biol 78:419–433
Peterson AT (2007) Why not why where: the need for more complex models of simpler environmental spaces. Ecol Model 203:527–530
Peterson AT, Nakazawa Y (2008) Environmental data sets matter in ecological niche modelling: an example with Solenopsis invicta and Solenopsis richteri. Glob Ecol Biogeogr 17:135–144
Peterson AT, Vieglais DA (2001) Predicting species invasions using ecological niche modeling: new approaches from bioinformatics attack a pressing problem. Bioscience 51:363–371
Peterson AT, Soberon J, Sanchez-Cordero V (1999) Conservatism of ecological niches in evolutionary time. Science 285:1265–1267
Peterson AT, Papes M, Eaton M (2007) Transferability and model evaluation in ecological niche modeling: a comparison of GARP and Maxent. Ecography 30:550–560
Peterson AT, Papes M, Soberon J (2008) Rethinking receiver operating characteristic analysis applications in ecological niche modeling. Ecol Model 213:63–72
Phillips SJ, Dudik M (2008) Modeling of species distributions with Maxent: new extensions and a comprehensive evaluation. Ecography 31:161–175
Phillips SJ, Anderson RP, Schapire RE (2006) Maximum entropy modeling of species geographic distributions. Ecol Model 190:231–259
Roura-Pascual N, Brotons L, Peterson AT, Thuiller W (2009) Consensual predictions of potential distributional areas for invasive species: a case study of Argentine ants in the Iberian peninsula. Biol Invasions 11:1017–1031
Sakai AK, Allendorf FW, Holt JS et al (2001) The population biology of invasive species. Ann Rev Ecol Syst 32:305–332
Sax DF, Stachowicz JJ, Brown JH et al (2007) Ecological and evolutionary insights from species invasions. Trends Ecol Evol 22:465–471
Segurado P, Araújo MB (2004) An evaluation of methods for modelling species distributions. J Biogeogr 31:1555–1568
Silva NM, Fantinel CD, Valente VLS et al (2005) Population dynamics of the invasive species Zaprionus indianus (Gupta) (Diptera: Drosophilidae) in communities of drosophilids of Porto Alegre city, southern of Brazil. Neotrop Entoml 34:363–374
Soberon J (2007) Grinnellian and Eltonian niches and geographic distributions of species. Ecol Lett 10:1115–1123
Steiner FM, Schlick-Steiner BC, VanDerWal J et al (2008) Combined modelling of distribution and niche in invasion biology: a case study of two invasive Tetramorium ant species. Divers Distrib 14:538–545
Stockwell DRB, Noble IR (1992) Induction of sets of rules from animal distribution data: A robust and informative method of data analysis. Math Comput Simul 33:385–390
Throckmorton LH (1975) The phylogeny, ecology, and geography of Drosophila. Invertebrates of genetic interest. Plenum Press, New York, pp 421–469
Thuiller W (2007) Biodiversity—climate change and the ecologist. Nature 448:550–552
Thuiller W, Richardson DM, Pysek P et al (2005) Niche-based modelling as a tool for predicting the risk of alien plant invasions at a global scale. Glob Chang Biol 11:2234–2250
Tidon R, Leite DF, Leão BFD (2003) Impact of the colonization of Zaprionus (Diptera, Drosophilidae) in different ecosystems of the Neotropical region: 2 years after the invasion. Biol Conserv 112:299–305
Tsoar A, Allouche O, Steinitz O et al (2007) A comparative evaluation of presence only methods for modelling species distribution. Diver Distrib 13:397–405
Van Der Linde K, Steck GJ, Hibbard K et al (2006) First records of Zaprionus indianus (Diptera: Drosophilidae), a pest species on commercial fruits from Panama and the United States of America. Fla Entomol 89:402–404
Vilela CR (1999) Is Zaprionus indianus Gupta, 1970 (Diptera, Drosophilidae) currently colonizing the Neotropical region? Drosoph Inf Serv 82:37–39
Whitney KD, Gabler CA (2008) Rapid evolution in introduced species, ‘invasive traits’ and recipient communities: challenges for predicting invasive potential. Divers Distrib 14:569–580
Wiens JJ, Graham CH (2005) Niche conservatism: Integrating evolution, ecology, and conservation biology. Ann Rev Ecol Evol Syst 36:519–539
Acknowledgments
We are grateful to Dr. Stephen Harris, from Oxford University, and one anonymous reviewer for the critical reading of this manuscript and to Paulo Oliveira de Souza for helping with sampling data. The Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) has provided R. A. Mata with a 1 year Pos-Doctoral fellowship (CNPq proc. 155203/2006-1). Work by J. A. F. Diniz-Filho, P. D. Marco Jr., and R. Tidon have been continuously supported by CNPq productivity fellowships.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
da Mata, R.A., Tidon, R., Côrtes, L.G. et al. Invasive and flexible: niche shift in the drosophilid Zaprionus indianus (Insecta, Diptera). Biol Invasions 12, 1231–1241 (2010). https://doi.org/10.1007/s10530-009-9542-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-009-9542-0