Abstract
Biological invasions are one of the main threats to biodiversity worldwide, and understanding the mechanisms allowing invasive species to colonize their new environments is critical to the management of invasive populations. One particular aspect of invasion biology is to define the environmental conditions within which invasive species can persist (i.e. their ecological niche) to get insights on the potential role of adaptation in successful invasions, as well as to predict future invasion. Here, we use multiple correlative species distribution models and metrics of niche expansion and stability to investigate the worldwide invasion of the spotted-wing drosophila, Drosophila suzukii. By modeling the climatic niche of D. suzukii from occurrence data in its native and invasive ranges, we tested if a shift in the realized niche has occurred during invasion. Furthermore, we use recent population genetics work on the invasion history of the species to test whether invasive populations have preferentially invaded areas with climatic conditions more similar to the ones in their precise area of origin. Overall, our results show that D. suzukii displays a wide climatic niche and suggest that the species’ success in the invaded ranges may result from the absence of environmental challenges upon colonization. Furthermore, we show that the use of different geographical backgrounds can impact the outputs of niche comparisons and advice using complementary methods in the study of niche dynamics during biological invasions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Adaptive evolution can facilitate biological invasions (Reznick and Ghalambor 2001; Lee 2002; Lambrinos 2004; Wares 2005; Prentis et al. 2008) but some invasive species may not always face adaptive challenges in the invaded range. In such case, similar environmental conditions between introduced and native ranges can allow invaders to persist in an area from which they were historically out of reach (Facon et al. 2006; Estoup et al. 2016). This suggests that a change in the dispersal ability of a species—or in the case of invasive species, dispersal means (Nathan 2006)—could explain its successful introduction in a newly available area if the environmental conditions match the ones from the source area (Facon et al. 2006; Hayes and Barry 2008). Investigating the degree of environmental differentiation between native and invaded ranges can thus shed light on the processes involved in successful invasions. Namely, absence of differentiation between native and invaded environments would suggest that invasive populations did not face adaptive challenges upon colonization whereas the identification of a niche shift (Guisan et al. 2014) would in turn hint towards other mechanisms at play in the new range (Hill et al. 2013).
Species distribution models (SDMs) are predictive statistical models which combine species spatial occurrences data to their environmental characteristics (Elith and Leathwick 2009). As such, they represent a tool to estimate the climatic envelope of species or populations in their current range (i.e. their realized niche; Hutchinson 1991). The contribution of niche theory to the study of biological invasions is particularly relevant to characterize invasive species’ niches across their distribution, assess the amplitude of environmental changes undergone during invasions (Broennimann et al. 2007; Medley 2010; Tingley et al. 2014) or predict future invasions (e.g. Lozier and Mills 2011). To this end, a classical approach is to project SDMs calibrated on the native range in other areas, either invaded or not (Broennimann and Guisan 2008). However, some invasions may require a finer niche modeling approach than SDM projections based on coarse resolution presence data from the entire native range. For instance, different populations within a large native geographic distribution can have different realized niches (Peterson and Holt 2003). In such case, SDMs calibrated on each populations’ environments rather than on the entire species’ distribution might be more accurate in predicting environmental suitability elsewhere. In a recent study, Godefroid et al. (2015) used SDMs in several species of the genus Dendroctonus, characterized by divergent niche requirements. Their results suggest that classical models based on species’ entire range calibration tend to under- or overestimate the potential range of the species when compared to a global prediction from lineage-based projections. Consequently, if only a limited proportion of the native distribution served as a source for the invasion, or, if multiple sources with divergent niche requirements are present in the native distribution, intraspecific niche differentiation could be overlooked and therefore bias niche projection on uncolonized areas (Godefroid et al. 2015; Lecocq et al. 2016). Finally, the presence of bridgehead populations (i.e. where primary invasive populations act as source(s) for subsequent invasions, Lombaert et al. 2010) should be taken into account when predicting future colonization. As these populations may have experienced niche shift in the invasive range, they have the potential to further colonize areas where environmental conditions are not suitable for ancestral populations. Predictive models should thus be calibrated not only on the historical native range, but also on the invasive range (Broennimann and Guisan 2008; Medley 2010).
Such a complex invasion scenario can be found in the worldwide spread of the spotted-wing Drosophila suzukii (Matsumura 1931). This drosophilid species native from south-eastern Asia has started to colonize Europe and the United States in 2008 (Hauser 2011; Calabria et al. 2012) and is now widely distributed across the globe, with the latest occurrences in the Southern hemisphere reported in Brazil (Deprá et al. 2014). D. suzukii is a major pest for stone fruits and berries crops, causing considerable damage for the fruit production economy in the infested cultures (Lee et al. 2011). Whereas the colonization history of D. suzukii is now well documented (Adrion et al. 2014; Fraimout et al. 2017) its remarkable ability to invade such a diverse array of environments remains puzzling. At the ecological level, D. suzukii is known to persist in a rather unoccupied niche within the Drosophila genus. Its preference for ripe fruits and unique ability to oviposit through hard fruit skin (Atallah et al. 2014; Karageorgi et al. 2017) suggest that the spread of the species should not be prevented by resource competition with closely related species (but see Dancau et al. 2017; Rombaut et al. 2017; Shaw et al. 2017). Furthermore, the wide range of host plants used by D. suzukii points toward a rather generalist status of the species (Lee et al. 2011; Poyet et al. 2014). The species’ very large distribution in its native range (Kimura 2004; Asplen et al. 2015) suggest that Asian populations experience a wide array of bio-climatic variation. Thus, native D. suzukii may represent either a pool of wide-tolerant populations or discrete units which may have evolved within differentiated niche among Asian localities. Consequently, the global spread of D. suzukii—initiated by multiple differentiated genetic and geographic sources (Fraimout et al. 2017)—may have been facilitated by its climatic tolerance regardless of the source populations, or initiated by specialized propagules having colonized areas with similar environmental conditions. So far, several studies have investigated the role of environmental variables in the spread of D. suzukii at regional scales within the different invaded ranges (e.g. Gutierrez et al. 2016; Langille et al. 2016) but few have considered the invasion history at a global scale to explain the remarkable success of this recent invasion.
Here we used an ensemble of SDMs associated with knowledge on the invasion history of D. suzukii to examine species-environment relationships along its invasion routes and address three specific questions: (1) What is the realized niche of D. suzukii in its native and invasive range? (2) Can we observe niche shifts between ancestral and invasive populations? (3) Do invasive populations show more similarity in their realized niche than with their most probable geographical sources? Given the efficiency of the species to have colonized such a broad range of environments is a relatively short time (i.e. less than 10 years, Asplen et al. 2015) we predict that high similarity between native and invasive niches could explain the success of D. suzukii’s invasion. Conversely, we expect that models predicting low environmental suitability in areas where the species is known to occur, would suggest a shift in the species’ niche, and that invasion success in such case may involve other factors invoking local adaptations. Overall, by addressing the three aforementioned questions we test the hypotheses that (1) D. suzukii might in fact be a generalist species, having colonized environments which were historically out of reach and (2) accounting for the invasion history of the species can bias the outputs of niche comparisons.
Material and methods
Occurrence data
We gathered a total of 344 presence data of D. suzukii from its native range in south-eastern Asia and in its invasive range where D. suzukii is consistently invasive through years in continental USA, Hawaii, Europe and the Southern hemisphere distributions (Brazil and the French island La Réunion) from published occurrence records, databases, and personal observations from the field (Fig. S1). To avoid any potential geographic sampling bias within continent, we applied a “systematic sampling” by enforcing a minimum distance of 50 km among nearby presences (Fourcade et al. 2014).
For continental USA occurrences, the EDDMapS county checklist (2016, www.eddmaps.org) provided a thorough record of the species. We thus adapted our modeling approach to the specificity and potential bias of this area (see “split” method from Fourcade et al. 2014) and specifically used from these data a downscaling strategy known as “envelope approach” (Bombi and D’Amen 2012). We randomly sampled 150 locations within the surface covered by counties where D. suzukii have been recorded and used these points as pseudo-presences. The number of pseudo-presences is thus similar to the number of presences used to model the invaded distribution in Europe. This approach allowed us to model the species distribution by taking advantage of the good coverage of the data at the county level and preventing the bias in space and time that can occur in occurrences records in USA from unbalanced search efforts.
Geographic backgrounds: accounting for invasion history
The worldwide invasion history of D. suzukii has been described in details in Fraimout et al. (2017). Briefly, it is characterized by two initial main routes of invasion from Asia to western USA (Hauser 2011) and southern Europe (Calabria et al. 2012). Subsequently, spread within USA was initiated by western USA propagules dispersing to eastern USA. Furthermore, genetic data revealed the existence of gene flow from eastern USA to Europe.
To test if presence in the invasive range could be better predicted knowing the invasion history of the species, the analyses were performed using two sets of geographic extents. First, we used broad-scale continental backgrounds defining the native area in south-eastern Asia (hereafter “Asia”), and both major invaded areas in USA and Europe (see colored boxes, Fig. S1). Second, we assigned occurrences to more precise geographical units corresponding to the genetic groups of D. suzukii described in Fraimout et al. (2017): Asia, Hawaii, western USA, eastern USA, Brazil, La Réunion and Europe (see colored occurrence points, Fig. S1). D. suzukii populations have initially invaded southern Europe and western USA (Hauser 2011; Calabria et al. 2012), most likely from continental Asian areas (see Fig. 1 in Fraimout et al. 2017). Thus—and although they represent a single genetic cluster—we refined the geographical background to focus on the occurrences in continental Asia by excluding Japan in the geographical extent (Fig. S1). This allowed us to perform comparisons along the invasion routes of D. suzukii between geographical areas of known history. First, we followed the two initial routes of invasion by comparing the native area (Asia and continental Asia) to western USA and Europe independently. Second, we followed the spread of D. suzukii in USA by comparing western to eastern USA. Finally, given the possibility of gene flow between USA and Europe, we performed comparisons between eastern USA and Europe. Unfortunately, the small record of presences in the most recent colonization in Brazil and the relative small areas that represent the two islands (Hawaii and La Réunion) prevented us from including them in the comparative analyses of niche modeling and niche overlap.
Environmental data and representation in the climatic space
The selection of the environmental predictors for niche modeling is a source of uncertainty in model predictions that can be reduced with sound statistical methods and ecological knowledge of the target species (Barbet-Massin et al. 2014; Petitpierre et al. 2017). A low number of predictive variables reduces the issues of model overfitting (i.e., when predictors are exceeding the number of occurrences) and multicollinearity (i.e., when the predictive variables are highly correlated, Heikkinen et al. 2006). Therefore, we focused on eight climatic variables to encompass the mean trends, the variability, and the extremes of temperature and precipitation at a given location: (1) mean annual temperature, (2) mean annual precipitation, (3) temperature seasonality, (4) precipitation seasonality, (5) temperature of the coldest month and (6) of the warmest month, (7) precipitation of the driest month and (8) of the wettest month. Similar sets of variables have proved their efficiency in applying SDMs to ectotherms organisms (Tingley et al. 2016) and particularly invasive insects (Hill et al. 2017) and are expected to be relevant for Drosophila species distributions (Kellermann et al. 2012). This set of predictors provided three more advantages. First, it makes the comparison possible with niche modelling of other taxa and with other insects (Hill et al. 2017). Second, Petitpierre et al. (2017) showed in a recent article that these eight climatic variables yield a high transferability of SDMs at macroclimatic scale when they projected the climatic niche of invasive plants in different environments. We extracted these climatic variables from the Worldclim Global Climate data on the current climate (version 1, 1960–1990; Hijmans et al. 2005) at a 10 arc-minute resolution (ca. 18.5 km × 18.5 km) to explore the climatic space of D. suzukii’s distribution.
In order to get a visual representation of the positions of the occurrences in the climatic space, we performed a principal component analysis (PCA) on the climatic predictors using the “FactoMineR” library (Husson et al. 2017) in R.
Ecological niche modeling
We first modeled the distribution of D. suzukii at a global scale (i.e. from its native and invasive distributions) following Beaumont et al. (2009). This approach seems particularly suitable to model distribution of invasive species as it provides more accurate assessment of the potential invasive distribution at that stage of the invasion, where expanding populations are not expected to be at equilibrium with the climate (Beaumont et al. 2009). Then, to evaluate the capacity of the models to predict the presence of the invasive populations from the presence records of the ancestral population, we modeled separately the native niche of D. suzukii (Asia), its invasive niche in USA, and its invasive niche in Europe. We then projected the native modeled niche on the invasive range (in the USA and in Europe). Conversely, the native range was also projected from the two invasive ranges, separately. All models were calibrated either using continental scale (i.e., Europe, USA) or intra-continental scale using the genetic groups and the geographical backgrounds described above.
We performed an ensemble of eight modeling techniques, all implemented in the biomod2 package (Thuiller et al. 2014) in R: (1) generalized linear models (GLM), (2) generalized boosted models (GBM), (3) generalized additive model (GAM), (4) flexible discriminant analysis (FDA), (5) classification tree analysis (CTA), (6) artificial neural networks (ANN), (7) random forests (RF) and (8) multivariate adaptive regression splines (MARS). Because our datasets do not provide true absences, we generated 10 sets of 1000 artificial absence records (pseudo-absences) by randomly sampling from the grid cells of each study area (Barbet-Massin et al. 2012). We replicated ten times the splitting of the data into a random subset including 70% of the data for calibration and one second subset with the remaining for evaluation by cross-validation. We used the true skill statistic (TSS) to evaluate the predictive performance of each modeling technique (Allouche et al. 2006). To account for the inter-model variability in predictive performance, and because they have been shown to provide the best predictive performance, we chose consensus methods (Araujo and New 2007; Marmion et al. 2009) by averaging the predictions of models from the eight modeling techniques, removing models with a TSS below the quality threshold of 0.5 (Marmion et al. 2009). We calculated the average values and standard deviation of TSS across pseudo-absences and data-splitting runs to evaluate the ensemble model.
Finally, we computed Multivariate Environmental Similarity Surfaces (MESS) to determine the extent of the environmental differences between model training in one range and model projections in another (Elith and Leathwick 2009). MESS output values (climatic similarity) were then reported at the presence records in the range where the model is projected.
Quantifying niche shifts between ranges
In addition to the crossed projections from niche modeling, we measured the niche overlap between native and invasive ranges. We used Broennimann et al.’s framework (2012), which provides a quantification of the niche similarity between two taxa that occurred in different geographic regions by correcting for bias related to the availability of environmental space. For each geographical background, we performed a PCA calibrated on the entire environmental space of the study area (hereafter called PCA-env). We used the same eight climatic predictors than we selected for ecological niche modeling. The scores of the occurrences on the first two axes of PCA-env were projected onto a 100 × 100 grid, and a smoothed density of occurrences in environmental space was calculated using a kernel density function to compare ecological niches independently of sampling effort and of the spatial resolution of the environmental grid (Broennimann et al. 2012).
To quantify the similarities in climatic requirements between ranges, we calculated Schoener’s D (Schoener 1968, reviewed in Warren et al. 2008), a measure of niche overlap which varies from 0 (no overlap) to 1 (complete overlap). We then performed statistical tests of niche equivalency and similarity (Warren et al. 2008; Broennimann et al. 2014). We tested the hypothesis of niche equivalency by comparing the niche overlap calculated on the observed datasets and with the overlaps calculated on 1000 sets of simulated datasets. Simulated datasets were built from a random sample of the same number of occurrences than the observed dataset of each range. We performed a two-way test of equivalency to determine whether the niche overlap is more or less equivalent than random. Then, we calculated the overlap between one range and the randomly shifted density of occurrences of another range, and vice versa. This process was repeated 1000 times. We then performed a two-way test of niche similarity to determine whether niches are more similar than expected at random. The null hypothesis (i.e., niches are no more similar to one another than expected at random) was rejected if the observed overlap was greater than 95% of the simulated values of overlap.
Finally, we calculated three components of niche changes: niche expansion, niche stability, and niche unfilling (Guisan et al. 2014). Niche expansion is the proportion of densities that occurred in climatic conditions only within the invaded niche (i.e. not found in the native one). Conversely, niche unfilling is the proportion of densities occurring in climatic conditions only within the native niche (Guisan et al. 2014). Finally, niche stability refers to the proportion of densities in climatic conditions occurring in both native and invaded ranges. To take into account the availability of climates between ranges when quantifying niche changes, we calculated the niche overlap within the most common environments shared between the two ranges by removing marginal climates (Guisan et al. 2014). We calculated the niche metrics at the intersection of backgrounds reduced with different percentiles of density overlap (75th 85th, 90th, 95th, and 100th) to test if the scores of niche metrics are consistent across the different environmental extents. All niche metrics have been calculated using the ecospat library in R (Broennimann et al. 2014).
Results
Variation in D. suzukii presence data for climatic variables
We visualized the distributions of the occurrence data and their associated environmental variables in the climatic space by performing a PCA on the dataset (Fig. 1). The two first axes explained a moderate amount of variation in the data (48.87%). Occurrences were discriminated along the first axis (25.57%) on average from an increasing gradient of precipitation with a decreasing seasonality and a decline of the maximum temperatures during the warmest month. The second axis discriminated the occurrences from the latitudinal gradient and an average increase in temperature seasonality and in minimum temperature during the coldest month.
Principal components analysis calibrated on the worldwide presence data of D. suzukii (left panel) and its associated climatic variables (right panel). Points represent each presence in the current native and invasive range of D. suzukii. Climatic variables were extracted from the Worldclim Global Climate database (http://www.worldclim.org) and were coded as follows: bio1 = mean annual temperature; bio4 = temperature seasonality; bio5 = maximum temperature of the warmest month; bio6: minimum temperature of the coldest month; bio12 = annual precipitation; bio13 = precipitation of the wettest month; bio14 = precipitation of the driest month; bio15 = precipitation seasonality
Projections of the niche of D. suzukii across biogeographical regions
The ensemble modeling of the worldwide current distribution of D. suzukii obtained a high evaluation score (TSS = 0.821 ± 0.047; mean ± SD; Fig. 2). Models computed from the backgrounds in Asia (TSS = 0.753 ± 0.05, Fig. 3), continental Asia (TSS = 0.759 ± 0.052, Fig. S4), or from the two invasive ranges (USA: TSS = 0.842 ± 0.067, Europe: TSS = 0.856 ± 0.076, Fig. 3) showed a high predictive performance. Models calibrated on the native range were projected across both invasive ranges (Fig. 3). These projections yielded environmental suitabilities on average higher or equal to 0.50 in central and western Europe and included all the described invasive range of D. suzukii. In USA, projections from the native model yielded climatic suitability on average superior to 0.65 but only in eastern USA. All models were reciprocally projected on each other (Fig. 3). Asia, USA, and Europe mostly displayed analog or sub-analog climate (Table 1, Fig S2). The MESS analysis identified non-analog climate conditions in Northern Europe and the Alps when compared with the Asian native range and the invaded range in USA (Fig. S2). Similarly, some regions at the margin of the invasive range in western USA were non-analog to the native range (Fig. S2). As expected, the occurrences in each invasive range were better predicted with the reference model (e.g. when European records were used to model European distribution). However, model calibrated on USA yielded fairly high environmental suitability in different parts of Europe whereas the reciprocal projection of the European model in USA did not yield environmental suitability values superior to 0.3.
Reciprocal model projection between the Asian native range and both invasive ranges in Europe and USA. Models were calibrated on the presence data from each of the focal ranges of D. suzukii (across) either Asia, USA or Europe, and reciprocally projected onto (down) each other. Color scale represents the environmental suitability from low (blue) to high (red)
Measures of broad-scale niche changes
We first investigated niche changes at a broad geographical scale between Asia and USA, Asia and Europe, and USA and Europe to account for the global invasion of D. suzukii. The environmental space available in the invasive ranges in USA and Europe were both widely included in the environmental space available in Asia (Fig. 4). The niche overlaps between Asia and the invasive ranges were low (D = 0.33 and D = 0.3 in USA and in Europe, respectively, Fig. 4, Table 1). We also found a low overlap between USA and Europe (D = 0.2, Fig. 5, Table 1). This classification of the values of Schoener’s D followed the suggestion of Rödder and Engler (2011) to facilitate interpretation of the results: no overlap or very limited overlap for D comprise between 0 and 0.2, low overlap between 0.2 and 0.4 and moderate overlap between 0.4 and 0.6. These overlaps were more equivalent than random and were more similar than expected by chance (Table 1).
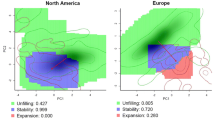
Niche dynamics between the Asian native range and both invasive ranges in Europe and USA. Principal components analyses were calibrated on environmental background (PCA-env) to compare the use of the environmental space from one region 1 (in blue) to one region 2 (in red). Solid and dotted lines correspond to 100% and 50% of the available environment space, respectively. We compared the use of the environmental space between the native range in Asia and USA (a), and Europe (b), and between the two invasive ranges, USA and Europe (c). The niche overlap is partitioned into three components: niche stability (in purple), the niche expansion (in yellow), and niche unfilling (in blue). The black dashed arrow indicates the change in the centroid of the climatic conditions from the region 1 to the region 2 and the red arrow the change in the centroid of the occurrences density of the population from the region 1 to the region 2. Correlation circles in the left column show the contribution of each environmental variable to the two first axes of the principal components analysis. Environmental variables are the mean annual temperature (bio1), mean annual precipitation (bio12), temperature seasonality (bio4), precipitation seasonality (bio15), temperature of the coldest month (bio6) and of the warmest month (bio5), precipitation of the driest month (bio14) and of the wettest month (bio13)
Niche dynamics along the invasive routes of D. suzukii in USA and Europe. Principal components analyses were calibrated on environmental background (PCA-env) to compare the use of the environmental space from one region 1 (in blue) to one region 2 (in red). Solid and dotted lines correspond to 100% and 50% of the available environment space, respectively. We compared the use of the environmental space (a) between Asia and western USA (b) western USA and eastern USA, and (c) eastern USA and Europe. The niche overlap is partitioned into three components: niche stability (in purple), the niche expansion (in yellow), and niche unfilling (in blue). The black dashed arrow indicates the change in the centroid of the climatic conditions from the region 1 to the region 2 and the red arrow the change in the centroid of the occurrences density of the population from the region 1 to the region 2. Correlation circles in the left column show the contribution of each environmental variable to the two first axes of the principal components analysis. Environmental variables are the mean annual temperature (bio1), mean annual precipitation (bio12), temperature seasonality (bio4), precipitation seasonality (bio15), temperature of the coldest month (bio6) and of the warmest month (bio5), precipitation of the driest month (bio14) and of the wettest month (bio13)
The values of the niche dynamic metrics were similar across percentiles (Fig S3). We report the results for the 75th percentiles here and the results for the other percentiles in Fig. S3. D. suzukii had high niche stability from the native range to its two main invaded ranges (97 and 100% from Asia into USA and Europe, respectively), and almost no niche expansion (Table 1). We identified niche unfilling from Asia to USA (18%) and from Asia to Europe (36%, Table 1). We found no signal of expansion from USA to Europe but substantial niche unfilling (43%, Table 1).
Measures of niche changes along the invasion routes
We first investigated niche changes following the two initial invasion routes of D. suzukii from Asia to western USA and Europe. We found no signal of niche expansion into western USA when considering native distributions in all Asia or continental Asia (Fig. 4, Table 1). Both comparisons revealed high niche stability (100%) and evidence for niche unfilling (39% and 20% from Asia and continental Asia to western USA, respectively). Measures of niche changes from continental Asia to Europe revealed lower niche stability (70%, Table 1) and higher niche expansion (30%) and niche unfilling (51%) relative to comparisons from all Asia to Europe. Western USA and Europe displayed more analog or sub-analog conditions when compared with Asia than when compared with continental Asia only (Table 1).
We then proceeded along the invasion routes across USA by measuring niche changes from western to eastern USA (Fig. 5, Table 1). The climatic conditions used by D. suzukii in western USA were almost not used in eastern USA (86% of niche unfilling, Table 1, Fig. 5). Consequently, we report a 64% niche expansion from western to eastern USA, associated with low niche stability (i.e. 36%), low niche overlap and mostly non-analog climate, Table 1). Finally, measurements of niche changes from eastern USA to Europe indicated a 54% niche expansion with moderate niche stability (54%), 29% niche unfilling, and mostly non-analog climate (Table 1). Niche overlaps between these two regions were not more similar than expected by chance (Table 1).
Generally, presences in the invasive ranges were better predicted when the models where calibrated on the whole south-eastern Asia than on continental Asia only (i.e. when we restricted the geographic extent to fit with the invasion history, Fig. S4). Moreover, all the measures of niche overlaps between the native ranges and the invasive ranges (in North America and Europe) were very limited when considering continental Asia only (Table 1). These niche overlaps were more equivalent than random. The niche overlap was more similar than expected by chance when density in USA were randomly shifted (p < 0.05, Table 1).
Discussion
In the present paper we aimed at assessing the realized niche of D. suzukii in its native and invasive range and sought to identify niche dynamics and potential niche shifts along its dispersion at a worldwide scale using multiple SDMs and niche metrics.
The generation of niche-based distribution models is subject to several uncertainties which need to be acknowledged prior to the interpretation of results. First, they inherently rely on the occurrence points used for calibration. Because our dataset incorporates occurrences from a wide distribution, it should encompass a sufficient amount of environmental variation to calibrate the models and predict invasive ranges. Second, a challenge in the generation of niche-based distributions from presence data is the lack of consideration for possible biotic interactions. Indeed, absence of occurrence of a species in a geographical area may be the result of out-competition by a conspecific, presence of predators, or other patterns of biotic interactions (Soberon and Peterson 2005; Hirzel and Le Lay 2008). It is thus primordial to consider that absence of distribution records may not be due solely to unsuitable environmental conditions. In the case of D. suzukii one of its main ecological characteristic is the evolution of its ovipositing behavior from an ancestral ‘Drosophila-like’ decaying substrate, to a wide variety of ripening stone fruit and berries (Asplen et al. 2015; Keesey et al. 2015; Karageorgi et al. 2017). This specialization in egg-laying behavior opened a new niche for the species which remains unoccupied by other drosophilid species (Poyet et al. 2015). Therefore, D. suzukii should not have direct competitors within drosophilid species (but see Dancau et al. 2017; Shaw et al. 2017; Rombaut et al. 2017), nor known predators, which relaxes the weight of potential biotic interactions in the calibration accuracy of the our models. However, it has been shown that the observed or modeled range of the plant host could improve distribution models of species in interaction (Barbet-Massin and Jiguet 2011). In the case of D. suzukii, the host plants specifically used by the species could then be used as an environmental variable in the model. However, D. suzukii specifically lays on ripening stonefruits and berries as strawberries, raspberries or grapes. The information on this type of crops is not available in land use data at global scale yet, but could be successfully used on smaller spatial extent. Considering this, it is also important to account for the effect of the targeted crops on D. suzukii’s distribution. Indeed, artificial environments such as those formed by agricultural crops—where temperature and humidity levels can be adjusted—may provide the species with some refuge (Tochen et al. 2016). Hence, the occurrence of natural populations of D. suzukii in areas where climatic conditions are not predicted to be suitable could be explained by the presence of such human altered habitats. However, previous field study suggest that D. suzukii in the wild makes use of natural habitats surrounding agricultural lands (Kenis et al. 2016; Klick et al. 2016). Thus we argue that our climatic descriptors are still relevant to explain the species distribution in most of its range. Lastly, the presence of non-analog climates—i.e. different combinations of environmental conditions—between regions may bias outputs of model projections by underestimating areas of environmental suitability in the projected region (Schwartz 2012). Here, the worldwide geographic scale of our study system implied to evaluate niche dynamics between areas with non-analog climates. Although the presence of non-analog climates should not ultimately prevent the use of model projections and niche metrics, one must treat the resulting values with caution and avoid over interpretation (Fitzpatrick and Hargrove 2009). Taking all the above points into consideration we propose several axes of discussion for our results, which can add to our understanding of the global invasion of D. suzukii.
Model projections at a global scale revealed high environmental suitabilities in all current invasive distribution of D. suzukii we could project onto (namely Europe, USA, and Brazil) suggesting a fair relevance of the selected climatic variables. The global model represents the realized niche of the species and provides a present-day snapshot at the current stage of the invasion (Fig. 2). As for other Asian invasive insects [e.g. Halyomorpha halys, Zhu et al. (2012); Vespa velutina, Arca et al. (2015); Harmonia axyridis, Poutsma et al. (2008)], and as expected from its broad distribution in Asia (Asplen et al. 2015), the realized native niche of D. suzukii is large, and encompasses a wide array of climates. This result is compatible with our first hypothesis suggesting that the large ancestral niche of D. suzukii would provide the species with high potential to colonize several areas without necessarily facing environmental challenges. To further explore this hypothesis we specifically investigated the niche dynamics of D. suzukii following its invasion history both spatially (i.e. in areas with recorded D. suzukii invasions) and temporarily (i.e. following the sequence of invasion from one area to another).
We did not detect signal of niche expansion from Asia to the two initially invaded ranges in USA and Europe but rather high levels of niche stability between these regions. This suggests that the first invasive D. suzukii propagules encountered similar climates as in their native range upon colonization of USA and Europe. More precisely, MESS analyses between Asia, Europe and USA revealed that the two first historical outbreaks of D. suzukii in northern California and southern Europe most likely occurred in areas with analog climates to the native area (Fig. S3). First records of D. suzukii in these two regions occurred almost simultaneously (Hauser 2011; Calabria et al. 2012), and evidences to explain the biological mechanisms underlying these successful introductions are still lacking. Although invasive populations may have independently adapted locally at these introduction points, our results provide a more parsimonious alternative by suggesting that D. suzukii did not face adaptive challenges—at least in regard to climatic conditions—during the first stages of invasion.
One of our main objectives was also to estimate the effect of incorporating information about the species invasion history in SDMs outputs and measurements of niche differentiation. Overall, reducing the native range of D. suzukii to its most probable area of origin in continental Asia did not yield higher suitabilities—rather lower suitabilities—of presence in the invasive European or North American ranges based on SDMs projections (Fig. S4). However, the invaded ranges were within non-analog conditions which might explain that model calibrated on the continental Asian native range poorly predicted the presence into the novel climates of invasive ranges (Fig S4). Additionally, whereas niche metrics scores and MESS analysis further confirmed the high level of niche stability between Asia and western USA, and confirmed the availability of analog climates, we found contrasting results in niche differentiation between Asia and Europe when using the continental Asian background only. Specifically, we observed a 30% niche expansion from continental Asia to Europe which was not previously detected using the broad-scale Asian background. This suggests that the reduction in spatial extent used for model calibration reduced the part of available and analog climates between ranges, making it difficult to forecast the invaded range from continental Asia only. Alternatively this could also suggest that invasive populations do not completely share climatic requirements with this particular part of the native range.
Accounting for the invasion history of D. suzukii by refining geographical backgrounds to western and eastern USA also revealed distinctive results compared with the use of broad-scale continental backgrounds. In western USA, model projections seemed to indicate poor environmental suitability, whichever background used for model calibration (Fig. 3). However, climates in this region are analog to the native Asian range (Fig. S2) and we found high niche stability between Asia and western USA (Table 1, Fig. 3). It is possible that the model projection failed to predict suitable areas in this region because occurrence points in western USA may represent marginal populations within the native area.
Furthermore reducing backgrounds to western and eastern USA allowed us to get insight on the niche dynamics of the species across USA which was previously inaccessible from the analysis of the entire USA background. Specifically we obtained high scores of niche expansion along the invasion routes across USA likely driven by non-analog climates. From a climatic point of view, these results suggest that D. suzukii experienced a large variation in precipitation levels between these particular regions, with higher mean annual precipitation (Fig. S5) and seasonality (Fig. 5) along this invasion route. Precipitation and humidity levels are expected to have an effect on the distribution of D. suzukii (dos Santos et al. 2017), but a previous laboratory and field study suggest that higher levels of humidity should promote rather than constrain the species activity (Tochen et al. 2016). From our results we can thus infer that propagules from western USA expanded their niche towards more favorable climate in eastern USA. Population genetics data on the invasion history of D. suzukii suggests unilateral gene flow from eastern USA to the northern part of Europe (Fraimout et al. 2017). We detected non-analog climates, and therefore a potential niche expansion from eastern USA to Europe, whereas no expansion was previously detected when considering the complete territory of the USA as source for the invasive populations in Europe, because, overall, USA and Europe share similar climates (Fig. 4, Fig S2). Furthermore, eastern USA and European climatic envelopes were not entirely differentiated by ordination in the two first axes of the PCA (see Fig. 1 for graphic representation on PC1 and PC2). It is thus possible that the expansion we observe might in fact be driven by the drier climates in southern regions of Europe—possibly explaining expansion towards lower mean precipitation (Fig. S5)—where introduction of D. suzukii from eastern USA most likely did not occur. As gene flow most likely occurred in northern Europe, similar niche dynamics analyses at a smaller geographic scale—i.e. focused on northern European climates—would be worth to validate our results.
Finally, our study raises some critical points on the use of SDMs for the study of biological invasions. Specifically, whereas the west coast of USA and the state of California particularly, did not yield high suitability values whichever the native niche used for model projections, measures of niche metrics indicated a high level of niche stability, analog climates, and no signal of niche expansion in this particular region. Such differences between methods have been previously reported also in Drosophilidae for the invasive Zaprionus indianus. In that case, whereas a niche shift had been reported from ancestral African niche to cooler climates in elevated regions in invaded region of India (Da Mata et al. 2010), Hill et al. (2017) conversely showed high environmental stability between the two regions using the niche shift metrics also used in the present study. Thus, interpretation of model-based projection might lead to misleading conclusions when climates in native and invaded ranges differ—e.g. in our case a niche shift in western USA—and our study emphasizes the complementary aspects of these methods for niche comparisons.
Concluding remarks
Our analysis revealed several points of interest in the understanding of the worldwide invasion of D. suzukii: (1) In addition to its wide trophic niche (Poyet et al. 2015) D. suzukii also displays a broad climatic niche inherent from its large native range in south-eastern Asia, and as a result our models calibrated at global scale predicted high environmental suitabilities in most of the invasive ranges. This result points towards a generalist status for D. suzukii (at least for climatic conditions) that could explain its successful introduction in areas which may have been historically constrained by a lack of migration means. (2) Overall, our results showed relative niche stability between the native and the two primarily invaded ranges in Europe in USA, and are compatible with the hypothesis that D. suzukii did not face climatic—and possibly adaptive—challenges upon colonization of these areas. (3) Whereas targeting geographical background specifically involved in the invasion process can help capturing detailed niche dynamics in invaded ranges—e.g. here between western and eastern USA—restricting the native range only to the most likely source of invasion may result in failure to predict invasive distributions. Accounting for invasion history in SDMs applied to biological invasion should thus be treated cautiously (Zhu et al. 2017). Furthermore we encourage the use of complementary methods such as SDMs and niche metrics in the analyses of niche dynamics during biological invasions along with the analysis of niche analogy that informs the reliability of niche scores and niche projections (Guisan et al. 2014). (4) Finally, although D. suzukii has widely colonized most continents of the world, the significant amounts of niche unfilling in USA and in Europe (> 10%, see Hill et al. 2017) suggest that the species is not in climatic equilibrium and have the potential to spread further within these two areas.
References
Adrion JR, Kousathanas A, Pascual M, Burrack HJ, Haddad NM, Bergland AO, Machado H, Sackton TB, Schlenke TA, Watada M et al (2014) Drosophila suzukii: the genetic footprint of a recent, worldwide invasion. Mol Biol Evol 31(12):3148–3163. https://doi.org/10.1093/molbev/msu246
Allouche O, Tsoar A, Kadmon R (2006) Assessing the accuracy of species distribution models: prevalence, kappa and the true skill statistic (TSS). J Appl Ecol 43(6):1223–1232. https://doi.org/10.1111/j.1365-2664.2006.01214.x
Araujo MB, New M (2007) Ensemble forecasting of species distributions. Trends Ecol Evol 22(1):42–47. https://doi.org/10.1016/j.tree.2006.09.010
Arca M, Mougel F, Guillemaud T, Dupas S, Rome Q, Perrard A, Muller F, Fossoud A, Capdevielle-Dulac C, Torres-Leguizamon M (2015) Reconstructing the invasion and the demographic history of the yellow-legged hornet, Vespa velutina, in Europe. Biol Invasions 17(8):2357–2371
Asplen MK, Anfora G, Biondi A, Choi D-S, Chu D, Daane KM, Gibert P, Gutierrez AP, Hoelmer KA, Hutchison WD et al (2015) Invasion biology of spotted wing Drosophila (Drosophila suzukii): a global perspective and future priorities. J Pest Sci 88(3):469–494. https://doi.org/10.1007/s10340-015-0681-z
Atallah J, Teixeira L, Salazar R, Zaragoza G, Kopp A (2014) The making of a pest: the evolution of a fruit-penetrating ovipositor in Drosophila suzukii and related species. Proc Biol Sci 281(1781):20132840. https://doi.org/10.1098/rspb.2013.2840
Barbet-Massin M, Jiguet F (2011) Back from a predicted climatic extinction of an island endemic: a future for the Corsican nuthatch. PLoS ONE 6(3):e18228
Barbet-Massin M, Jiguet F, Albert CH, Thuiller W (2012) Selecting pseudo-absences for species distribution models: how, where and how many? Methods Ecol Evol 3(2):327–338. https://doi.org/10.1111/j.2041-210X.2011.00172.x
Barbet-Massin M, Jetz W, Heikkinen R (2014) A 40-year, continent-wide, multispecies assessment of relevant climate predictors for species distribution modelling. Divers Distrib 20(11):1285–1295. https://doi.org/10.1111/ddi.12229
Beaumont LJ, Gallagher RV, Thuiller W, Downey PO, Leishman MR, Hughes L (2009) Different climatic envelopes among invasive populations may lead to underestimations of current and future biological invasions. Divers Distrib 15(3):409–420. https://doi.org/10.1111/j.1472-4642.2008.00547.x
Bombi P, D’Amen M (2012) Scaling down distribution maps from atlas data: a test of different approaches with virtual species. J Biogeogr 39(4):640–651
Broennimann O, Guisan A (2008) Predicting current and future biological invasions: both native and invaded ranges matter. Biol Lett 4(5):585–589
Broennimann O, Treier UA, Müller-Schärer H, Thuiller W, Peterson AT, Guisan A (2007) Evidence of climatic niche shift during biological invasion. Ecol Lett 10(8):701–709
Broennimann O, Fitzpatrick MC, Pearman PB, Petitpierre B, Pellissier L, Yoccoz NG, Thuiller W, Fortin M-J, Randin C, Zimmermann NE et al (2012) Measuring ecological niche overlap from occurrence and spatial environmental data. Glob Ecol Biogeogr 21(4):481–497. https://doi.org/10.1111/j.1466-8238.2011.00698.x
Broennimann O, Petitpierre B, Randin C, Engler R, Breiner F, D’Amen M, Pellissier L, Pottier J, Pio D, Mateo R (2014) ecospat: Spatial ecology miscellaneous methods. R package version, 1
Calabria G, Máca J, Bächli G, Serra L, Pascual M (2012) First records of the potential pest species Drosophila suzukii (Diptera: Drosophilidae) in Europe. J Appl Entomol 136(1–2):139–147. https://doi.org/10.1111/j.1439-0418.2010.01583.x
Da Mata RA, Tidon R, Côrtes LG, De Marco P, Diniz-Filho JAF (2010) Invasive and flexible: niche shift in the drosophilid Zaprionus indianus (Insecta, Diptera). Biol Invasions 12(5):1231–1241
Dancau T, Stemberger TL, Clarke P, Gillespie DR (2017) Can competition be superior to parasitism for biological control? The case of spotted wing Drosophila (Drosophila suzukii), Drosophila melanogaster and Pachycrepoideus vindemmiae. Biocontrol Sci Technol 27(1):3–16
Deprá M, Poppe JL, Schmitz HJ, De Toni DC, Valente VLS (2014) The first records of the invasive pest Drosophila suzukii in the South American continent. J Pest Sci 87(3):379–383. https://doi.org/10.1007/s10340-014-0591-5
dos Santos LA, Mendes MF, Krüger AP, Blauth ML, Gottschalk MS, Garcia FR (2017) Global potential distribution of Drosophila suzukii (Diptera, Drosophilidae). PLoS ONE 12(3):e0174318
Elith J, Leathwick JR (2009) Species distribution models: ecological explanation and prediction across space and time. Annu Rev Ecol Evol Syst 40:677–697
Estoup A, Ravigné V, Hufbauer R, Vitalis R, Gautier M, Facon B (2016) Is there a genetic paradox of biological invasion? Annu Rev Ecol Evol Syst 47(1):51–72. https://doi.org/10.1146/annurev-ecolsys-121415-032116
Facon B, Genton BJ, Shykoff J, Jarne P, Estoup A, David P (2006) A general eco-evolutionary framework for understanding bioinvasions. Trends Ecol Evol 21(3):130–135. https://doi.org/10.1016/j.tree.2005.10.012
Fitzpatrick MC, Hargrove WW (2009) The projection of species distribution models and the problem of non-analog climate. Biodivers Conserv 18(8):2255
Fourcade Y, Engler JO, Rödder D, Secondi J (2014) Mapping species distributions with MAXENT using a geographically biased sample of presence data: a performance assessment of methods for correcting sampling bias. PLoS ONE 9(5):e97122
Fraimout A, Debat V, Fellous S, Hufbauer RA, Foucaud J, Pudlo P, Marin JM, Price DK, Cattel J, Chen X et al (2017) Deciphering the routes of invasion of Drosophila suzukii by means of ABC random forest. Mol Biol Evol 34(4):980–996. https://doi.org/10.1093/molbev/msx050
Godefroid M, Cruaud A, Rossi JP, Rasplus JY (2015) Assessing the risk of invasion by Tephritid fruit flies: intraspecific divergence matters. PLoS ONE 10(8):e0135209. https://doi.org/10.1371/journal.pone.0135209
Guisan A, Petitpierre B, Broennimann O, Daehler C, Kueffer C (2014) Unifying niche shift studies: insights from biological invasions. Trends Ecol Evol 29(5):260–269
Gutierrez AP, Ponti L, Dalton DT (2016) Analysis of the invasiveness of spotted wing Drosophila (Drosophila suzukii) in North America, Europe, and the Mediterranean Basin. Biol Invasions 18(12):3647–3663. https://doi.org/10.1007/s10530-016-1255-6
Hauser M (2011) A historic account of the invasion of Drosophila suzukii (Matsumura) (Diptera: Drosophilidae) in the continental United States, with remarks on their identification. Pest Manag Sci 67(11):1352–1357. https://doi.org/10.1002/ps.2265
Hayes KR, Barry SC (2008) Are there any consistent predictors of invasion success? Biol Invasions 10(4):483–506
Heikkinen RK, Luoto M, Araújo MB, Virkkala R, Thuiller W, Sykes MT (2006) Methods and uncertainties in bioclimatic envelope modelling under climate change. Prog Phys Geogr 30(6):751–777
Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A (2005) Very high resolution interpolated climate surfaces for global land areas. Int J Climatol 25(15):1965–1978. https://doi.org/10.1002/joc.1276
Hill MP, Chown SL, Hoffmann AA (2013) A predicted niche shift corresponds with increased thermal resistance in an invasive mite. Halotydeus destructor. Glob Ecol Biogeogr 22(8):942–951
Hill MP, Gallardo B, Terblanche JS (2017) A global assessment of climatic niche shifts and human influence in insect invasions. Glob Ecol Biogeogr 26(6):679–689
Hirzel AH, Le Lay G (2008) Habitat suitability modelling and niche theory. J Appl Ecol 45(5):1372–1381. https://doi.org/10.1111/j.1365-2664.2008.01524.x
Husson F, Josse J, Le S, Mazet J (2017) FactoMineR: multivariate exploratory data analysis and data mining. R Package Version 1.32. Available at: https://CRAN.R-project.org/package=FactoMineR
Hutchinson GE (1991) Population studies: animal ecology and demography. Bull Math Biol 53(1–2):193–213
Karageorgi M, Bracker LB, Lebreton S, Minervino C, Cavey M, Siju KP, Grunwald Kadow IC, Gompel N, Prud’homme B (2017) Evolution of multiple sensory systems drives novel egg-laying behavior in the fruit pest Drosophila suzukii. Curr Biol 27(6):847–853. https://doi.org/10.1016/j.cub.2017.01.055
Keesey IW, Knaden M, Hansson BS (2015) Olfactory specialization in Drosophila suzukii supports an ecological shift in host preference from rotten to fresh fruit. J Chem Ecol 41(2):121–128
Kellermann V, Overgaard J, Hoffmann AA, Fløjgaard C, Svenning J-C, Loeschcke V (2012) Upper thermal limits of Drosophila are linked to species distributions and strongly constrained phylogenetically. Proc Natl Acad Sci 109(40):16228–16233
Kenis M, Tonina L, Eschen R, van der Sluis B, Sancassani M, Mori N, Haye T, Helsen H (2016) Non-crop plants used as hosts by Drosophila suzukii in Europe. J Pest Sci 89(3):735–748
Kimura MT (2004) Cold and heat tolerance of drosophilid flies with reference to their latitudinal distributions. Oecologia 140(3):442–449. https://doi.org/10.1007/s00442-004-1605-4
Klick J, Yang W, Walton V, Dalton D, Hagler J, Dreves A, Lee J, Bruck D (2016) Distribution and activity of Drosophila suzukii in cultivated raspberry and surrounding vegetation. J Appl Entomol 140(1–2):37–46
Lambrinos JG (2004) How interactions between ecology and evolution influence contemporary invasion dynamics. Ecology 85(8):2061–2070
Langille AB, Arteca EM, Ryan GD, Emiljanowicz LM, Newman JA (2016) North American invasion of spotted-wing Drosophila (Drosophila suzukii): a mechanistic model of population dynamics. Ecol Model 336:70–81. https://doi.org/10.1016/j.ecolmodel.2016.05.014
Lecocq T, Rasmont P, Harpke A, Schweiger O (2016) Improving international trade regulation by considering intraspecific variation for invasion risk assessment of commercially traded species: the Bombus terrestris Case. Conserv Lett 9(4):281–289. https://doi.org/10.1111/conl.12215
Lee CE (2002) Evolutionary genetics of invasive species. Trends Ecol Evol 17(8):386–391
Lee JC, Bruck DJ, Curry H, Edwards D, Haviland DR, Van Steenwyk RA, Yorgey BM (2011) The susceptibility of small fruits and cherries to the spotted-wing drosophila, Drosophila suzukii. Pest Manag Sci 67(11):1358–1367. https://doi.org/10.1002/ps.2225
Lombaert E, Guillemaud T, Cornuet JM, Malausa T, Facon B, Estoup A (2010) Bridgehead effect in the worldwide invasion of the biocontrol harlequin ladybird. PLoS ONE 5(3):e9743. https://doi.org/10.1371/journal.pone.0009743
Lozier JD, Mills NJ (2011) Predicting the potential invasive range of light brown apple moth (Epiphyas postvittana) using biologically informed and correlative species distribution models. Biol Invasions 13(10):2409
Marmion M, Parviainen M, Luoto M, Heikkinen RK, Thuiller W (2009) Evaluation of consensus methods in predictive species distribution modelling. Divers Distrib 15(1):59–69. https://doi.org/10.1111/j.1472-4642.2008.00491.x
Mateo RG, Broennimann O, Petitpierre B, Muñoz J, van Rooy J, Laenen B, Guisan A, Vanderpoorten A (2015) What is the potential of spread in invasive bryophytes? Ecography 38(5):480–487
Matsumura S (1931) 6000 illustrated insects of Japan-Empire, 1931, 8 + 1497-I-23 pp., 10 pis, Tokyo
Medley KA (2010) Niche shifts during the global invasion of the Asian tiger mosquito, Aedes albopictus Skuse (Culicidae), revealed by reciprocal distribution models. Glob Ecol Biogeogr 19(1):122–133. https://doi.org/10.1111/j.1466-8238.2009.00497.x
Nathan R (2006) Long-distance dispersal of plants. Science 313(5788):786–788
Peterson AT, Holt RD (2003) Niche differentiation in Mexican birds: using point occurrences to detect ecological innovation. Ecol Lett 6(8):774–782
Petitpierre B, Broennimann O, Kueffer C, Daehler C, Guisan A (2017) Selecting predictors to maximize the transferability of species distribution models: lessons from cross-continental plant invasions. Glob Ecol Biogeogr 26(3):275–287. https://doi.org/10.1111/geb.12530
Poutsma J, Loomans A, Aukema B, Heijerman T (2008) Predicting the potential geographical distribution of the harlequin ladybird, Harmonia axyridis, using the CLIMEX model. Biocontrol 53(1):103–125
Poyet M, Eslin P, Héraude M, Le Roux V, Prévost G, Gibert P, Chabrerie O (2014) Invasive host for invasive pest: when the Asiatic cherry fly (Drosophila suzukii) meets the American black cherry (Prunus serotina) in Europe. Agric For Entomol 16(3):251–259. https://doi.org/10.1111/afe.12052
Poyet M, Le Roux V, Gibert P, Meirland A, Prévost G, Eslin P, Chabrerie O (2015) The wide potential trophic niche of the asiatic fruit fly Drosophila suzukii: The key of its invasion success in temperate Europe? PLoS ONE 10(11):e0142785
Prentis PJ, Wilson JR, Dormontt EE, Richardson DM, Lowe AJ (2008) Adaptive evolution in invasive species. Trends Plant Sci 13(6):288–294. https://doi.org/10.1016/j.tplants.2008.03.004
Reznick DN, Ghalambor CK (2001) The population ecology of contemporary adaptations: what empirical studies reveal about the conditions that promote adaptive evolution. In: Microevolution rate, pattern, process. Springer, Berlin, pp 183–198
Rödder D, Engler JO (2011) Quantitative metrics of overlaps in Grinnellian niches: advances and possible drawbacks. Glob Ecol Biogeogr 20(6):915–927. https://doi.org/10.1111/j.1466-8238.2011.00659.x
Rombaut A, Guilhot R, Xuéreb A, Benoit L, Chapuis MP, Gibert P, Fellous S (2017) Invasive Drosophila suzukii facilitates Drosophila melanogaster infestation and sour rot outbreaks in the vineyards. R Soc Open Sci 4(3):170117
Schoener TW (1968) Anolis lizards of Bimini: resource partitioning in a complex fauna. Ecology 49:704–726
Schwartz MW (2012) Using niche models with climate projections to inform conservation management decisions. Biol Conserv 155:149–156
Shaw B, Brain P, Wijnen H, Fountain MT (2017) Reducing Drosophila suzukii emergence through inter-species competition. Pest Manag Sci. https://doi.org/10.1002/ps.4836
Soberón J, Peterson AT (2005) Interpretation of models of fundamental ecological niches and species’ distributional areas. Biodiv Inf 2:1–10. https://doi.org/10.17161/bi.v2i0.4
Thuiller W, Georges D, Engler R (2014) biomod2: Ensemble platform for species distribution modeling. R package version 3.1-64. http://CRAN.R-project.org/package=biomod2. Accessed Feb 2015
Tingley R, Vallinoto M, Sequeira F, Kearney MR (2014) Realized niche shift during a global biological invasion. Proc Natl Acad Sci USA 111(28):10233–10238. https://doi.org/10.1073/pnas.1405766111
Tingley R, Thompson MB, Hartley S, Chapple DG (2016) Patterns of niche filling and expansion across the invaded ranges of an Australian lizard. Ecography 39(3):270–280
Tochen S, Woltz J, Dalton D, Lee J, Wiman N, Walton V (2016) Humidity affects populations of Drosophila suzukii (Diptera: Drosophilidae) in blueberry. J Appl Entomol 140(1–2):47–57
Wares J (2005) Mechanisms that drive evolutionary change. Insights from species introduction and invasions. In: Sax DF, Stachowicz JJ, Gaines SD (eds) Species invasions: insights into ecology, evolution, and biogeography. Sinauer Associates, Sunderland, Massachusetts, pp 229–257
Warren DL, Glor RE, Turelli M (2008) Environmental niche equivalency versus conservatism: quantitative approaches to niche evolution. Evolution 62(11):2868–2883. https://doi.org/10.1111/j.1558-5646.2008.00482.x
Zhu G, Bu W, Gao Y, Liu G (2012) Potential geographic distribution of brown marmorated stink bug invasion (Halyomorpha halys). PLoS ONE 7(2):e31246
Zhu G, Gariepy TD, Haye T, Bu W (2017) Patterns of niche filling and expansion across the invaded ranges of Halyomorpha halys in North America and Europe. J Pest Sci 90(4):1045–1057
Acknowledgements
AF was supported by state funding by the Agence Nationale de la Recherche, through the LabEx ANR-10-LABX-0003-BCDiv, of the program “Investissements d’avenir” (ANR-11-IDEX-0004-02) and the ANR SWING (ANR-16-CE02-0015). The authors thank three anonymous reviewers for their constructive comments on previous versions of this manuscript. They also thank Blas M. Benito for helpful discussion and comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fraimout, A., Monnet, AC. Accounting for intraspecific variation to quantify niche dynamics along the invasion routes of Drosophila suzukii. Biol Invasions 20, 2963–2979 (2018). https://doi.org/10.1007/s10530-018-1750-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-018-1750-z