Abstract
To model the invasion of Prunus serotina invasion within a real forest landscape we built a spatially explicit, non-linear Markov chain which incorporated a stage-structured population matrix and dispersal functions. Sensitivity analyses were subsequently conducted to identify key processes controlling the spatial spread of the invader, testing the hypothesis that the landscape invasion patterns are driven in the most part by disturbance patterns, local demographical processes controlling propagule pressure, habitat suitability, and long-distance dispersal. When offspring emigration was considered as a density-dependent phenomenon, local demographic factors generated invasion patterns at larger spatial scales through three factors: adult longevity; adult fecundity; and the intensity of self-thinning during stand development. Three other factors acted at the landscape scale: habitat quality, which determined the proportion of the landscape mosaic which was potentially invasible; disturbances, which determined when suitable habitats became temporarily invasible; and the existence of long distance dispersal events, which determined how far from the existing source populations new founder populations could be created. As a flexible “all-in-one” model, PRUNUS offers perspectives for generalization to other plant invasions, and the study of interactions between key processes at multiple spatial scales.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
For the last few decades, biological invasions have offered a stimulating field of research, both in basic community ecology, to test existing theories and models of species assemblages (Callaway and Maron 2006; Sax et al. 2007), and from a more applied perspective, to prevent their deleterious effects on biodiversity, ecosystem functioning, economical activities and public health (Pimentel et al. 2005). A huge body of literature has tackled the biological invasion challenge, searching for general rules that could help predict which species are more likely to become invaders (‘invasiveness’, e.g. Kolar and Lodge 2001) and which ecosystems are more susceptible to invasion (‘invasibility’, e.g. Alpert et al. 2000). Early prospects for generalizations often failed, concluding that no general rule could be found (Lonsdale 1999; Williamson 1999). Maybe the incredible diversity of both invaders and their recipient ecosystems impaired such attempts, but also many analyses may have been flawed by focusing separately on different components of a system, instead of considering their combined interactive effects (Higgins and Richardson 1998, see also Richardson and Pyšek 2006 for a critical analysis of the dyad invasibility-invasiveness).
The exploration of single processes which contribute to plant invasions are common among a large number of published observational case studies and experiments (Richardson and Pyšek 2006). However, in recent years, modelling approaches have led to important insights by integrating elements in a more holistic way (Higgins and Richardson 1998; Thuiller et al. 2006). Several models have been developed to understand how different factors interact to determine invasion dynamics in particular systems (Marco et al. 2002; Cannas et al. 2003). All provide fascinating results that are interpreted in different ways, but still with limited scope for comparing fundamental results between studies. We consider that four key processes offer valuable perspectives for generalizations.
-
1.
Disturbance has long been recognized as a major factor which may promote biological invasion (Elton 1958; Crawley 1987), by releasing both space and resources for establishment (Davis et al. 2000; Shea and Chesson 2002). Invaders may also be more facilitated by disturbance in their exotic range than in their native one (Hierro et al. 2006). Using a spatially explicit simulation model, Pausas et al. (2006) found that the spatial spread of the wind-dispersed Cortaderia selloana (pampas grass) was increased with disturbance frequency, and also that spatial patterns of disturbance were more important than frequency. Consistently, using a stochastic matrix model to explore the influence of disturbance frequency on Prunus serotina (black cherry) demography, Sebert-Cuvillier et al. (2007) analytically demonstrated the existence of a critical threshold value, above which the invasion could start. These results highlight that when integrating a disturbance regime into a model, all aspects should be considered (Moloney and Levin 1996), including intensity (i.e. proportion of removed biomass), spatial extent (i.e. size and shape), temporality (i.e. frequency) and spatio-temporal patterns (i.e. temporal and spatial autocorrelation among individual disturbances).
-
2.
Propagule pressure (i.e., increased probability of invasion with the number of propagules arriving in a given location; Hierro et al. 2006; Lockwood et al. 2005; Colautti et al. 2006) and residence time (i.e., increased probability of invasion with time since introduction; Rejmánek 2000; Wilson et al. 2007) have been found to compensate for low inherent species invasiveness and/or low intrinsic ecosystem invasibility (Richardson and Rejmánek 2004; Richardson and Pyšek 2006). Thus, the classical view, which states that only some species have a high ‘invasiveness’ defined by a particular set of life-history traits, and only recipient ecosystems have a high ‘invasibility’ as they provide a given set of biotic and abiotic characteristics (Lonsdale 1999; Alpert et al. 2000), is progressively shifting towards a more elusive interacting continuum between those two components (Richardson and Pyšek 2006). In particular, the mass action of local dispersal has been shown to increase the chance for an invader to successfully establish, even in a priori lowly invasible habitats (Snyder and Chesson 2003; Sebert-Cuvillier et al. 2008).
-
3.
Long distance dispersal (LDD) has received growing attention in the literature. Although a rare event often involving a very small proportion of propagules, LDD has been found to ultimately control the rate of spread for a huge number of invasive plants (Higgins and Richardson 1999; Nathan et al. 2005; Trakhtenbrot et al. 2005; Garnier and Lecomte 2006). Long distance dispersal alone accounts for the usually observed exponential growth of the invasion front (Neubert and Caswell 2000; Cannas et al. 2006), as well as for the creation of new founder populations in distant suitable areas (Nehrbass et al. 2007; Sebert-Cuvillier et al. 2008).
-
4.
Environment heterogeneity was initially neglected in models of biological invasions (Skellam 1951), before being incorporated into spatially explicit models (Kot et al. 1996; Neubert et al. 2000). Heterogeneity has been shown to influence not only dispersion in both space and time, but also the impact on recipient ecosystems (Melbourne et al. 2007). The position of founder populations in relation to potentially invasible patches, and their spatial distribution are crucial to invasion patterns (Wilson et al. 2007). Spatially explicit models revealed that environment heterogeneity, especially connectivity between patches of suitable habitats, increased the speed of invasion (Söndgerath and Schröder 2002), but decreased the final spatial extent (Sebert-Cuvillier et al. 2008). Moreover, even when dispersal is incorporated as a multidirectional process, landscape heterogeneity makes the invasion highly directional (Sebert-Cuvillier et al. 2008).
In this paper, we describe a flexible general model, which incorporated the four key processes mentioned above (namely disturbance, propagule pressure and residence time, LDD, environmental heterogeneity), and explore their influence on the invasion dynamics of plant species. For this purpose, we incorporated a Lefkovitch matrix model accounting for local population growth (population sub-model) into a non-linear version of a Markov chain accounting for spatial spread (landscape sub-model), both being linked by dispersal functions. The ultimate goal was to provide a model that can be adapted to use with various invasive plant species to explore general hypotheses. However, to calibrate the model we used data collected for the American black cherry (Prunus serotina Ehrh.), an invasive tree spreading in European temperate forests. Then, to search for the relative importance of each component of the model on invasion, we conducted sensitivity analyses by varying the different parameters in the simulations. More specifically, we tested the following research hypotheses: the spatial spread of an invader at a landscape (regional) scale increases when (1) disturbance frequency and/or intensity increases; (2) local demographical processes increase propagule pressure and/or residence time; (3) habitat suitability increases with respect to landscape heterogeneity; (4) long-distance dispersal increases in intensity and/or maximal distance.
The PRUNUS model
Conceptual background
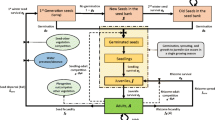
Our mathematical model relies on the conceptual model of alien plant spread proposed by Higgins and Richardson (1996; Fig. 1). In their model, the demographic process, which primarily takes place at a local scale, is the outcome of the interaction between the invader’s autecological attributes (invasiveness) and the recipient ecosystem attributes, including resource availability (invasibility). Alien abundance in both space and time typically involves a landscape or even regional scale, which is linked to the local scale through dispersal. The model also includes feedback effects of alien abundance on resource fluctuations, speeding up or slowing down the invasive spread.
Conceptual framework of alien plant spread (adapted from Higgins and Richardson 1996)
Here, we use a Lefkovitch matrix to model the demographic process. This approach ensures that any life cycle of the target species can be taken into account, with respect to their autecological attributes. This local model also includes stochastic environmental fluctuations of resource over time, to represent the unpredictable heterogeneity at a site (e.g., storm, fire).
The landscape is made discrete using a lattice of cells. A suitability index, which accounts for habitat suitability (i.e., local scale) with respect to the invader’s autecology, is applied to each cell. This index represents the predictable heterogeneity in both space and time (e.g., soil type, management-related disturbances). This index balances the transition probabilities of the population sub-model.
Finally, the local population sub-model is linked to the landscape model using a set of dispersal functions. The stage abundances for each cell of the discrete landscape are determined by a nonlinear version of a Markov chain using the dispersal functions.
Transition probabilities (population sub-model), suitability indices (landscape sub-model) and dispersal kernels (dispersal functions) are species-specific. For the purpose of this study, we developed our model for Prunus serotina, which is among the most intensively studied invasive plant species in Europe (Starfinger 1991, 1997; Deckers et al. 2005; Godefroid et al. 2005; Pairon et al. 2006; Chabrerie et al. 2007a, b; 2008; Closset-Kopp et al. 2007; Verheyen et al. 2007; Sebert-Cuvillier et al. 2007, 2008). This species has the added advantage of exhibiting a complex life-history cycle and invading complex forest ecosystems. Therefore, we believe that our ‘PRUNUS’ model could be applied to many other plant invasive plant species after several simplifications, particularly in the population sub-model.
Case study and study site
The American black cherry (Prunus serotina Ehrh.) is a gap-dependent tree species native to North America, which has been introduced in many European forests for ornamental, timber production, and soil amelioration purposes (Starfinger 1997). For at least three decades it has largely spread throughout the temperate forests of Western and Central Europe, particularly on well-drained, nutrient-poor soils (Starfinger 1997; Chabrerie et al. 2007a; Verheyen et al. 2007; Chabrerie et al. 2008). Its population dynamics have been studied in detail in northern France (Closset-Kopp et al. 2007). The seeds of P. serotina are able to enter closed-canopy forests and form a long-living sapling bank (‘Oskar syndrome’: no height growth, diameter increment <0.06 mm year−1, longevity > several decades). When a canopy gap occurs, saplings are released from suppression and grow rapidly (>56 cm year−1) to reach the canopy and fill the gap. In clearcuts and large gaps P. serotina often forms a low, closed carpet of small trees, which impedes natural regeneration of other species (Starfinger 1991; Chabrerie et al. 2007a).
Once established, an individual of P. serotina can self-maintain indefinitely by actively resprouting from its roots and stumps. Individuals become fertile at an average age of 8 years and produce numerous seeds (6,011 per tree on average), of which 42% are able to germinate (Closset-Kopp et al. 2007) whereas 55% have their kernel eaten by snout beetles (unpublished data). Several studies have shown that ca. 95% of the seeds are dispersed by gravity or after local regurgitation by birds in a radius of 5 m around the parent tree (Starfinger 1997; Deckers et al. 2005; Pairon et al. 2006). The remaining seeds (ca. 5%) are dispersed via mid- and long-distance dispersal events, by birds and mammals (especially foxes in the study area). The mean dispersion distance in forests has been measured at 100 and 918 m on average, for birds and foxes respectively. These species characteristics were used throughout the population sub-model of PRUNUS (Fig. 2) and have previously been used to construct a demographic model (Sebert-Cuvillier et al. 2007).
Flowchart showing the conceptual background of PRUNUS model, where a population submodel (inside the dash zone) is incorporated into a landscape submodel through dispersal. Numbered bold arrows indicate where are located the processes that potentially influence the invasion dynamics: 1 density of vectors, 2 habitat quality, 3 germination rate, 4 light availability, 5 longevity of suppressed saplings (“Oskars”) under shade conditions, 6 probability for “Oskars” to be released from suppression and grow to sapling2 stage, 7 light availability, 8 resprouting rate, 9 intensity of self-thinning during the aggradation phase, 10 adult longevity, 11 number of seeds which is annually produced, 12 post-dispersal predation rate
The PRUNUS model was developed as a ‘spatially realistic’ model, which could be used to study real populations in real landscapes. In this article we used field data collected from the forest complex of Compiègne-Laigue, which is located in northern France (49°22′N; 2°54′E; 32–148 m altitude). It was chosen because it contains a wide range of habitat conditions and is currently the most heavily invaded site by P. serotina in France. It is also representative of other ecosystems that are likely to be invaded by P. serotina in temperate Europe. Compiègne-Laigue forest is a mixed forest covering 18,244 ha, which is currently managed as an even-aged plantation of common beech (Fagus sylvatica), oaks (Quercus robur, Quercus petraea) and Scots pine (Pinus sylvestris). The clearcut-return interval for the forest is 180 year for Q. robur and Q. petraea, 110 year for F. sylvatica and 100 year for P. sylvestris. During each time interval, thinnings are conducted every 4–10 years. Natural disturbances mainly consist of treefalls and windthrows; three unusual storm episodes have damaged the SE part of the forest in 1984, 1990 and 1999. Prunus serotina was probably first introduced around 1850 in a former arboretum located inside the forest (‘Les Beaux Monts’ area).
Construction of the landscape sub-model
We first converted the real landscape into a raster map, following the approach developed in Sebert-Cuvillier et al. (2008), with the notable difference that each cell consisted of an ecologically homogeneous unit (i.e., relief, soil type, soil moisture, and canopy composition were assumed to be the same over the entire cell area), matching our definition of the local scale. Using Geographic Information System (GIS) technology, we superimposed a lattice of 50 × 50 m cells over the forest vector map of Compiègne-Laigue. This generated a grid of 401 × 425 cells, among which 72,900 and 97,525 corresponded to forest and non-forest cells, respectively.
A habitat suitability index (V i ) was then assigned to each forest cell i, ranging from 0 (cell resistant to invasion whatever the diaspore pressure, including non-forest cells) to 2 (cell which would be invaded with certainty if an invader disperses into it; Appendix 1). To elaborate this habitat suitability index, we used the establishment probabilities of Prunus serotina according to both soil type and soil moisture, as calculated by Chabrerie et al. (2007a). At each time step the number of Prunus serotina individuals in cell i that are released from suppression is multiplied by V i . All 170,425 suitability indices V i defined the time-independent ‘suitability vector’ V.
Construction of the local population sub-model
We used the stochastic matrix model with stage distribution (i.e., Lefkovitch matrix) elaborated by Sebert-Cuvillier et al. (2007) to describe population dynamics in homogeneous environments. Eleven life stages were used to account for the population structure: ‘seed-2’ and ‘seed-1’ are seeds able to germinate 2 or 1 year after setting (i.e., dormant seeds of the transient seed bank); ‘seedling’ corresponds to germinating seeds; ‘sapling1’ (“Oskar”) to ‘sapling7’ are saplings which are 1–7-year old (8 years representing the average delay before sexual maturity); and ‘adult’ corresponds to reproducing tall shrubs and trees. All these stages are linked by transition probabilities, i.e., a probability for an individual of a given stage to reach the stage after. Stages ‘sapling1’ and ‘adult’ have a further probability to be stationary, to account for the ‘Oskar syndrome’ (i.e. suppressed saplings) and the mean tree life span, respectively.
Resprouting capacity was considered as the possibility for individuals from stage ‘sapling1’ to stage ‘adult’ of decreasing their size to return to the ‘sapling1’ stage, after their aerial stem has died or been cut down.
As light represents the main critical resource for the gap-dependent Prunus serotina, it was incorporated as a binary variable: present (gap) or absent (shade). Light may be available both periodically, in the case of forestry-related cutting rotations, and stochastically, after a storm-induced treefall, for example. We considered a mean duration of Per i = 7 years of light availability, until the gap was filled-in by one or several trees. After this time-lag, the canopy is closed and the light is switched off (i.e. resource is no longer available until the next disturbance).
Let A, B, C and D be the transition matrices corresponding to shade state, treefall-induced light state, canopy closure state, and clearcut-induced light state of the environment, respectively. Full details about these four matrices are given in the section “Appendix 1”.
When a storm-induced canopy gap occurs, matrix B is repeated 7 times and followed 1 time by matrix C; the matrix B 7 C is thus applied.
Forest management-related disturbances are periodic and depend on the target canopy tree species (see “Case study and study site”). Each cell i, which support a single target tree species, is thus characterised by 3 silvicultural parameters: the clearcut return-interval (Cl i ), the thinning return-interval (Th i ), and the proportion of gaps after a thinning (Pth i ). Hence:
-
when a clearcut is conducted in cell i, matrix D is implemented once and followed by matrix \( B^{{t_{i} - 1}} C, \) where t i is the time-lag before canopy closure, which is species-specific (see “Appendix 1”). The matrix \( DB^{{t_{i} - 1}} C \) is thus applied.
-
when a thinning is conducted in cell i, only a proportion Pth i of the forest floor is reached by light; the matrix Pth i B + (1 − Pth i ) A is thus applied.
At each time step n in cell i the environment determines which one of the three matrices is used:
-
\( A_{n} = DB^{{t_{i} - 1}} C \) when a clearcut is conducted, i.e., when the time interval T i (n) between n and the last clearcut equals Cl i .
-
A n = Pth i B + (1 − Pth i ) A when a thinning is conducted, i.e., when T i (n) is a multiple of Th i .
-
else A n = A (no disturbance since at least 8 years) with probability 1 − P or A n = B 7 C with probability P, for P ∈ [0,1], the latter giving the probability of occurrence of a (stochastic) natural disturbance (e.g., treefall, windthrow).
For each cell i, we created an 11 component-vector P i (n). For 1 ≤ j ≤ 11, P i,j (n) gives the number of individuals of stage j in cell i at time n. Hence, P(n) is a matrix with 170,425 rows and 11 columns, and the product of P i (n) by matrix A n gives the number of individuals in cell i at time n + 1; hence, \( P(n + 1) = A_{n} P(n). \) This number was forced not to exceed the carrying capacity C max of cell i, which was defined as the average maximum Prunus serotina stem density supported by a 2,500 m2-area. In accordance to our field measures and data presented by Sebert-Cuvillier et al. (2007), C max was set at: 630 stems per cell for adult and sapling7 stages; 1,465 stems per cell for sapling6 stage; 21,265 stems per cell for sapling5 stage; 57,950 stems per cell for sapling4 stage; 135,090 stems per cell for sapling3 stage; 177,750 stems per cell for sapling 2 stage; 250,000 stems per cell for sapling1 (Oskar) stage; and 375,000 stems per cell for seedling stage. No limitation applied to stages seed-2 and seed-1.
We also created a coefficient G i (n) containing the number of seeds which are produced each year by the mature trees in cell i (i.e. the number of seeds participating to dispersion). G(n) is a vector containing 170,425 components.
Linking population and landscape sub-models through seed dispersion
Three dispersal types were distinguished: long-distance (corresponding to mammals, especially foxes), mid-distance (corresponding to birds exporting seeds outside a given cell) and short-distance (corresponding to gravity and local regurgitation by birds), with proportions P f = 0.5%, P b = 1.5% and 1 − P f − P b = 98% respectively, to fit field measures (see “Case study and study site”) and to take into account the cell size. Mid- and long-distance dispersal were described by two lognormal functions: f b and f f (see “Appendix 1”). Hence, our model simulates both the mass action of local dispersal and the random nature of long-distance dispersal.
PRUNUS is thus a quantitative model describing, for each cell i of a real landscape, the number of Prunus serotina individuals per development stage j (from dormant seeds to mature trees). The time step of the model is 1 year since both seed production and tree growth are rhythmic, discrete processes with a 1 year period. The model output is dependent upon eleven variables (Fig. 2): density of dispersal vectors, habitat quality, germination rate, light availability (which acts at two steps), probability of suppressed saplings survival in the shade, probability of transition from Oskar stage to sapling2 stage, resprouting capacity, growth rate (incorporating competition for light), adult survival rate, number of produced seeds, and seed mortality rate (incorporating both seed predation and embryo viability). Variables and parameters of the model are summarized in Table 1. Full information about the model, transition matrices, invasibility indices, deterministic measures of each dominant tree species and dispersal is given in Appendix 1.
Rules
We used a split-step method to compute the abundance of Prunus serotina in each stage j for cell i at time n + 1. At each time step n:
Rule 0: for each cell i at time n, one matrix from A, B, C or D is selected to match the environmental state.
Rule 1: Local evolution in cell i in one time step:
In order not to exceed the carrying capacity of the cells: for j>2
Creation of the vector G, which contains the number of seed produced during the year:
where Stot is the total number of seeds produced per tree.
Rule 2: Seed dispersal: For each cell i, P f G i (n) and P b G i (n) seeds are dispersed outside the cell by foxes and birds, respectively, while (1 − P f − P g )G i (n) seeds are staying in cell i. For each seed escaping cell i, the dispersal distance is given by the dispersal lognormal functions while the direction is randomly chosen from 0° to 360° to determine which cell will receive this seed. Dispersal is restricted to seed stages (j ≤ 3). Hence, the abundance of individuals at the sapling and adult stages does not change: for j > 3, P i,j (n + 2/3) = P i,j (n + 1/3).
Rule 3: Establishment: An individual can grow from sapling2 up to the canopy in cell i if the habitat suitability index V i of cell i is greater than 1. With regards to the demographic strategy of our studied species (see above), we applied this index V i to stage 5 (i.e. sapling-2 stage):
Simulations
Model verification and validation
The model was verified by checking whether it behaved as expected and gave plausible outputs. Unfortunately, we could not conduct a rigorous validation of the model, since detailed accounts of the initial invasion dynamics of P. serotina at the study site are unknown. The current distribution and abundance of the species is also likely to be biased by measures used to control its expansion since ca. 1975, especially since these control measures have varied considerably in their frequency, intensity, spatial extent, and methods. Hence, we restricted our validation procedure to a quantitative comparison of the maps showing the current observed distribution of P. serotina (in 2003, see below) with the predictive distribution maps produced by the simulation initiated at the presumed point of introduction in 1850 (cell i 0 = 94,447 at n = 0, ‘Les Beaux Monts’) and running for 153 iterations.
Sensitivity analysis
To measure how much parameters and forcing functions contribute to the model response, we conducted a sensitivity analysis of the model. For this purpose, we simply ran simulations in which each input variable, parameter or forcing function was varied while the others were kept fixed, and recorded the corresponding change in the state variables. The relative change in parameters was chosen so that the range largely exceeded the uncertainty. For example, if a given parameter was known within ±10%, we varied it within at least ±50% up to extreme values of the entire range. Following this procedure, we tested the relative importance of each potentially critical step (Fig. 2).
Step 1: sensitivity to dispersal parameters—The relative proportions of seeds dispersed by birds (P b ) and foxes (P f ), that were estimated to 1.5 and 0.5% respectively, were successively increased from 0 to 4%.
Step 2: sensitivity to landscape suitability—All indices V i of the suitability vector were successively multiplied by 0.5, 0.67, 0.8, 1.25, 1.5 and 2 (i.e. from 50 to 200% of their real value), with respect of the landscape heterogeneity.
Steps 3, 5, 6, 8, 9, 10, 11, and 12: sensitivity to demographic parameters—Depending on the life stage under consideration, we applied the following ranges of values for the corresponding input parameter:
-
step 3 (germination rate): from 0.05 to 1;
-
step 5 (survival probability of sapling-1 “Oskar” stage under shade conditions): from 0 to 1;
-
step 6 (transition probability from sapling-1 “Oskar” to sapling 2 stage): from 0.1 to 0.9;
-
step 8: sensitivity to the resprouting response of saplings was investigated by varying the 7 resprouting coefficients in matrix B from 0 to 1 (self-thinning process) and the 6 resprouting coefficients in matrix C from 0 to 1 (canopy closure), both independently and simultaneously, and then separately for the 6 coefficients in matrix D by varying these from 0 to 1;
-
step 9 (intraspecific competition for light): from 0.1% to 10%;
-
step 10 (adult survival probability): from 0.94 to 0.99;
-
steps 11 (reproduction rate) and 12 (seed survival probability): those two parameters were studied simultaneously, by multiplying the mean annual number of seeds Stot by 0.1, 0.2, 0.5, 2, 5 and 10 successively.
Moreover, we investigated the impact of the carrying capacity Cmax by multiplying it by 0.01, 0.1, 0.5, 2, 10 and 100 successively.
Steps 4 and 7: sensitivity to resource availability—Light availability at the forest floor was investigated separately for natural and anthropogenic disturbances. For the former, stochastic light arrival probability P was varied from 0 to 1. For the latter, periodic light arrival was varied by multiplying Cl i by 0.33, 0.67, 1.5 and 3 for each cell i.
Computation and graphical representations
All simulations used C++ language. For the sake of simplicity all simulations were started with ten reproducing trees already established in the same cell at time n = 0 (i.e., cell i 0 = 94,447 in 1850 to match real conditions of our case study). For each simulation round, we averaged the number of individuals per stage and per cell at each time step over 30 iterations. Moreover, we distributed the 11 stages among the four following groups to clarify the figures: (1) seed stage (including seed-2, seed-1 and seedling stages), (2) Oskar stage (i.e., sapling1 stage), (3) sapling stages (incl. sapling2 to sapling7 stages), (4) adult stage.
For the purpose of the model validation, output maps were made binary (i.e. presence/absence maps), assuming that species distribution has been less impacted upon by control operations than species abundance. As reliable field data were available only for the Compiègne forest, we restricted our statistical analysis to this forest, which represents 79% of the whole area (14,417 ha). Observed distribution maps were built using data from an intensive field survey conducted in 2003, during which presence/abundance of P. serotina among tree, shrub and herb layers was scored for each stand of the forest (Chabrerie et al. 2007a). When a stand was supporting several soil types and/or several management units, each “homogeneous” unit had been scored independently. The resulting map comprised 6,434 polygons ranging from 8 to 563,600 m2 (mean ± standard deviation = 22,800 ± 30,300 m2).
To cope with the difference of spatial grain between the two sets of maps (2,500 vs. 8 to 563,600 m2), we converted the observed distribution (vector) maps into binary raster maps by superimposing a lattice of 50 × 50 m cells on them. All cells that matched an occupied polygon were considered ‘invaded’. Three statistical measures of goodness-of-fit were subsequently computed: overall agreement, i.e., the percentage of cells that were correctly predicted by the model, Κstandard, i.e., the kappa statistic, and Κlocation, i.e., the component of Κstandard quantifying the proportion of agreement due to location (Pontius 2000).
We plotted two descriptors against time to represent the invasion dynamics: propagation distance Pr n , which was calculated as the Euclidian distance between i 0 and the furthest cell from i 0 where at least one individual is, and invasion extent I n which is the percentage of the 72,900 cells with more than one individual at time n.
In sensitivity analyses, outputs were graphically represented, by the invasion extent I n against time, only at the adult stage, and only for model parameters/functions responsible for significant relative changes.
Results
Model verification and validation
The model behaved as expected. Seeds quickly reached numerous cells whereas the establishment of saplings and trees was clearly slower (Fig. 3). The proportion of the forest which became inhabited by the light-independent life stages of P. serotina increased logarithmically from approximately 25 and 45 years for seeds and Oskars, respectively, after the plantation of the first founder population (Fig. 3a). Conversely, this proportion increased linearly for light-dependent life stages, from ca. 50 and 75 years for saplings and adults respectively, following the first introduction. At the end of the simulation, almost all cells contained seeds (89%) and suppressed saplings (85%), but fewer were colonised by saplings (17%) and adults (11%).
Quantitative descriptors showing the spatial spread of four life-stages of Prunus serotina (seed, sapling 1, saplings and adult) in the Compiègne-Laigue forest following the introduction of 10 fertile trees in cell i 0 = 94,447 at n = 0 (year 1850). a invasion extent (percentage of the 72,900 forest cells that are invaded). b propagation distance (distance from the cell of first introduction in kilometres)
Before the maximum value is reached, the propagation distance of Oskars and adults increased almost linearly with time, after a lag of ca. 10 and 45 years respectively, indicating a constant progression of the invasion edge (Fig. 3b). Conversely, although increasing with time, the propagation distance of seeds exhibited important fluctuations which only ceased in ca. 1895 when the adult population started to spread. This irregularity in seed dispersal suggests that the founder population is particularly sensitive to environmental stochasticity during this time lag. The propagation distance of saplings also show important fluctuations until the maximal value is reached, towards year 1970, due to the dependence of those states upon light availability.
The model produced predicted distribution maps that roughly match the observed distribution maps, given the difference in spatial resolution (Fig. 4). The overall agreement is 74, 69 and 64% for the tree, shrub and herb layer respectively. Kappa standard/Kappa location statistics are 0.40/0.62, 0.39/0.54 and 0.32/0.68 for the tree, shrub and herb layer, respectively. Overall, predicted maps tended to overestimate the number of invaded cells, especially at the SW and NE forest margins, often where the aging coppice-with-standards have not yet been converted into high forests. The overestimation was more important for the tree and, to a lesser degree for the shrub, than for the herb layer. Conversely, the model slightly underestimated the invasion into the SE quarter of the forest, which is the section of forest which has been severely damaged by storms during the last three decades.
Binary maps showing the presence (in black) and absence (in grey) of Prunus serotina in the Compiègne forest, among the tree (top), shrub (middle) and herb (bottom) layers, as predicted by the PRUNUS model (on the left) and recorded on the field (on the right). Predicted maps were obtained by introducing 10 mature trees in cell i 0 = 94,447 at n = 0 (year 1850) and running 153 iterations
Sensitivity to the different processes
Dispersion parameters
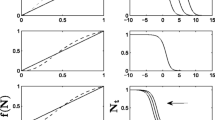
Variations in the proportion of seeds dispersed by birds altered neither the invasion extent nor the propagation distance. Conversely, the proportion of seeds dispersed by fox was crucial to the invasion dynamics. When the fox dispersal proportion was set to 0%, the invasion extent only reached 4%. As soon as LDD was incorporated, the invasion extent increased linearly, up to values between 13 and 18%, irrespective of the value of this proportion (Fig. 5a).
Sensitivity of Prunus serotina invasion to variations of different variables of the model PRUNUS. The invasion extent, taken as the percentage of the 72,900 forest cells that are invaded, is plotted against time, following the introduction of 10 mature trees in cell i 0 = 94,447 at n = 0 (year 1850) and running 153 iterations. a Sensitivity to the proportion of seeds (%) undergoing long-distance dispersal by foxes; b sensitivity to the variations (%) of the habitat suitability index, landscape heterogeneity being conserved; c sensitivity to the proportion of viable seeds; d sensitivity to the seed survival rate (%); e sensitivity to the variations (%) in the intensity of self-thinning; f: sensitivity to the probability of treefall occurrence
Habitat suitability
While the invasion extent reached 11% at the end of the simulation run in the real landscape, it averaged 6, 8, 9, 13, 14 and 17% when all invisibility indices were multiplied by 0.5, 0.67, 0.8, 1.25, 1.50 and 2 respectively (Fig. 5b).
Demographic parameters
Among the eight demographic parameters tested, only three significantly influenced the outcome of the invasion, namely the seed germination capacity, the proportion of seeds participating in dispersion, and the intensity of the self-thinning process.
The germination capacity (step 3) and seed survival rate (combining the number of viable seeds produced (i.e., step 11) and the proportion of seeds surviving predation and natural death (i.e., step 12) were the most sensitive components, with higher values resulting in greater extent of invasion (Fig. 5c, d). For example, the invasion extent increased from 2.5 to 29% as the amount of seeds participating in dispersal is multiplied by 0.1 to 10.
With regards to the intensity of the self-thinning process (step 9), the invasion extent exponentially increased along the range of tested values, despite the forcing function of carrying capacity (Fig. 5e). Invasion extent increased from 5 to 20% for an overall transition rate ranging from 1 to 10% between sapling2 and adult stages.
The proportion of sapling1 “Oskars” able to reach the sapling2 stage (step 6) and, to a lesser degree, the Oskar survival rate (step 5), impacted upon the invasion process only when they were set to unrealistic values close to 0.
Neither variation of the resprouting rates, nor ‘reasonable’ variation of adult longevity impacted upon the invasion process. However, for the latter, extremely low values strongly decreased the invasion extent. For instance, when adult longevity was set to 1 year, it only reached 0.1%.
Resource availability
As expected, the invasion process was highly sensitive to light availability. The invasion extent increased from 6 to 85% when the probability P of disturbance-induced treefall (steps 4 and 7) was increased from 0 to 1 (Fig. 5f). Even when P = 0 the invasion extent was not zero, since we considered a managed forest and hence disturbances were included in the model. It is noteworthy that management programmed disturbances were less influential on the invasion process than stochastic natural disturbances: the invasion extent averaged 6, 9, 13 and 14%, when the clearcutting frequency was divided by 3 and 1.50 and multiplied by 1.50 and 3, respectively.
Discussion
Our main goal was to build a flexible general model of plant invasion dynamics (PRUNUS), which incorporated four key processes (disturbance, propagule pressure/residence time, long-distance dispersal, and environment heterogeneity) that we had identified from the literature as having an important influence on the establishment, spread and distribution of invading species. The holistic inclusion of these processes allowed us to explore the role of each specific process as well as their combined interactive effects on invasion dynamics. Moreover, as a spatially realistic model (sensu Hanski 1999), PRUNUS incorporates landscape heterogeneity and thus the spatial autocorrelation of habitat suitability (i.e., suitability of two similar habitats may differ according to their spatial location in the landscape). In the following discussion, we address the realism of the model for the special case of P. serotina, examine the processes driving the invasion dynamics of this species, and discuss what generalisations PRUNUS makes towards the theory of invasive plant ecology.
How closely does PRUNUS match the real world?
PRUNUS was calibrated with empirical data from P. serotina and the Compiègne-Laigue forest and behaved as expected. Spatial spread patterns at the landscape scale, as well as the distribution of individuals among life stages, were consistent with field observations and confirm earlier results that were obtained with a matrix population model and a simple mechanistic model of spatial spread (Sebert-Cuvillier et al. 2007, 2008).
Goodness-of-fit statistics gave satisfactory results between observed and predicted distributions, even if the predicted maps tended to overestimate the extent of species invasion. This overestimation increased with distance away from the site of the first introduction. Hence, prediction accuracy also declined with time. These discrepancies may be accounted for by the exclusion, within the model, of the plant invasion control measures performed in the area during the last three decades. These operations are expected to have significantly slowed the spread of the invader, hence to have reduced its spatial extent and its abundance in invaded stands.
Also, the observed data used for validation may be misleading. Even though an intensive field survey has been carried out to check for presence/abundance of Prunus serotina in the Compiègne forest, the area is so vast (14,417 ha) that some stands may have been falsely considered as uninvaded during field investigations. The probability of such an underestimation error is expected to increase when the visibility is low, a stand is weakly invaded, when the invader occurs only as saplings. This is particularly expected on the margins of the forest, where the relief is the most uneven and where many stands are still supporting aging, dense coppices-with-standards. From a more technical point of view, when the vector map was converted into a raster map (see “Computation and graphical representations”), we may have overestimated the number of invaded cells by considering all cells matching an occupied polygon without respect to its size and population density.
Several approximations may have also contributed to this overestimation. Firstly, we considered that the proportion of seeds undergoing LDD (i.e. fox-dispersed seeds) was constant over time, thus neglected fluctuations of vector density and a potential ceiling to seed density above which the number of LDD-seeds would not increase. However, our sensitivity analysis showed that all LDD intensities greater than zero would not significantly alter the invasion dynamics.
Secondly, to account for the self-thinning process during stand development (i.e. density dependence) we applied the same values of carrying capacity and transition coefficients to seedling/sapling/adult stages for all cells, irrespective of soil or vegetation type. As those values were measured in pure stands of P. serotina, also neglected was competitive exclusion by other species (only the dominant canopy tree species has been incorporated into the habitat suitability index), as well as variations in water and nutrient availability. As the model is found to be sensitive to those transition coefficients, this approximation may have indeed impacted upon the results.
Thirdly, we ignored demographic stochasticity and Allee effects which are thought to be able to slow down biological invasion (Renshaw 1991; Buckley et al. 2003; Drake and Lodge 2006). Compared to environmental stochasticity, they have been found to play a minor role in the population growth of large sized populations (Haccou and Iwasa 1996; Snyder 2003). However, our sensitivity analysis revealed that both the number of produced seeds and their germination capacity were important controls of the invasion dynamics. Although not included in the model, pest attacks and extreme climatic events (e.g. seedling mortality due to severe summer drought, low fecundity due to spring frost) are not rare in the study area, so may have reduced the observed population density, and hence slowed the invasion, especially at the leading edge.
Key processes driving Prunus serotina invasion
Several key processes emerged from our sensitivity analysis, which were also the elements of the model which needed to match the field situation most accurately in order to produce reasonable model estimates.
Firstly, as expected for such a gap-dependent species, disturbance frequency (steps 4 and 7) emerged as the most influential factor on the spread of P. serotina within our model. The results supported the first part of our research hypothesis by showing that the spatial spread of an invader at a landscape scale increases with disturbance frequency and/or intensity. Consistent with earlier studies, both invasion speed and extent increased with disturbance frequency (Alpert et al. 2000; Lake and Leischman 2004; Pausas et al. 2006). More frequent disturbances lead to a higher chance of arrival for an invader’s offspring and therefore its subsequent establishment in a temporarily invasible habitat. Surprisingly, management-associated disturbances (i.e. the cutting/thinning rotation length) had only a limited impact on the invasion speed, compared to natural disturbance (i.e., frequency of storm-induced treefalls). Studying invasion-disturbance relationships on a floodplain, Pyle (1995) reported opposing results, with large-scale anthropogenic disturbances enhancing invasion while small-scale natural disturbances had no effect. This may be explained by the pattern and regularity in the type of disturbances modelled in the two studies. In our study, natural disturbances were stochastic events, whereas in the Pyle study (1995), anthropogenic events were stochastic. Therefore these events could be described as ‘real’ external disturbances. Conversely, the more or less regular periodicity of anthropogenic (this study) or natural (Pyle 1995) disturbances ensures they form part of the normal ecosystem function, and even of their ‘evolutionary’ history (Briske et al. 2003; Decocq et al. 2004). This regular disturbance regime then acts as a stress on the system rather than as a real disturbance, but is still sufficient to allow a progressive invasion of the forest. Whilst Pausas et al. (2006) concluded that disturbance regularity rather than disturbance frequency, modified invasion pattern assessed simultaneously in this study, these authors assessed these parameters in separate simulation sets, and over much shorter time intervals than those used in this model.
Secondly, we found strong support for the second part of our research hypothesis. Local demographical processes, which increased propagule pressure and/or residence time, enhanced the spatial spread of an invader at the landscape scale. The invasion process was highly sensitive to the number of viable seeds, which takes into account both the number of seeds which is annually produced by a mature tree (step 11), and the proportion of those seeds surviving post-dispersion predation (step 12). The resulting number (i.e. number of individuals reaching the Oskar stage) can be directly related to propagule pressure or “mass effect” (Williamson 1996). To a lesser degree, the adult tree longevity (step 10) was also found to influence the invasion process, with shorter tree life-spans resulting in a slower invasion. Adult tree longevity can be related to both residence time (Rejmánek 2000; Wilson et al. 2007) and propagule pressure. Every year an established mature tree produces a number of viable seeds; hence the cumulated number of seeds, and therefore its propagule pressure will increase with its residence time. As most seeds are dispersed either beneath the canopy of the mother tree or in its close vicinity, the risk of local extinction of an already established population decreases as the number of seeds produced increases, and the probability of establishing in adjacent, less invasible areas increases (Snyder and Chesson 2003; Sebert-Cuvillier et al. 2008). Also, the chance of the invader’s offspring finding a distant suitable habitat to create new founder populations increases as the number of seeds capable of undergoing LDD increases.
The model was also highly sensitive to two other demographical characteristics, namely the germination rate of seeds (step 3) and the intensity of the self-thinning process (step 9). These results contrasted with those of an elasticity analysis of the local sub-model, which failed to find a crucial role of those two rates on the local population dynamics; instead, both adult and Oskar survival probabilities were the most influential parameters (Sebert-Cuvillier et al. 2007). Therefore the factors controlling the invasion varied with the spatial scale, as formerly suspected (Pauchard and Shea 2006). In addition, the model successfully generated invasion patterns at larger scales from local demographical processes.
As the germination rate increased, the sapling bank accumulated more individuals per time unit. Hence, when a gap was created the number of individuals participating in the aggradation phase was higher, and thus the time taken to reach the carrying capacity of the cell was shorter. Similarly, the number of individuals which reached the canopy increased as self-thinning intensity was decreased, and therefore the carrying capacity of the cell would also be achieved earlier. In both cases, the local density of reproducing adults increased more rapidly. In addition an increase was observed in the overall number of seeds participating in dispersion, especially in LDD, thus contributing to an increase in the “mass effect” of the local populations (Williamson 1996). As a result, the probability for the invader to disperse outside the cell and subsequently establish new local populations into distant suitable habitats is increased (Martinez-Ghersa and Ghersa 2006), speeding-up its spatial spread at the landscape scale. This density-dependent emigration rate has been recently recognized in animal metapopulation biology (Hanski 1999; Saether et al. 1999; Amarasekare 2004). Surprisingly, it seems to have been largely ignored in plant invasion ecology, with the notable exception of Neubert and Caswell (2000) who have shown, using a matrix population model coupled to integrodifference equations, that invasion speed increases with intrinsic population growth rate. It should also be noted that the model was not sensitive to the value of the carrying capacity. This indicates that crucial to the model is not so much the maximal density that the species can achieve at a local scale, but the rate at which maximal density is achieved.
Thirdly, consistent with the third part of our research hypothesis, when landscape heterogeneity was kept constant, varying habitat quality influenced the invasion speed more or less linearly. As habitats were simultaneously made more suitable for Prunus serotina, the invasion speed increased. A higher proportion of the landscape mosaic became potentially invasible and areas that would impair the invasion spread were reduced. This is consistent with the simulation results of Söndgerath and Schröder (2002) that showed that the total amount of suitable habitat and connectivity between patches of suitable habitats increased the invasion speed.
Finally, consistent with a growing body of literature, we found that long-distance dispersal played a crucial role in the spatial spread of P. serotina (Kot et al. 1996; Clark et al. 1998; Higgins and Richardson 1999; Neubert and Caswell 2000; Nathan et al. 2005; Trakhtenbrot et al. 2005; Garnier and Lecomte 2006; Sebert-Cuvillier et al. 2008). This confirms the results of Neubert and Caswell (2000), which showed that when dispersal was composed of long- and short-distance dispersal, it was the long-distance component that governs the invasion speed, even when LDD was rare. Furthermore, it confirms that it is not so much the intensity of LDD which accounts for the invasion spatial patterns, but its very existence (Garnier and Lecomte 2006). Therefore, with reference to the final part of our research hypothesis, the model showed that the spatial spread of an invader at a landscape increased with maximal dispersion distance, but was independent of LDD intensity. Hence, knowing the tail of the dispersal kernel is much more critical to understand invasion spread than knowing the proportion of seeds undergoing LDD, making it an important attribute of plant invasiveness (Trakhtenbrot et al. 2005; Richardson and Pyšek 2006).
Towards generalizations using a universal model of plant invasions?
The PRUNUS model incorporates both local processes, using a stage-structured population matrix, and regional processes, through a spatially explicit matrix and seed dispersal kernels. The coupling of the two sub-models (local and landscape sub-models) was tractable because their mathematical formalism were similar. All factors that potentially influence the invasion spread of a plant species are thus integrated into a single model and sensitivity to each of these factors can be easily tested. Although we restricted our analysis to the individual influence of each parameter, the model also allows for simultaneous testing of the key processes to explore interactive effects that may be compensatory (e.g. how far adult longevity can compensate for a low fecundity?), antagonistic (e.g. would a decrease in natural disturbance frequency reduce the effects of an increasing propagule pressure?) or synergistic (e.g. does an increasing local population density magnify the effects of an increasing disturbance frequency?).
Throughout this article we have described the application of PRUNUS to a case-specific scenario, with the model results presented relating to a specific set of initial conditions, organism (P. serotina) and landscape (Compiègne-Laigue forest). However, we believe that PRUNUS can be extended to many other situations, involving different focal species and sites, offering perspectives for comparisons and generalizations, but also for the management of plant invasions. To be more illustrative, we take hereafter the example of the Giant Hogweed (Heracleum mantegazzianum Sommier & Levier), one of the most problematic invasive species in Europe, using data from Nehrbass et al. (2007), Pergl et al. (2007) and Jongejans et al. (2008).
For the landscape sub-model of PRUNUS the only requirements are a map indicating habitat suitability and information on disturbances at the study site. Both sets of data are commonly available for a number of areas and used in GIS technology, which has become a popular tool for management and planning. In this form, the grain and the scale of the map-derived lattice can be easily fitted (e.g. 5 × 5 m for H. mantegazzianum). The habitat suitability index is then determined according to the autecology of the focal species (e.g. from 0 for forest to 2 for road verges in the case of H. mantegazzianum).
The population sub-model theoretically requires a huge amount of information about all life stages of the focal species and the carrying capacity of habitats, which is rarely available for most species. However, our sensitivity analysis revealed that few parameters are of utmost importance in explaining invasion spread. We found the most influential factors to be the quantity of seeds produced as well as their survival and germination rates (20,500 seeds, 3 years and 91% respectively for H. mantegazzianum). These can be summarized into a single transition probability accounting for the overall fecundity of a mature plant (i.e. number of germinating seeds per mature individual per year: 6,218 seeds year−1). In addition, the intensity of the self-thinning process can be incorporated as a single transition probability accounting for the proportion of germinating seeds reaching maturity (1 m−2 for H. mantegazzianum). Hence, the local sub-model can be easily reduced to a 2 dimension-matrix (i.e. germinating seeds and adults), and the length of the 2 life stages can be altered to match the corresponding average time observed for each specific focal species (e.g. 4 years on average for H. mantegazzianum).
The most crucial step in applying PRUNUS to other species is to determine the shape of their dispersal kernel(s), especially the length of the tail (Jongejans et al. 2008). When several vectors are involved, the model can be simplified by only including the one involved in the longest dispersal distance (e.g. 2.5% of the 20,500 seeds produced by an adult are dispersed in a range of 10 m to 10 km around the parent H. mantegazzianum).
To develop our model, we chose a very heterogeneous landscape, a highly stochastic environment and a target species exhibiting a complex life-history cycle. Thus, we expect that PRUNUS can be applied to many other cases of plant invasion by undertaking several simplifications and using a limited set of parameters. For example, applying PRUNUS to H. mantegazzianum would require only one transition matrix (no gap-dependence) with fewer lifestage probabilities needed than those used for P. serotina: since H. mantegazzianum is monocarpic (no adult survival) and does not possess a sapling bank, all stationary probabilities and all resprouting probabilities associated to adult and sapling stages could be excluded.
Conclusion
Our results clearly show that local demography, dispersal and spatio-temporal landscape characteristics strongly interacted to control both the speed and extent of a plant invasion. Three local processes were found to be influential at the landscape scale: adult longevity; adult fecundity; and intensity of the self-thinning process. All three controlled the local population density, and therefore influenced the total number of seeds participating in dispersion, when offspring emigration was represented by a density-dependent phenomenon. Three landscape processes were found to control invasion patterns: LDD; habitat quality; and disturbance frequency. LDD and habitat quality influenced the ability of the species to create new founder populations, whilst disturbance frequency determined the distance between the new local populations and their parent founder population. Furthermore, PRUNUS showed that it was the local processes which controlled the intensity of the seed rain, whilst landscape processes determined the success of establishment.
References
Alpert P, Bone E, Holzapfel C (2000) Invasiveness, invasibility and the role of environmental stress in the spread of non-native plants. Perspect Plant Ecol Evol Syst 3:52–66
Amarasekare P (2004) The role of density-dependent dispersal in source-sink dynamics. J Theor Biol 226:159–168
Briske DD, Fuhlendorf SD, Smeins FE (2003) Vegetation dynamics on rangelands: a critique of the current paradigms. J Appl Ecol 40:601–614
Buckley YM, Briese DT, Rees M (2003) Demography and management of the invasive plant species Hypericum perforatum. II. Construction and use of an individual-based model to predict population dynamics and the effects of management strategies. J Appl Ecol 40:494–507
Callaway RM, Maron JL (2006) What have exotic invasions taught us over the past twenty years? Trends Ecol Evol 21:369–374
Cannas SA, Marco DE, Páez SA (2003) Modelling biological invasions: species traits, species interactions, and habitat heterogeneity. Math Biosci 183:93–110
Cannas SA, Marco DE, Montemurro MA (2006) Long range dispersal and spatial pattern formation in biological invasions. Math Biosci 203:155–170
Chabrerie O, Hoeblich H, Decocq G (2007a) Déterminisme et conséquences écologiques de la dynamique invasive du cerisier tardif (Prunus serotina Ehrh.) sur les communautés végétales de la forêt de Compiègne. Acta Bot Gall 154:383–394
Chabrerie O, Roulier F, Hoeblich H, Sebert E, Closset-Kopp D, Leblanc I, Jaminon J, Decocq G (2007b) Defining patch mosaic functional types to predict invasion patterns in a forest landscape. Ecol Appl 17:464–481
Chabrerie O, Verheyen K, Saguez R, Decocq G (2008) Disentangling relationships between habitat conditions, disturbance history, plant diversity and American Black cherry (Prunus serotina Ehrh.) invasion in a European temperate forest. Divers Distrib 14:204–212
Clark JS, Fastie C, Hurtt G, Jackson ST, Johnson C, King GA, Lewis M, Lynch J, Pacala S, Prentice C, Schupp EW, Web T III, Wyckoff P (1998) Reid’s paradox of rapid plant migration. Bioscience 48:13–24
Closset-Kopp D, Chabrerie O, Valentin B, Delachapelle H, Decocq G (2007) When Oskar meets Alice: does a lack of trade-off in r/K-strategies make Prunus serotina a successful invader of European forests? For Ecol Manag 247:120–130
Colautti RI, Grigorovich IA, MacIsaac HJ (2006) Propagule pressure: a null model for biological invasions. Biol Invasions 8:1023–1037
Crawley MJ (1987) What makes a community invasible? In: Gray AJ, Crawley MJ, Edwards PJ (eds) Colonization, succession and stability. Blackwell, Oxford, pp 429–453
Davis MA, Grime JP, Thompson K (2000) Fluctuating resources in plant communities: a general theory of invasibility. J Ecol 88:528–536
Deckers B, Verheyen K, Hermy M, Muys B (2005) Effects of landscape structure on the invasive spread of black cherry Prunus serotina in an agricultural landscape in Flanders, Belgium. Ecography 28:99–109
Decocq G, Aubert M, Dupont F, Alard D, Saguez R, Wattez-Franger A, de Foucault B, Delelis-Dussolier A, Bardat J (2004) Plant diversity in a managed temperate deciduous forest: understorey response to two silvicultural systems. J Appl Ecol 41:1065–1079
Drake JM, Lodge DM (2006) Allee effects, propagule pressure and the probability of establishment: risk analysis for biological invasions. Biol Invasions 8:365–375
Elton CS (1958) The ecology of invasions by animals and plants. Methuen, London
Garnier A, Lecomte J (2006) Using a spatial and stage-structured invasion model to assess the spread of feral populations of transgenic oilseed rape. Ecol Model 194:141–149
Godefroid S, Phartyal S, Koedam N (2005) Ecological factors controlling the abundance of non-native invasive Black Cherry (Prunus serotina) in deciduous forest understory in Belgium. For Ecol Manag 210:91–105
Greene DH, Canham CD, Coates D, Lepage PT (2004) An evaluation of alternative dispersal functions for trees. J Ecol 92:758–766. doi:10.1111/j.0022-0477.2004.00921.x
Haccou P, Iwasa Y (1996) Establishment probability in fluctuating environments: a branching process model. Theor Popul Biol 50:254–280
Hanski I (1999) Metapopulation ecology. Oxford University Press, Oxford
Hierro JL, Villarreal D, Eren O, Graham JM, Callaway RM (2006) Disturbance facilitates invasion: the effects are stronger abroad than at home. Am Nat 168:144–156
Higgins SI, Richardson DM (1996) A review of models of alien plant spread. Ecol Model 87:249–265
Higgins SI, Richardson DM (1998) Pine invasions in the southern hemisphere: modelling interactions between organism, environment and disturbance. Plant Ecol 135:79–93
Higgins SI, Richardson DM (1999) Predicting plant migration rates in a changing world: the role of long-distance dispersal. Am Nat 153:464–475
Jongejans E, Skarpaas O, Shea K (2008) Dispersal, demography and spatial population models for conservation and control management. Persp Plant Ecol Evol Syst 9:153–170
Kolar CS, Lodge DM (2001) Progress in invasion biology: predicting invaders. Trends Ecol Evol 16:199–204
Kot M, Lewis M, van den Driessche P (1996) Dispersal data and the spread of invading organisms. Ecology 77:2027–2042
Lake JC, Leischman MR (2004) Invasion success of exotic plants in natural ecosystems: the role of disturbance, plant attributes and freedom from herbivores. Biol Conserv 117:215–226
Lockwood JL, Cassey P, Blackburn T (2005) The role of propagule pressure in explaining species invasion. Trends Ecol Evol 20:223–228
Lonsdale WM (1999) Global patterns of plant invasions and the concept of invasibility. Ecology 80:152–153
Marco DE, Páez SA, Cannas SA (2002) Species invasiveness, habitat invasibility and species interactions: a modelling approach. Biol Invasions 4:193–205
Marquis MA (1975) Seed germination and storage under northern hardwood forests. Can J Res 5:478–484. doi:10.1139/x75-065
Martinez-Ghersa MA, Ghersa CM (2006) The relationship of propagule pressure to invasion potential in plants. Euphytica 148:87–96
Melbourne BA, Cornell HV, Davies KF, Dugaw CJ, Elmendorf S, Freestone AL, Hall RJ, Harrison S, Hastings A, Holland M, Holyoak M, Lambrinos J, Moore K, Yokomizo H (2007) Invasion in a heterogeneous world: resistance, coexistence or hostile takeover? Ecol Lett 10:77–94
Moloney KA, Levin SA (1996) The effect of disturbance architecture on landscape-level population dynamics. Ecology 77:375–394
Nathan R, Sapir N, Trakhtenbrot A, Katul GG, Bohrer G, Otte M, Avissar R, Soons MB, Horn HS, Wikelski M, Levin SAL (2005) Long-distance biological transport processes through the air: can nature’s complexity be unfolded in silico? Divers Distrib 11:131–137
Nehrbass N, Winkler E, Müllerovà J, Pergl J, Pysek P, Perglovà I (2007) A simulation model of plant invasion: long-distance dispersal determines the pattern of spread. Biol Invasions 9:383–395
Neubert MG, Caswell H (2000) Demography and dispersal: calculation and sensitivity analysis of invasion speed for structured populations. Ecology 81:1613–1628
Neubert GN, Kot M, Lewis MA (2000) Invasion speeds in fluctuating environments. Proc R Soc Lond B 267:1603–1610
Pairon M, Jonard M, Jacquemart AL (2006) Modeling seed dispersal of black cherry (Prunus serotina Ehrh.) an invasive tree: how microsatellites may help? Can J For Res 36:1385–1394
Pauchard A, Shea K (2006) Integrating the study of non-native plant invasions across spatial scales. Biol Invasions 8:399–413
Pausas JG, Keeley JE, Verdú M (2006) Inferring differential evolutionary processes of plant persistence traits in Northern Hemisphere Mediterranean fire-prone ecosystems. J Ecol 94:31–39
Pergl J, Hüls J, Perglovà I, Eckstein RL, Pyšek P, Otte A (2007) Population dynamics of Heracleum mantegazzianum. In: Pyšek P, Cock MJW, Nentwig W, Ravn HP (eds) SpecEcology and management of Giant hogweed (Heracleum mantegazzianum). CAB International, Wallingford, pp 92–111
Pimentel D, Zuniga R, Morrison D (2005) Update on the environmental and economic costs associated with alien-invasive species in the United States. Ecol Econ 52:273–288
Pontius RG (2000) Quantification error versus location error in comparison of categorical maps. Photogramm Eng Remote Sens 66:1011–1016
Pyle L (1995) Effects of disturbance on herbaceous exotic plant species on the floodplain of the Potomac River. Am Midl Nat 134:244–254
Rejmánek M (2000) Invasive plants: approaches and predictions. Austral Ecol 25:497–506
Renshaw E (1991) Modelling biological populations in space and time. Cambridge University Press, Cambridge
Richardson DM, Pyšek P (2006) Plant invasions—merging the concepts of species invasiveness and community invasibility. Prog Phys Geogr 30:409–431
Richardson DM, Rejmánek M (2004) Invasive conifers: a global survey and predictive framework. Divers Distrib 10:321–331
Saether BE, Engen S, Lande R (1999) Finite metapopulation models with density-dependent migration and stochastic local dynamics. Proc R Soc Lond B 266:113–118
Sax DF, Stachowicz JJ, Brown JH, Bruno JF, Dawson MN, Gaines SD, Grosberg RK, Hastings A, Holt RD, Mayfield MM, O’Connor MI, Rice WR (2007) Ecological and evolutionary insights from species invasions. Trends Ecol Evol 22:465–471
Sebert-Cuvillier E, Paccaut F, Chabrerie O, Endels P, Goubet O, Decocq G (2007) Local population dynamics of an invasive tree species with a complex life-history cycle: a stochastic matrix model. Ecol Model 201:127–143
Sebert-Cuvillier E, Simon-Goyheneche V, Paccaut F, Chabrerie O, Goubet O, Decocq G (2008) Spatial spread of an alien tree species in a heterogeneous forest landscape: a spatially realistic simulation model. Landsc Ecol 23:787–801
Shea K, Chesson P (2002) Community ecology theory as a framework for biological invasions. Trends Ecol Evol 17:170–176
Skellam JG (1951) Random dispersal in theoretical populations. Biometrika 38:196–218
Snyder RE (2003) How demographic stochasticity can slow biological invasions. Ecology 84:1333–1339
Snyder RE, Chesson P (2003) Local dispersal can facilitate coexistence in the presence of permanent spatial heterogeneity. Ecol Lett 6:301–309
Söndgerath D, Schröder B (2002) Population dynamics and habitat connectivity affecting spatial spread of populations—a simulation study. Landscape Ecol 17:57–70
Starfinger U (1991) Population biology of an invading tree species-Prunus serotina. In: Seitz A, Loeschcke V (eds) Species conservation: a population-biological approach. Birkhaüser, Basel, pp 171–184
Starfinger U (1997) Introduction and naturalization of Prunus serotina in Central Europe. In: Brock JH, Wade M, Pyšek P, Green D (eds) Plant invasions: studies from North America and Europe. Backhuys, Leiden, pp 161–171
Thuiller W, Midgley GF, Rouget M, Cowling RM (2006) Predicting patterns of plant species richness in megadiverse South Africa. Ecography 29:733–744
Trakhtenbrot A, Nathan R, Perry G, Richardson DM (2005) The importance of long-distance dispersal in biodiversity conservation. Divers Distrib 11:173–181
Verheyen K, Vanhellemont M, Stock T, Hermy M (2007) Predicting patterns of invasion by black cherry (Prunus serotina Ehrh.) in Flanders (Belgium) and its impact on the forest understorey community. Divers Distrib 13:487–497
Williamson M (1996) Biological invasions. Chapman and Hall, London
Williamson M (1999) Invasions. Ecography 22:5–12
Wilson JRU, Richardson DM, Rouget M, Proches S, Amis MA, Henderson L, Thuiller W (2007) Residence time and potential range: crucial considerations in modelling plant invasions. Divers Distrib 13:11–22
Acknowledgments
We are grateful to Olivier Chabrerie, Marie Pairon and Jean Boucault for their help in parameterizing the model and to Jérôme Jaminon (Office National des Forêts) for facilities during field data collection. We thank Mark Bilton for revising the language. This study was financially supported by the French ‘Ministère de l’Ecologie et du Développement Durable’ (INVABIO II program, CR n°09-D/2003).
Author information
Authors and Affiliations
Corresponding author
Appendix 1: further information about the PRUNUS model
Appendix 1: further information about the PRUNUS model
Life cycle graph and transition matrices
After Sebert-Cuvillier et al. (2007), we retained the following 11 stages to account for the life cycle of Prunus serotina (Fig. 6):
Life cycle graph of Prunus serotina as considered in the model (adapted from Sebert-Cuvillier et al. 2007)
-
seed stages: to incorporate seed dormancy, which can attain 2 years (Marquis 1975), we distributed the annual seed production per mature tree among three life stages: seed-2, seed-1 and seedling, corresponding to seeds germinating on the 3rd, 2nd and 1st spring following release, respectively. The proportion of seeds that never germinate to reach the seedling stage, which includes seeds without embryo and seeds that die at seed-1 or seed-2 stage, was removed from the total number of produced seeds.
-
sapling stages: sterile plants without cotyledons and taller than 10 cm were considered as saplings. Under dense shade (e.g., in the understorey of a closed-canopy forest), saplings become rapidly suppressed and form a long-living sapling bank (Starfinger 1997; Closset-Kopp et al. 2007). Such suppressed saplings (i.e., saplings remaining at sapling1 stage) are called ‘Oskars’ since they develop a “sit and wait” strategy (Starfinger 1997). Conversely, in full light conditions (e.g., in a gap or a clearcut), sapling1 can grow to reach superior sapling stages and ultimately the canopy. As time to reach the canopy averages 7 years, saplings were distributed among 7 stages: sapling1, sapling2, sapling3, sapling4, sapling5, sapling6, and sapling7, corresponding to saplings high of <25, 25–50, 50–150, 150–250, 250–350, 350–450, and 450–550 cm, respectively. Individuals can neither stay more than 1 year in a given sapling stage nor skip any intermediate stage, except for ‘Oskars’ which are able to remain at the sapling1 stage for several decades.
-
adultstage: tall shrubs and trees that are sexually mature, survive several decades during which they produce seeds annually, were grouped into a single adult stage.
To account for the resprouting capacity of Prunus serotina individuals through stump and root suckers, we included further transition probabilities for stages sapling2, sapling3, sapling4, sapling5, sapling6, sapling7 and adult to go back to the sapling1 stage after their aerial parts have suffered dieback, especially under closed canopy conditions.
This 11-stage life cycle has been mathematically translated into four 11 × 11 transition matrices (Sebert-Cuvillier et al. 2007):
-
Matrix A (Table 2): shaded environmental conditions—This matrix includes two positive elements on the diagonal, corresponding to individuals remaining in the same stage (i.e., sapling1-stage and adult-stage), and six null rows, since saplings1 cannot reach the next stage under shade conditions. The total number of seeds that are yearly produced by an adult tree averages 6,011, but only 42% are viable and thus able to become seedlings (Closset-Kopp et al. 2007). After Marquis (1975), we distributed 2.5, 26 and 13.5% of seeds among seed-2, seed-1 and seedling stages, respectively. Moreover, to incorporate post-release seed predation, from which only 55.4% of the released seeds escape (unpublished data), we set the mean annual number of seeds entering seed-2, seed-1 and seedling stages to 6011 × 0.025 × 0.554 = 83.1, 6,011 × 0.26 × 0.554 = 865.9, and 6011 × 0.135 × 0.554 = 449.3, respectively. Both probabilities for seed-2 individuals to reach the seed-1 stage in a year, and for seed-1 individuals to reach the seedling stage in a year, were set to 1 since the mortality has already been taken into account. The probability for seedlings to reach the sapling1 stage was set to 0.1105.
Table 2 The transition matrix A accounting for shaded environmental conditions -
Matrix B (Table 3): treefall-induced canopy gap—The new probability for sapling1 individuals to remain in this stage in full light conditions equals 0.1. The 7 transition coefficients on the sub-diagonal describe the self-thinning process due to competition for space and light during the aggradation phase. All 7 coefficients of the sapling1 row equal 0.1 to account for the resprouting of overtopped saplings during the aggradation phase, which lasts 7 years in our model.
Table 3 The transition matrix B accounting for canopy gap conditions -
Matrix C (Table 4): canopy closure—This matrix is applied when environment conditions shift from full light to shade, on the 8th year after gap creation. All individuals of the seven sapling stages suffer from aerial part death but actively resprout from stumps and/or roots. Matrix C is nearly the same as matrix A, but with six additional coefficients in the sapling1 row, all equalling 0.8. As adults already reached the canopy, they do not resprout.
Table 4 The transition matrix C accounting for the canopy closure phase -
Matrix D (Table 5): clearcut-induced full light conditions—The clearcut affects all individuals at both sapling and adult stages, so that their aerial parts die back. Hence, matrix D has seven null rows. The resprouting probabilities are the same as in matrix C, but here adults also resprout.
Table 5 The transition matrix D accounting for clearcut conditions
Habitat suitability index
A vector ‘habitat suitabiliy’ V has been elaborated by assigning a habitat suitability index V i to each cell of the matrix. For this purpose, we followed the approach proposed by Chabrerie et al. (2007b) and already applied in Sebert-Cuvillier et al. (2008), with the notable exception that all cells were ecologically homogeneous. We retained two environmental variables that were available as maps in a geographic information system (GIS): soil types and soil drainage classes. Partial indices were derived for each of them:
-
The partial soil type index (I soiltype) ranged from 0.2 to 1.8 (Table 6), a value inside the interval [0,1] indicating a cell limiting Prunus serotina establishment, while a value inside the interval [1,2] characterizes a cell promoting it;
Table 6 Values of the partial soil type index -
The partial soil drainage index (I drainage) ranged from 0 to 1 (Table 7), drainage classes greater than 2 preventing from establishment, while classes 1 and 2 have no influence.
Table 7 Values of the partial drainage coefficient
We then combined those two partial indices into a single index V i quantifying the overall cell suitability for Prunus serotina establishment as mature trees. For each cell, this index is computed as follow:
-
If I drainage = 1, then V i = I soiltype
-
Else, \( V_{i} = \sqrt {I_{\text{soiltype}} \times I_{\text{drainage}} } \)
Hence, when a cell has a partial soil drainage index equal to 0, the tree cannot develop whatever the soil type. The habitat suitability index V i ranged from 0 to 2. The time-independent vector V has 170,425 components that can be mapped (Fig. 7). At each time step, in each cell i the number of individuals at the Sapling-2 stage is multiplied by V i .
Characteristics of forest-management related periodic disturbances
Four deterministic parameters were specified for each cell i, depending on its dominant canopy tree species (Table 8): the clearcut-return interval (Cl i ), the percentage of light due to a small-scale thinning (Pth i ), the time between two small-scale thinnings (Th i ) and the duration of the disturbance induced by the clearcut (t i ).
Dispersal functions
Dispersal functions incorporated both the mass action of local dispersal and the stochastic nature of long-distance dispersal. At each time step n and for each cell i, P f G i (n) and P b G i (n) seeds are dispersed outside cell i by foxes and birds, respectively, according to the dispersal functions. Hence, (1 − P f − P g )G i (n) seeds are dispersed by gravity inside cell i.
The probability for a seed coming from a cell i to be dispersed by a vector v (birds or foxes) at the distance x was given by the dispersal lognormal function f v that depended on two parameters: the shape parameter S v and the scale parameter L v :
where x is the distance (in grid units).
Following the recommendations of Greene et al. (2004), we chose S v = 1, and two values were selected for L v that approximate the mean dispersal distance by birds and foxes. After Deckers et al. (2005), Pairon et al. (2006) and personal field observations, we retained a mean dispersal distance of 100 m for birds (i.e. L b = 2 grid units) and 918 m for foxes (i.e. L f = 18.36 grid units).
The integral \( {\int_{0}^{x} {\tilde{f}_{v} (r){\text{dr}}} }, \) where the integration variable is the distance r and \( {\tilde{f}}_{v} \) is the dispersal function (\( {\tilde{f}_{v} = \tilde{f}_{b} } \) for birds and \( {\tilde{f}_{v} = \tilde{f}_{f} } \) for foxes), which has been scaled such as \( {\int_{0}^{\infty } {\tilde{f}_{v} (r){\text{dr}}} } = 1, \) gave the probability for a seed to be dispersed by the vector v at a distance inferior to x at each time step. This probability, also called “distribution function”, is an increasing continuous function. Hence, we have two distribution functions, one for birds and another for foxes.
For each seed picked by a vector, we randomly selected a number P between 0 and 1. We computed the unique antecedent R of this number by the following vector distribution function: \( x \mapsto {\int_{0}^{x} {\tilde{f}_{v} (r){{dr}}} .} \) R gave the distance at which the seed would be dispersed. Next, a direction ϑ was randomly chosen between 0 and 360° to determine which cell will receive this seed. The seed was thus added to a “sink” cell j (j ≠ i) at the distance R from “source” cell i following direction ϑ.
Rights and permissions
About this article
Cite this article
Sebert-Cuvillier, E., Simonet, M., Simon-Goyheneche, V. et al. PRUNUS: a spatially explicit demographic model to study plant invasions in stochastic, heterogeneous environments. Biol Invasions 12, 1183–1206 (2010). https://doi.org/10.1007/s10530-009-9539-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-009-9539-8