Abstract
Since 1998 the non-indigenous Pacific oyster Crassostrea gigas (Thunberg 1793) has been invading the Wadden Sea of Lower Saxony, southern German Bight. C. gigas settles predominantly on intertidal Mytilus-beds (M. edulis L.) and subsequently create rigid reef-like structures. Both bivalve species are ecosystem engineers in sedimentary tidal flats. They provide hard substrate for sessile species, mobile organisms find refuge within the habitat matrix of dense suspension feeders, and biodeposits enrich the sediments with organic matter. The transformation of Mytilus-beds into Crassostrea-reefs gives rise to the question whether the invader may affect the native community. We investigated two parts of a changing bivalve bed in the backbarrier area of the island of Juist in March 2005. One part was still dominated by M. edulis whereas the other part was already densely colonized by C. gigas. Crassostrea-reefs compensate for the conceivable loss of Mytilus-beds in the intertidal of the Wadden Sea by replacing the ecological function of M. edulis. There was no indication of a suppression of indigenous species. This even applied to M. edulis, which persisted at the site invaded by C. gigas. The associated macrofaunal community showed increased species richness, abundance, biomass, and diversity in the Crassostrea-reef. The latter particularly favored sessile species like anthozoans, hydrozoans, and barnacles. Higher abundance and biomass for vagile epizoic species like the shore crab Carcinus maenas and the periwinkle Littorina littorea also occurred among oysters. Abundance of deposit feeding oligochaetes was enhanced by oysters as well. More opportunistic, facultative filter-feeding polychaetes occurred in the Crassostrea-reef.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
More and more marine species have been transported across natural barriers in recent times (Carlton and Geller 1993; Carlton 1987; Nehring 1998; Gollasch 2002). When they become established, the non-indigenous species may threaten native biodiversity as well as ecosystem functions of the region (Kolar and Lodge 2001). However, introduced species may also create habitat structures which locally may enhance biological diversity (Crooks 1998).
For the purpose of aquaculture, the Pacific oyster Crassostrea gigas (Thunberg 1793) was first imported 1964 into the Easter Scheld estuary (The Netherlands) which is connected to the open North Sea. Natural reproduction was not expected due to low water temperatures. But after spatfalls in the warm summers of 1975, 1976, and 1982, C. gigas established itself outside the culture plots in the Easter Scheld estuary (Drinkwaard 1999). In 1983 the first Pacific oyster was found in the Dutch Wadden Sea near Texel (West Frisian Island), which appeared to have been deliberately introduced (Dankers et al. 2004). First specimens in the East Frisian Wadden Sea (Wadden Sea of Lower Saxony, southern German Bight) were recorded in 1998 from the backbarrier area of the island of Baltrum most likely originating from eastward larval drift via Texel (Wehrmann et al. 2000; Brandt et al. 2008). Massive spatfalls of Crassostrea in 2003 and 2004 resulted in an exponential increase in abundance. In 2005, up to 700 individuals m−2 were found in the backbarrier of the island of Juist (Wehrmann et al. 2006).
In the Wadden Sea, the colonization by invasive Pacific oysters occurred predominantly on intertidal Mytilus-beds (M. edulis L.), which provide secondary hard substrate for the settlement of C. gigas larvae (Reise 1998; Wehrmann et al. 2000; Diederich 2005). The indigenous but extirpated European oyster Ostrea edulis L. was found in subtidal environments of the Wadden Sea (Möbius 1877). Thus, C. gigas is not occupying the open niche of the native oyster but intertidal areas already occupied and affected by M. edulis (Reise 1998).
Mytilus edulis is an ecosystem engineer in the sediment dominated tidal flats of the Wadden Sea. Compared to ambient tidal flats, Mytilus-beds are “Islands of biodiversity” (Buschbaum and Nehls 2003). They offer habitats for several sessile species like barnacles, anthozoans, hydrozoans, bryozoans and macroalgae. They provide shelter and offer food resources to various mobile epibenthic organisms like crustaceans and molluscs. Mytilus-beds have been termed a biosedimentary system (Hertweck and Liebezeit 1996). Biodeposits enrich the sediments of patches and open spaces with organic matter (Dittmann 1987), enhancing infaunal diversity. Even areas adjacent to a Mytilus-bed are influenced by biogenic deposition (Kröncke 1996; Bergfeld 1999; Hild and Günther 1999). Additionally, Mytilus-beds are an important food resource for many fish species, a variety of bird species and humans.
According to Linke (1954) mature Mytilus-beds are composed of Mytilus-covered “patches” and interspersed bare sediments as Mytilus-free “open spaces”. M. edulis creates flexible three-dimensional structures as multiple individuals connect and disconnect their shells by byssus threads. The Mytilus-meshwork covers the sediment of the patches in horizontally oriented layers. Due to their mobility, the individuals mostly manage to stay above the sediment.
In contrast, Crassostrea-reefs constitute a new biogenic structure in the Wadden Sea (Reise et al. 2005). C. gigas larvae prefer settling on conspecifics (Arakawa 1990). The process of multiple settlement leads to the creation of stable clusters as the individuals stay cemented to their substrate of settlement. In patches of high oyster density, the rigid reef-like structures tend to be vertically oriented. Over time, the lower part in Crassostrea-patches becomes buried with sediment whereas the upper part remains exposed. By constructing reefs and producing biodeposits, C. gigas may influence habitat features in a similar way like M. edulis (Reise 2002; Ruesink et al. 2005). However, differences in ecosystem engineering may alter the resident biocoenosis.
The aim of this study was to investigate whether and how the bioinvasion of the Pacific oyster affects the recipient biota. To analyze community effects we chose one bivalve bed in the backbarrier area of the island of Juist (Fig. 1) where patches of high Crassostrea density co-occurred with still remaining Mytilus-patches. We focused on the following question: are there differences in species number, abundance, biomass and diversity of the associated macrofauna between Mytilus-beds and Crassostrea-reefs?
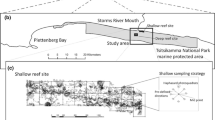
Top: Study area in the Wadden Sea of Lower Saxony, south of the East Frisian Island of Juist, southern German Bight, North Sea. Blow-up shows the bivalve bed and the locations of the sampling sites S (sand flat, 100 m outside the bivalve bed), M (Mytilus-bed) and C (Crassostrea-reef). Bottom: Sampling design shows the sampling station S (sand flat), Mytilus-patch (covered by Mytilus), M COV (underlying sediment) and M OS (bare open spaces between the Mytilus-patches), Crassostrea-patch (covered by Crassostrea), C COV (underlying sediment), C OS (bare open spaces between the Crassostrea-patches)
Methods
Study site, sampling, and sample treatment
The Wadden Sea of Lower Saxony covers an area of 1200 km². 108 intertidal bivalve beds are recorded which cover approx. 1% of the tidal flats. Sampling took place in March 2005 on one 37 ha large intertidal bivalve bed, located in the backbarrier tidal sand flats south of the East Frisian Island of Juist, southern German Bight, North Sea (N 53°38′; E 006°56′; Fig. 1). In the area we find a salinity of ~30 psu, an average temperature of 10.9°C (04/2004-03/2005) and an average tidal range of 2.4 m. Compared to other intertidal bivalve beds in the area this bed is characterized by a consistently high occurrence of M. edulis over the last decades (Herlyn and Millat 2004). First individuals of C. gigas were found in 1999 but a massive spread occurred not till 2003. The positive feedback in Crassostrea settlement (Diederich 2005; Wehrmann et al. 2006) led to a rapid reef formation in the western part of the bivalve bed in 2003/2004 (Crassostrea-reef, site C, Fig. 1), while at the time of the investigation the eastern part still remained dominated by M. edulis (Mytilus-bed, site M, Fig. 1). Over a distance of 700 m from one site to the other a continuous transition in C. gigas density occurred. Tidal elevation with a mean exposure time at low tide of about 3.5 h per tidal cycle and sediment characteristics were similar at both sites; thus no obvious differences between the sites occurred which could entail changes in associated fauna. Both sites consist of bivalve-covered patches (“Mytilus-patches”, “Crassostrea-patches”) and of interspersed epibenthic bivalve-free bare sediment (“open spaces”).
We took epibenthos samples in Mytilus-patches and Crassostrea-patches (Fig. 1) with a 15 cm diameter tube (~177 cm−2). Directly after clearing the patches from epibenthic bivalve coverage, the underlying sediment (MCOV, CCOV) was taken with a 5.9 cm diameter tube (~27 cm−2) down to 30 cm sediment depth (Fig. 1). Additionally, we took sediment samples with a 5.9 cm diameter tube down to 30 cm sediment depth in the epibenthic bivalve-free open spaces at site M and site C (MOS, COS) and in the epibenthic bivalve-free adjacent sand flat (site S) (Fig. 1). The latter was located 100 m outside the bivalve bed area. Tidal elevation and sediment characteristics of the sand flat were similar as at site M and C. By taking three replicates per station (Mytilus-patch, Crassostrea-patch, MCOV, CCOV, MOS, COS and S) a total of 21 samples were analyzed.
All samples were fixed in 4% buffered formaldehyde and stored for at least 3 months to level off weight losses (Hamilton and Kingston 1985; Böttger and Schnack 1986; Brey 1986). The samples were then sieved through 0.5 mm mesh size in the laboratory. All remaining organisms were collected from the recovered material. Shell lengths of M. edulis and C. gigas specimens were measured to the nearest mm. The flesh of each specimen was stored separately in 70% ethanol before determining ash free dry weight in gram (g AFDW) after drying and combusting in a furnace using standard methods (Rumohr 1990). All other macrobenthic fauna of the epibenthos samples and of the sediment samples were determined to species level, counted and stored in 70% ethanol. Biomass (g AFDW) was calculated from wet weight using published conversion factors (Brey 2001; Ricciardi and Bourget 1998). For barnacles a conversion factor was calculated based on data of this study.
Data analysis
In this survey individuals of M. edulis <6 mm shell length were defined as juveniles of the previous spatfall (late summer 2004) and individuals of C. gigas <21 mm were defined as juveniles of the spatfall in early autumn 2004. To evaluate differences between the sites M and C, we quantified abundance and biomass (g AFDW) of adult M. edulis and adult C. gigas as arithmetic means per m² patch with standard deviation (±), calculated mean shell length (mm) of both adult bivalve species (Table 1) as well as the shell length frequency distribution of M. edulis in Mytilus-patches and Crassostrea-patches.
Mytilus edulis and Crassostrea gigas were excluded from the analysis of the associated macrofauna of the epibenthos samples. Species richness results from total species number of all replicates, respectively. Species were categorized after their mode of life as sessile and vagile epifauna and infauna (Tables 2, 3). Colonies forming sessile taxa were grouped separately, noted as present/absent and were excluded from quantitative analysis. Abundance and biomass are presented as arithmetic means per m² (rounded values). To detect similarities in community structure ordination (Multi-Dimensional Scaling, MDS) and classification (Hierarchical cluster) methods (Kruskal and Wish 1978) were applied, using the Bray–Curtis similarity coefficients (Bray and Curtis 1957). Diversity of Mytilus-patches and Crassostrea-patches was determined by the Shannon–Wiener Index (Weaver and Shannon 1949) and by the Evenness (Pielou 1966), calculated for biomass data.
To evaluate differences between all stations, we compared the infauna of the sand flat (S) with the infauna of the Mytilus-bed (M) and the Crassostrea-reef (C), sites M and C subdivided into the stations “patch” and “open space”, respectively. C. gigas clusters were sticking up to 5 cm in the sediment of the patches. Therefore, we picked up more sediment by taking the epibenthos samples in Crassostrea-patches than in Mytilus-patches. To obtain comparable data for the infauna analysis, we added the infauna data of the epibenthos samples to the infauna data of the sediment samples, which we took beneath the Mytilus- and Crassostrea-cover in the patches (MCOV, CCOV), respectively. Species richness of the infauna communities at the five stations results from total species numbers, respectively. Abundance of infauna is presented as arithmetic means per m² (rounded values). We classified infauna species into higher taxonomical groups. Polychaetes, the main group of the macrobenthic species assemblages at all stations, were categorized into feeding modes (Fauchald and Jumars 1979; Hartmann-Schröder 1996).
Results
Ecosystem engineers: Mytilus edulis and Crassostrea gigas
Total mean abundance of both ecosystem engineers in Crassostrea-patches was almost three times higher and total mean biomass was more than twice higher than in Mytilus-patches (Table 1).
Mytilus-patches were dominated by its ecosystem engineer Mytilus which accounted for 92% of all adult individuals and 95% of the total biomass (Fig. 2). In Mytilus-patches, adult Crassostrea stayed low in abundance and due to small individuals as well biomass of the oysters was low (Table 1).
In contrast, Crassostrea-patches were built up by 54% adult oysters and 46% adult blue mussels. Due to large individuals (Table 1), Crassostrea dominated the Crassostrea-patches in biomass (Fig. 2). The largest C. gigas specimen in Crassostrea-patches was 179 mm in length.
The abundance of adult Mytilus in Crassostrea-patches was higher than in Mytilus-patches but was represented by smaller individuals (Table 1; Figs. 2, 3). At both stations, Mytilus was found in all size classes up to 70 mm but we detected a distinct gap in medium size classes between 26 and 45 mm in Mytilus-patches whereas these size classes were abundant in Crassostrea-patches (Fig. 3). Hence, biomass of Mytilus was lower in Crassostrea-patches than in Mytilus-patches (Fig. 2). Juvenile Mytilus were abundant at both stations but Mytilus-patches had 1.5 times more juveniles than Crassostrea-patches. Juveniles accounted for 73% of the Mytilus population in Mytilus-patches (3597 ± 887 juvenile Mytilus m−2) and 55% of the Mytilus population in Crassostrea-patches (2335 ± 974 juvenile Mytilus m−2).
Associated macrofaunal communities
In total we identified 59 macrofaunal taxa (Tables 2, 3). According to their mode of life, 31 taxa were assigned to the infauna and 28 taxa to the epifauna. The latter were subdivided in 12 vagile and 16 sessile taxa. The sessile epibiont community included 9 colonies forming taxa (ascidian, bryozoans, hydrozoans), which were noted as present/absent and are excluded from quantitative analysis (Table 2). Most common were the polychaetes with 21 taxa.
Epibenthos samples: species richness, abundance and biomass of associated macrofauna in Crassostrea- and Mytilus-patches
We identified 28 macrofaunal taxa in Mytilus-patches and 45 in Crassostrea-patches. Six species were found exclusively in Mytilus-patches and 23 exclusively in Crassostrea-patches, while 22 taxa were found at both stations (Table 2). Infauna had highest species numbers at both stations, followed by vagile epifauna, colonies forming sessile taxa and other sessile epifauna (Fig. 4). The higher total number of species in Crassostrea-patches was reflected in all groups. Infauna increased from 14 to 19 species, vagile epifauna from 7 to 10 species, colonies forming taxa from 4 to 9 species and other sessile epifauna from 3 to 7 species. Infauna and vagile epifauna decreased in relative species numbers in Crassostrea-patches, whereas the sessile epibiont species assemblage was emphasized (Fig. 4). Anthozoans were exclusively found in Crassostrea-patches (Table 2).
Total mean abundance and total mean biomass in Crassostrea-patches were twice as high as they were in Mytilus-patches (Table 2; Fig. 5). An increased abundance was detected for all functional groups in Crassostrea-patches whereas the increase in biomass was related to vagile epifauna.
Sessile epifauna was most abundant at both stations and dominated the associated macrofauna community with over 80% at both stations (Fig. 5). In Crassostrea-patches, 1.4 times more sessile epifauna individuals were found. Vagile epifauna had lowest abundances at both stations but as well 1.4 times more individuals were found in Crassostrea-patches. Whereas total biomass of associated macrofauna was still dominated by sessile epifauna in Mytilus-patches (75%), sessile epifauna biomass only made up 45% of the total biomass in Crassostrea-patches (Fig. 5). Despite of more but smaller barnacles, sessile epifauna had a lower biomass in Crassostrea-patches than in Mytilus-patches. The higher total biomass in Crassostrea-patches (Table 2; Fig. 5) was related to higher abundances of large individuals of vagile epifauna species like the periwinkle Littorina littorea and the shore crab Carcinus maenas as well as the exclusive occurrence of the polychaete Harmothoe imbricata and the starfish Asterias rubens (Table 2). Biomass of vagile epifauna was four times higher in Crassostrea-patches and contributed with 50% to the biomass of the associated macrofauna (Table 2; Fig. 5).
Infauna showed the highest increase in abundance. 3.4 times more infaunal individuals were encountered in Crassostrea-patches than in Mytilus-patches. In contrast, infauna biomass was only twice higher in Crassostrea-patches. Due to the high occurrence of oligochaetes, Tubificoides benedii being dominant, infauna abundance gained in importance in Crassostrea-patches and reached 16% of associated macrofauna individuals (Fig. 5).
Epibenthos samples: multivariate analyses and diversity
Multi-Dimensional Scaling performed with abundance data after 4th root transformation resulted in a separation of the species assemblages “Mytilus-patch” and “Crassostrea-patch”. The corresponding cluster analysis confirmed this grouping. The cluster of the three replicates in Crassostrea-patches had a similarity of 70% and a similarity of even 80% in Mytilus-patches (Fig. 6).
With reference to a mean density of 26.3 ± 1.2 species in Crassostrea-patches, the Evenness for biomass was 0.44 ± 0.06. In Mytilus-patches mean species density was only 17.7 ± 1.2, Evenness was 0.35 ± 0.07. The mean Shannon–Wiener index was higher in Crassostrea-patches (1.43 ± 0.21) than in Mytilus-patches (0.99 ± 0.24; Fig. 7).
Sediment samples: species richness and abundance of infauna
In total we identified 31 infaunal taxa. The sand flat and stations covered by epibenthic bivalves, the ecosystem engineers Mytilus edulis or Crassostrea gigas, tended to be higher in species number than the sediments in the open spaces of the Mytilus-bed and the Crassostrea-reef. Endobenthic amphipods were only found in the sand flat, with Urothoe poseidonis being dominant. Nemertines only occurred at stations covered by the epibenthic bivalves, with Lineus ruber being dominant. Only the sediments in the open spaces of the Mytilus-bed and the Crassostrea-reef were free of endobenthic bivalves. Annelids were found at all stations. With 16 species polychaetes were the most diverse infaunal group and dominated the species assemblages of all stations (Table 3; Fig. 8).
Total species numbers (left) and mean abundance with standard deviation per m² (right) of infauna in the sand flat (S), in Mytilus covered areas (MCOV), in Crassostrea covered areas (CCOV) and in the respective bare open spaces of the bivalve bed (MOS and COS); taxonomical composition based on species number (left), based on abundance (right) and proportional occurrence in circle diagrams
In terms of total infauna abundance, we detected highest values in patches and open spaces of the Crassostrea-reef. Abundance was conspicuously lower in the open spaces of the Mytilus-bed and in the sand flat, whereas the Mytilus-patches had an intermediate density (Table 3; Fig. 8). Annelids were the main taxa in structuring infauna abundance. They had a share of over 95% at all stations but the sand flat. Here amphipodes achieved a high proportion of 35%. Endobenthic bivalves and nemertines stayed low in abundance at all stations of occurrence.
Oligochaetes dominated stations covered by the epibenthic bivalves. The relative abundance of oligochaetes increased from the sand flat (5%) via the open spaces (MOS 20% und COS 39%) to the stations covered by the epibenthic bivalves (MCOV 53%, CCOV 76%). Highest infauna abundance was found in Crassostrea-patches. A comparable lower abundance as in Mytilus-patches was encountered in the open spaces of the Crassostrea-reef. Lowest oligochaete abundance was found in the open spaces of the Mytilus-bed and in the sand flat (Table 3; Fig. 8). At all stations Tubificoides benedii was the most abundant species.
Polychaetes dominated the stations without epibenthic bivalve coverage (Fig. 8). The highest abundance of polychaetes was found in the open spaces of the Crassostrea-reef. Almost all polychaete species had highest abundances at this station (Table 3). In the open spaces of the Mytilus-bed we found the lowest abundance of all stations. An intermediate abundance of polychaetes was found in Crassostrea-patches, in Mytilus-patches and in the sand flat (Table 3; Fig. 8). The latter was dominated by Pygospio elegans. All stations of the bivalve bed were dominated by the abundance of Aphelochaeta marioni (Table 3). Highest abundance of A. marioni was detected in the open spaces of the Crassostrea-reef. Capitella capitata was a common species in the sand flat, in Mytilus-patches and in the open spaces of the Crassostrea-reef. Neanthes succinea as an exclusive species in Crassostrea-patches was of notable importance. The predatory polychaete ranked third after A. marioni and the spionid polychaetes of the genus Polydora. Scoloplos armiger was only encountered at stations without epibenthic bivalve coverage. Highest abundance was found in the sand flat (Table 3).
Polychaetes: feeding mode
Considering infaunal and epifaunal polychaetes, surface deposit feeders dominated all stations. Facultative filter feeders, resp. Polydora cornuta and P. ciliata, reached a high proportion of 44% in Crassostrea-patches. Subsurface deposit feeders attained a marked percentage only in the sand flat (Fig. 9).
Discussion
Ecosystem engineers: Mytilus edulis and Crassostrea gigas
The Mytilus-patches were dominated by M. edulis. In contrast, the Crassostrea-patches were characterized by almost equal abundances of both ecosystem engineers. We even found more Mytilus in Crassostrea-patches than in Mytilus-patches but we detected differences in shell length frequency distribution of M. edulis at the two stations.
This study revealed a spatfall of M. edulis across the entire bivalve bed. Whereas Diederich (2005) stated that recruitment of Mytilus larvae was similar in Crassostrea-reefs and in Mytilus-beds, we found a lower abundance of juvenile Mytilus in the Crassostrea-patches. Troost et al. (2004) reported a more diffuse inhalant feeding current of C. gigas. Low swimming velocities of Mytilus veligers might increase the chance of being filtered by C. gigas.
However, mortality of juvenile Mytilus after successful recruitment seems to be lower in Crassostrea-invaded areas than in Mytilus-dominated areas. Hiding-places between individuals of C. gigas may have protected M. edulis from predation. However, up to the time of the investigation, birds as main predators were avoiding biogenic structures dominated by C. gigas (Wehrmann et al. 2006). The absence of medium size classes of M. edulis in Mytilus-beds was reported by Zens et al. (1997) as related to increasing bird populations, as well as to increasing predation caused by a decrease of Mytilus-bed coverage in the area (Herlyn and Michaelis 1996). Specimens of Mytilus <20 mm and >50 mm are rejected by oystercatchers (Haematopus ostralegus) (Meire and Ervynck 1986; Meire 1996; Zwarts et al. 1996). Birds like oystercatchers, hunting by vision control (Zwarts et al. 1996), might have been subjected to optical delusion. At first glance, Mytilus seems to be absent in Crassostrea-patches. Therefore, predation by birds which appears to be stronger during winter (Meire 1996) coincides with the higher abundance of medium sized M. edulis in the Crassostrea-patches and the sparse occurrence of 25–50 mm Mytilus in the Mytilus-patches (Fig. 3). In the meantime, oystercatchers in the Wadden Sea of Lower Saxony are frequently observed on the reefs and now prey predominantly on Crassostrea (unpublished data).
Associated macrofaunal communities
Crassostrea-reefs compensate for the conceivable loss of Mytilus-beds in the intertidal of the Wadden Sea by replacing the ecological function of M. edulis. The reefs seem to be an alternative habitat for species typically found in former Mytilus-beds. At the time of the investigation we did not observe a displacement of native macrofaunal species by the invasion of the non-indigenous Pacific oyster C. gigas. Besides C. gigas, no new invasive species was observed within this study. Except for Corynactis viridis, all species identified in this study have already been reported for Mytilus-beds of tidal flats in the German Wadden Sea. However, the literature data about macrofaunal species associated with Mytilus in the intertidal of the Wadden Sea originates from different references and is a sum of occurring species over the last 20–30 years (e.g. Dörjes 1978, 1992; Grotjahn 1987; Herlyn and Millat 2004). The composition of the community associated with “Mytilus-beds” has been shown to be highly variable over time. Whereas species are found in high abundances in 1 year they are absent in other years. In contrast, Crassostrea-reefs seem to provide a suitable habitat for a synchronous appearance of more common species. We found a statistical separation of the macrofaunal communities “Mytilus-patch” and “Crassostrea-patch”. We calculated higher values for species richness, abundance, biomass and diversity in Crassostrea-patches. In the following, these findings are discussed in terms of differences in ecosystem engineering by C. gigas versus M. edulis.
The Crassostrea-reef might have influenced the frequency of epibenthic organisms by providing a more complex habitat matrix with an extended hard substrate surface. Increased biodeposition might have led to an enhanced enrichment of the sediments in the Crassostrea-patches and in the open spaces between the patches, affecting the infauna.
Availability of hard substrate
The tidal flats of the Wadden Sea are dominated by soft sediments. Thus, M. edulis and C. gigas function as ecosystem engineers, offering a suitable hard substrate for the settlement of sessile organisms like barnacles (Buschbaum and Nehls 2003). The triple density of both ecosystem engineers in the Crassostrea-reef and the mean shell length of 67 mm of C. gigas implies an extended shell surface area and therefore led to the higher abundance of sessile organisms, e.g. of the barnacle E. modestus. We found anthozoans only in the Crassostrea-reef and hydrozoans as well as bryozoans were more diverse. The extended surface area of available hard substrate in the newly created habitat may as well have promoted the higher abundance of the periwinkle L. littorea.
Habitat matrix
The topography and structural complexity of biogenic habitats affects the ecological role of a habitat (Bell 1991). At low tide bivalve beds represent a moist habitat which diminishes the risk of dehydration. Compared to the flexible Mytilus-meshwork, the rigid structure of coalesced Crassostrea shells form new stable microhabitats. Regarding the Crassostrea-patches, the topography and in particular a vertical orientation of Crassostrea shells may amplify alteration in physical properties. Within the matrix of vertically oriented surfaces the influence of solar radiation decreases with increasing depth. This may explain the higher abundance of C. maenas as well as the exclusive occurrence of anthozoans, M. palmata, H. imbricata and A. rubens in the Crassostrea-reef (Table 2).
The geometry of the Crassostrea shells offers various cryptic microhabitats most suitable for colonization by several vermicular organisms. Sediment gets caught up in the petalled margins caused by shell proliferation. Through the settlement of Crassostrea larvae on conspecifics, sessile barnacles on these individuals happen to be overgrown by the extent of shell accretion of the young oyster. Sediment gets washed into the remaining space between oyster shells and plates of dead barnacles. In these crevices, between coalesced oyster shells and in the petalled margins of the oyster shells, we found T. benedii, N. succinea, and Polydora-species (Fig. 10). This may have resulted in the high abundance of oligochaetes, the exclusive and marked occurrence of N. succinea and the shift in the composition of polychaetes to more opportunistic facultative filter feeders in the Crassostrea-patches.
In contrast to mainly horizontal surfaces which occur in Mytilus-patches, vertically oriented Crassostrea shells show complex patterns of current flow. Soniat et al. (2004) reported that flow between vertically oriented shells was reduced to zero, whereas higher values were measured at the lateral extent of the shells and above the surface. Higher current velocities can favor food ingestion and growth of oysters as well as food ingestion and growth of associated filter feeders like Polydora-species (Lenihan et al. 1996). Also barnacles, anthozoans, hydrozoans and ascidians may benefit from these environmental conditions resulting in the enhanced colonization by sessile suspension feeding organisms in the Crassostrea-reef and, hence, may have resulted in a higher diversity of the epibiont community within this study.
Flow rates increase from bottom level towards the top of an oyster reef (Lenihan 1999). Accordingly, sedimentation near the reef base is favored by a strong reduction of hydrodynamic energy, whereas the upper parts of the shells remain free of sediment deposition due to high turbulence. M. edulis are more frequently affected by burial (Soniat et al. 2004). They are able to move back to the surface but attached organisms may suffer. The epifaunal reef community is continuously protected against sedimentation because of a permanently sediment-free upper shell surface in the Crassostrea-patch. This may have as well contributed to the richer epibenthos in Crassostrea-patches.
According to Asmus (1987), grazers and suspension feeders hold the highest share in total biomass of the associated macrofauna of intertidal Mytilus-beds. Predatory species appear to be less important. We found a similar distribution in Mytilus-patches. In contrast, we detected a shift in the distribution of sessile and vagile epifauna in the Crassostrea-reef. The strong increase in total biomass in Crassostrea-patches was associated with the vagile epifauna which contributed to 50% to the biomass, predominantly provided by L. littorea and C. maenas. Thus, suspension feeders like barnacles and anemones were of minor importance regarding their biomass, whereas predators (e.g. C. maenas, H. imbricata, A. rubens) became more important. While providing refuge to intermediate predators, habitat complexity appears to reduce interference competition among predators (Grabowski and Powers 2004). The evolving Crassostrea-reefs may promote the establishment of communities characterized by organisms of higher trophic levels. Already Dittmann (1990) reported a variety of trophic groups co-occurring in a Mytilus-bed as an attribute of structural heterogeneity of epibenthic bioherms.
Biodeposition
Walne (1972) reported higher clearance rates for C. gigas compared with equal sized M. edulis. Other studies revealed a higher clearance rate for M. edulis in springtime (Deslous-Paoli et al. 1987; May 2006; Wehrmann et al. 2006). However, the clearance rate and the corresponding biodeposit production depend on the size, respectively on the biomass of a bivalve. Biomass of both ecosystem engineers in the Crassostrea-patches was twice as high as it was in the Mytilus-patches. This implies a higher biodeposit production in Crassostrea-patches than in Mytilus-patches within this study.
The presence of biodeposits favors colonization by endobenthic organisms (Dittmann 1987). Kröncke (1996) showed that the amount of organic material in sediments constitutes the main factor in structuring endobenthic macrofaunal communities. The high food supply to the sediment benefits organisms feeding on organic particles or organic-rich sediments from the surface (surface deposit feeders). Mixing of the sediment column, for example by bioturbation, results in an enrichment of even deeper sediment layers with organic matter. Thus, subsurface deposit feeders are also favored. Resuspension transports biodeposits into the adjacent sandflats of Mytilus-beds (Kröncke and Bergfeld 1996; Hild and Günther 1999). Compared to a Mytilus-bed, the superficial structure of a Crassostrea-reef increases bottom roughness and water turbulences (Reise 2002). Thus, more biodeposits could have been exported from Crassostrea-patches than from Mytilus-patches.
The organic enrichment of sediments by biodeposits causes an increase in bacteria density (Dittmann 1987; Villbrandt et al. 1999) altering the geochemical sedimentary environment. High oxygen consumption by bacterial degradation of organic material leads more and more to anoxic conditions and causes an increase in toxic H2S produced by sulphur bacteria even in upper sediment layers (Little-Gadow 1978; Kristensen 2000). Dittmann (1990) and Kröncke (1996) showed that sand flats are dominated by polychaetes, whereas the underlying sediments of Mytilus-patches are dominated by oligochaetes. Likewise, in this study the sand flat and the sediments in the open spaces of the bivalve bed were dominated by polychaetes whereas the stations covered by epibenthic bivalves were dominated by oligochaetes. T. benedii, the most common oligochaete of this study is known to be indicative for areas of extremely high organic pollution (Pearson and Rosenberg 1978; Dittmann 1987). The detoxification of sulfides allows T. benedii to live in environments characterized by high H2S content (Hartmann-Schröder 1996). The strong appearance of oligochaetes in Crassostrea-patches and in the open spaces between these patches indicates increasing oxygen depletion and H2S production resulting from maximum exploitation of biodeposits.
Several authors (Dittmann 1987; Kröncke 1996; Bergfeld 1999; Villbrandt et al. 1999) predict that polychaete composition is affected by the accumulation of biodeposits and related changes in sediment chemistry. Dittmann (1987) reported that A. marioni, C. capitata and Microphthalmus sp. indicate enrichment of sediments by biodeposits. A. marioni prefers sandy sediments rich in fine grained muddy material. Enhanced organic enrichment of the sediments, especially in the open spaces between the Crassostrea-patches, may be responsible for the dominance of A. marioni. Besides C. capitata, M. sczelkowii and S. armiger frequently occurred in the open spaces between the Crassostrea-patches, also indicating a high concentration of organic material. According to Dittmann (1987) polychaetes respond very sensitively to epibenthic bivalve coverage. In her field experiments the composition of polychaetes changed within one year at stations artificially covered by Mytilus. At the beginning of the experiment A. marioni and S. armiger were the most abundant species. The species composition after one year was dominated by C. capitata, whereas the abundance of A. marioni and P. elegans decreased markedly. The frequencies of polychaetes in Mytilus-patches of our study did not correspond to the distribution found by Dittmann (1987) after one year. We counted more individuals of A. marioni and P. elegans in Mytilus-patches than in the respective open spaces. However, C. capitata became more important.
The influence of the C. gigas coverage on the sediment chemistry and subsequently on the composition of the infauna in Crassostrea-patches seems to be more distinct than the influence of the M. edulis cover in the Mytilus-patches. All polychaetes were found in low abundances in the Crassostrea-patches. Even C. capitata was rare and P. elegans was absent, perhaps due to enhanced accumulation of organic material. A. marioni dominated in Crassostrea-patches, even though with very low abundance. The internal fabric of the Crassostrea-patches, characterized by various microhabitats and a specific flow regime through vertical oriented shells (see section ‘Habitat matrix’), may result in a highly variable small-scale distribution pattern of sediment types differing in geochemistry. This is supported by the high species number but low abundance of polychaetes found in Crassostrea-patches. Most common were Polydora-species, added values for P. ciliata and P. cornuta reached 43% of the total number of polychaetes, which we found predominantly in the petalled margins of Crassostrea shells. N. succinea was also common, exclusively found between coalesced oyster shells. This may imply a distribution of these species independent of the geochemical conditions of the underlying sediments and may more likely be controlled by the availability of suitable habitats associated with the structure and formation of the oyster shells in the Crassostrea-patches.
The sand flat was predominantly characterized by the absence of nemertines and the common occurrence of S. armiger, A. marina and amphipods of the genus Bathyporeia, all of them typical residents of sandflats (Dörjes 1978). The latter, along with all other identified infaunal amphipods, were found exclusively in the sand flat. The dominant species, however, was P. elegans, known to prefer sandy tidal flats (Bergfeld 1999). C. capitata, an indicator species of nutrient-rich sediments, was found in moderate abundance. The sediments in the sand flat 100 m outside the bivalve bed seem to be influenced by biodeposits produced within the bivalve bed. This is also supported by the occurrence of large individuals of C. edule and M. balthica, both typical representatives of mudflats with intermediate mud content.
Outlook
According to Jones et al. (1994) ecosystem engineers are organisms that directly or indirectly modulate the availability of resources to other species. Major impact originates in high abundance of an ecosystem engineer influencing vast areas over a long period. The biodiversity of a habitat increases with its structural complexity (MacArther and MacArther 1961). Tsuchiya and Nishihira (1986) reported higher species richness and higher biodiversity with increasing structural complexity even within Mytilus-beds. The conceivable change from intertidal Mytilus-beds to Crassostrea-reefs in the Wadden Sea may promote the indigenous fauna. Long-term stability of a Crassostrea-reef and progressing habitat modulation as a consequence seems to be likely. This may lead to further increase in diversity.
References
Arakawa KY (1990) Natural spat collecting in the Pacific oyster Crassostrea gigas (Thunberg). Mar Behav Physiol 17:95–128
Asmus H (1987) Secondary production of an intertidal mussel bed community related to its storage and turnover compartments. Mar Ecol Prog Ser 39:251–266. doi:10.3354/meps039251
Bell WJ (1991) Searching behaviour. The behavioural ecology of finding resources. Chapman and Hall, New York
Bergfeld C (1999) Macrofaunal community pattern in an intertidal sandflat: effects of organic enrichment via biodeposition by mussel beds. First results. Senckenbergiana marit 29(suppl):23–27
Böttger R, Schnack D (1986) On the effect of formaldehyde fixation on the dry weight of copepods. Meeresforschung/reports on marine research. Sonderdruck 31:141–152
Brandt G, Wehrmann A, Wirtz KW (2008) Rapid invasion of Crassostrea gigas into the German Wadden Sea by larval supply. J Sea Res 59:279–296. doi:10.1016/j.seares.2008.03.004
Bray JR, Curtis JT (1957) An ordination of the upland forest communities of Southern Wisconsin. Ecol Monogr 27:325–349. doi:10.2307/1942268
Brey T (1986) Formalin and formaldehyde depot chemicals: effects on dry weight and ash free dry weight of two marine bivalve species. Meeresforsch./reports on marine res. Sonderdruck 31:52–57
Brey T (2001) Population dynamics in benthic invertebrates—a virtual handbook. Version 01.2. http://www.awi-bremerhaven.de/Benthic/Ecosystem/FoodWeb/Handbook/main.html Alfred Wegener Institute for Polar and Marine Research, Germany
Buschbaum C, Nehls G (2003) Effekte der Miesmuschel- und Garnelenfischerei. In: Lozán JL, Rachor E, Reise K, Sündermann J, von Westernhagen H (eds) Warnsignale aus Nordsee & Wattenmeer. Hamburg. Wissenschaftliche Auswertungen, pp 250–255
Carlton JT (1987) Patterns of transoceanic marine biological invasions in the Pacific ocean. Bull Mar Sci 41:452–465
Carlton JT, Geller JB (1993) Ecological roulette. The transport of nonindigenous marine organisms. Science 261:78–82. doi:10.1126/science.261.5117.78
Crooks JA (1998) Habitat alteration and community-level effects of an exotic mussel, Musculista senhousia. Mar Ecol Prog Ser 162:137–152. doi:10.3354/meps162137
Dankers NMJA, Dijkman EM, de Jong JL, de Kort G, Meijboom A (2004) De verspreiding en uitbreiding van de Japanese Oester en de Nederlandse Waddenzee. Alterra-rapport 909, Alterra, Wageningen
Deslous-Paoli JM, Heral M, Goulletquer P, Boromthanarat W, Razet D, Granier J, Prou J, Barille L (1987) Evolution saisonniere de la filtration de bivalves intertideaux dans des conditions naturelles. Oceanis 13(4–5):575–579
Diederich S (2005) Invasion of Pacific oysters (Crassostrea gigas) in the Wadden Sea: competitive advantage over native mussels. Dissertation, Universität Kiel
Dittmann S (1987) Die Bedeutung der Biodeposite für die Benthosgemeinschaft der Wattsedimente. Unter besonderer Berücksichtigung der Miesmuschel Mytilus edulis L. Dissertation, Universität Göttingen
Dittmann S (1990) Mussel beds – amensalism or amelioration for intertidal fauna? Helgoländer Meeresunters 44:335–352. doi:10.1007/BF02365471
Dörjes J (1978) Das Watt als Lebensraum. In: Reineck HE (ed) Das Watt. Ablagerungs- und Lebensraum. Verlag W Kramer, Frankfurt a.M.
Dörjes J (1992) Langzeitentwicklung makrobenthischer Tierarten im Jadebusen (Nordsee) während der Jahre 1974 bis 1987. Senckenbergiana marit 22:37–57
Drinkwaard AC (1999) Introductions and developments of oysters in the North Sea area: a review. Helgoländer Meeresunters 52:301–308. doi:10.1007/BF02908904
Fauchald K, Jumars PA (1979) The diet of worms: a study of polychaete feeding guilds. Oceanogr Mar Biol Ann Rev 17:193–284
Gollasch S (2002) The importance of ship hull fouling as a vector of species introductions into the North Sea. Biofouling 18:105–121. doi:10.1080/08927010290011361
Grabowski JH, Powers SP (2004) Habitat complexity mitigates trophic transfer on Crassostrea-reefs. Mar Ecol Prog Ser 277:291–295. doi:10.3354/meps277291
Grotjahn M (1987) Sedimente und Makrofauna der Watten bei der Insel Spiekeroog. Untersuchungen im Rahmen des „Sensitivitätsrasters Deutsche Nordseeküste. Forschungsstelle Küste: pp 97–119
Hamilton S, Kingston PF (1985) The effects of the preservatives alcohol, formalin and propylene phenoxetol on the wet weights of some marine animals. ICES Benthos working Group
Hartmann-Schröder G (1996) Polychaeta. In: Dahl F (ed) Die Tierwelt Deutschlands und der angrenzenden Meeresteile nach ihren Merkmalen und nach ihrer Lebensweise. 58. Teil, 2. Auflage. Gustav Fischer Verlag, Jena
Herlyn M, Michaelis H (1996) Untersuchung zur Entwicklung von Miesmuschelbänken der niedersächsischen Watten, unter besonderer Berücksichtigung der Miesmuschelfischerei. Abschlussbericht der A-Hauptphase, Teilprojekt A 3.3 des Teilvorhabens ÖSF Nds. Wattenmeer
Herlyn M, Millat G (2004) Wissenschaftliche Begleituntersuchungen zur Aufbauphase des Miesmuschelmanagements im Nationalpark „Niedersächsisches Wattenmeer”. Forschungsprojekt der Niedersächsischen Wattenmeerstiftung, Abschlussbericht März
Hertweck G, Liebezeit G (1996) Biogenic and geochemical properties of intertidal biosedimentary deposits related to Mytilus beds. Mar Ecol 17(1–3):131–141
Hild A, Günther C-P (1999) Ecosystem engineers: Mytilus edulis and Lanice conchilega. In: Dittmann S (ed) The Wadden Sea ecosystem – stability properties and mechanisms. Berlin. S, Springer, pp 43–49
Jones CG, Lawton JH, Shachak M (1994) Organisms as ecosystem engineers. Oikos 69(3):373–386. doi:10.2307/3545850
Kolar CS, Lodge DM (2001) Progress in invasion biology: predicting invaders. Trends Ecol Evol 16(4):199–204. doi:10.1016/S0169-5347(01)02101-2
Kristensen E (2000) Organic matter diagenesis at the oxic/anoxic interface in costal marine sediments, with emphasis on the role of burrowing animals. In: Liebezeit G, Dittmann S, Kröncke I (eds) Life at Interfaces and Under Extreme Conditions. Kluwer Dordrecht. Hydrobiologia 426:1–24
Kröncke I (1996) Impact of Biodeposition on Macrofaunal Communities in Intertidal Sandflats. P.S.Z.N.I. Mar Ecol 17(1–3):159–174. doi:10.1111/j.1439-0485.1996.tb00497.x
Kröncke I, Bergfeld C (1996) Makrofaunauntersuchungen auf der Swinnplate. In: Bartholomä A, Boysen-Ennen E, Delafontaine MT, Flemming BW, Hertweck G, Kröncke I, Wolf F, Bergfeld C (eds) Zur Elastizität makrofaunistischer biosedimentärer Systeme im Spiekerooger Watt: Wechselwirkungen zwischen Organismen, Sediment und Wasserkörper. Ökosystemforschung Niedersächsisches Wattenmeer (ELAWAT), Abschlussbericht des Teilprojektes B6, pp 117–152
Kruskal JB, Wish M (1978) Multidimensional scaling. Sage Publishers, Berverly Hills
Lenihan HS (1999) Physical-biological coupling on Crassostrea-reefs: how habitat structure influences individual performance. Ecol Monogr 69(3):251–275
Lenihan HS, Peterson CH, Allen JM (1996) Does flow speed also have a direct effect on growth of active suspension-feeders: an experimental test on oysters. Limnol Oceanogr 41(6):1359–1366
Linke O (1954) Die Bedeutung der Miesmuscheln für die Landgewinnung im Wattenmeer. Natur Volk 84(8):253–261
Little-Gadow S (1978) Sedimente und Chemismus. In: Reineck HE (ed) Das Watt – Ablagerungs- und Lebensraum. 2. neubearb. Auflage. Dr Waldemar Kramer, Frankfurt a. M
MacArther RH, MacArther JW (1961) On species diversity. Ecology 42:594–598. doi:10.2307/1932254
May P (2006) Nahrungskonkurrenz zwischen Crassostrea gigas (Thunberg 1793) und Mytilus edulis Linnaeus, 1758. Master thesis, Universität Oldenburg
Meire PM (1996) Using optimal foraging theory to determine the density of Mussels Mytilus edulis that can be harvested by hammering oystercatchers Haematopus ostralegus. Ardea 84A:141–152
Meire PM, Ervynck A (1986) Are oystercatchers (Haematopus ostralegus) selecting the most profitable mussels (Mytilus edulis)? Anim Behav 34:1427–1435. doi:10.1016/S0003-3472(86)80213-5
Möbius K (1877) Die Auster und die Austernwirthschaft. Verlag von Wiegandt, Hempel & Parey, Berlin
Nehring S (1998) Neozoa an der Nordseeküste – Ein bislang wenig beachtetes Phänomen!. DGM-Mitteilungen 3:3–6
Pearson TH, Rosenberg R (1978) Macrobenthic succession in relation to organic enrichment and pollution of the marine environment. Oceanogr Mar Biol Ann Rev 16:229–311
Pielou EC (1966) Shannon’s formula as a measurement of species diversity: it’s use and disuse. Am Nat 100:463–465. doi:10.1086/282439
Reise K (1998) Pacific oysters invade mussel beds in the European Wadden Sea. Senckenbergiana marit 28(4/6):167–175
Reise K (2002) Sediment mediated species interactions in costal waters. J Sea Res 48(2):127–140. doi:10.1016/S1385-1101(02)00150-8
Reise K, Dankers N, Essink K (2005) Quality Status Report Wadden Sea—introduced species. Int. Wadden Sea quality status report 2005. Wadden Sea ecosystem 19
Ricciardi A, Bourget E (1998) Weight-to-weight conversion factors for marine benthic macroinvertebrates. Mar Ecol Prog Ser 163:245–251. doi:10.3354/meps163245
Ruesink JL, Lenihan HS, Trimble AC, Heiman KW, Micheli F, Byers JE, Kay MC (2005) Introduction of non-native oysters: ecosystem effects and restoration implications. Annu Rev Ecol Evol Syst 36:643–689. doi:10.1146/annurev.ecolsys.36.102003.152638
Rumohr H (1990) Soft bottom macrofauna: collection, treatment and quality assurance of samples. ICES Techniques in Marine Environmental Sciences, vol 8, 18 pp
Soniat TM, Finelli CM, Ruiz JT (2004) Vertical structure and predator refuge mediate oyster reef development and community dynamics. J Exp Mar Biol Ecol 310:163–182
Troost K, Kamermans P, Stamhuis EJ, Wolff WJ (2004) Are introduced oysters (Crassostrea gigas) hampering the recruitment of indigenous bivalve filter feeders? ICES Council Meeting 2004/K: 10
Tsuchiya M, Nishihira M (1986) Islands of Mytilus as a habitat for small intertidal animals: effects of Mytilus age structure on the species composition of the associated fauna and community organisation. Mar Ecol Prog Ser 31:171–178. doi:10.3354/meps031171
Villbrandt M, Hild A, Dittmann S (1999) Biogeochemical processes in tidal flat sediments and mutual interactions with macrobenthos. In: Dittmann S (ed) The Wadden Sea ecosystem. Springer, Berlin
Walne PR (1972) The influence of current speed, body size and water temperature on the filtration rate of five species of bivalves. Mar Biol Assoc UK 52:345–374
Weaver W, Shannon CE (1949) The mathematical theory of communication. University of Illinois Press, Urbana, p 111
Wehrmann A, Herlyn M, Bungenstock F, Hertweck G, Millat G (2000) The distribution gap is closed–first record of naturally settled Pacific oysters Crassostrea gigas in the East Frisian Wadden Sea, North Sea. Senckenbergiana marit 30(3/6):153–160
Wehrmann A, Markert A, May P, Schieck P, Schmidt A (2006) Gefährdungspotential der eulitoralen Miesmuschelbänke im Niedersächsischen Wattenmeer durch die Bioinvasion der Pazifischen Auster Crassostrea gigas. Abschlussbericht Projekt 7/02 der Niedersächsischen Wattenmeer-Stiftung, 110 pp
Zens M, Michaelis H, Herlyn M, Reetz M (1997) Die Miesmuschelbestände der niedersächsischen Watten im Frühjahr 1994. Ber. Forschungsstelle Küste. Norderney 41:141–155
Zwarts L, Cayford JT, Hulscher JB, Kersten M, Meire PM, Triplet P (1996) Prey size selection and intake rate. In: Goss-Custard JD (ed) The oystercatcher: from individuals to populations. Oxford University Press, Oxford
Acknowledgments
The study was carried out within the framework of the project “Bioinvasion” which is financially supported by the SNG Senckenbergische Naturforschende Gesellschaft and the Niedersächsische Wattenmeer Stiftung. Thanks are due to Torsten Janßen for helping during field work. We are grateful to Norbert Dankers, Frouke Fey and Karsten Reise for valuable remarks which considerably improved an earlier version of the manuscript. The paper benefits essentially from constructive comments of two anonymous reviewers. Thanks to Charles Ver Straeten (New York State Museum) for correcting the English.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Markert, A., Wehrmann, A. & Kröncke, I. Recently established Crassostrea-reefs versus native Mytilus-beds: differences in ecosystem engineering affects the macrofaunal communities (Wadden Sea of Lower Saxony, southern German Bight). Biol Invasions 12, 15–32 (2010). https://doi.org/10.1007/s10530-009-9425-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-009-9425-4