Abstract
Effective marine resource management requires knowledge of the distribution of critical habitats that support resource populations and the processes that maintain them. Reefs that host diverse macrobenthic communities are important habitats for fish. However, detailed information on macrobenthic communities is rarely available and is usually limited to SCUBA diving depths. To establish depth-related distribution patterns and drivers that structure reef communities, the macrobenthos situated in a warm-temperate marine protected area (MPA; 34°01′24S; 23°54′09E) was sampled between 2009 and 2012. Comparison of shallow (11–25 m) and deep (45–75 m) sites revealed significantly different communities, sharing only 27.9 % of species. LINKTREE analysis revealed a changeover of species along the depth gradient, resulting in four significantly different assemblage clusters, each associated with particular environmental variables. High light intensity supported benthic algae at shallow depths, and as light availability decreased with depth, algal cover diminished and was eventually absent from the deep reef. Upright growth forms and settled particulate matter were positively related to depth and dominated the deep reef. Reduced wave action and currents on the deep reef can explain the increased settling of suspended particles. Under such conditions, clogging of feeding parts of the encrusting species is expected, and upright growth would be favoured. Considering that most MPAs are restricted to shallow coastal habitats and that macrobenthic communities change significantly with depth, it is probable that many unique deep reef habitats are currently afforded no protection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Marine protected areas (MPAs) are important for the conservation of biodiversity and the development of marine resource management strategies. However, to effectively manage our marine resources, critical habitats that support commercially and ecologically important species throughout their life cycles need to be identified and protected (Fitzpatrick et al. 2012; Seitz et al. 2014). In particular, hard-bottom communities, such as reefs, are especially important to include in MPA networks, since reefs host diverse assemblages of invertebrates, which in turn support higher-order consumers.
Sessile species (including macroalgae and suspension feeders) that inhabit reefs construct diverse and intricate three-dimensional habitats. As such, they increase the complexity of the reef topography and ecosystem functioning through interspecific facilitation (Gili and Coma 1998; Cardinale et al. 2002). These complex biogenic reef structures provide habitats for many commercially important fish species (Brouwer 2002; Griffiths and Wilke 2002; Brouwer and Griffiths 2004; Sink et al. 2006). Besides providing habitat for higher consumers, suspension feeders have major impacts on marine ecosystems through the regulation of primary production (Barange and Gili 1988; Coma et al. 1994) and are responsible for the bulk of the energy transfer from pelagic to benthic systems (Gili and Coma 1998).
Reef communities situated deeper than the conventional SCUBA diving depth limit (30 m) have received very little scientific attention (Sink et al. 2006; Virgilio et al. 2006). The lack of research on deeper reefs is because traditional deep water sampling methods are either not suited to sample complex high-profile reefs (e.g., dredges and grabs) or too expensive for most research budgets (e.g., remotely operated vehicles; ROVs). Only recently have some aspects of the ecology of deep nearshore reef communities been addressed, with the bulk of the research occurring in tropical seas (Lesser et al. 2009; Bongaerts et al. 2010; Hinderstein et al. 2010; Kahng et al. 2010; Locker et al. 2010; Sherman et al. 2010). Tropical research of this nature has focused on the light-dependent reef-building zooxanthellate corals occurring between 50 and 120 m depth, known as mesophotic coral reefs (Lesser et al. 2009). However, only a handful of studies have been conducted in the same bathymetric belt in temperate seas, most of which were conducted in the Mediterranean Sea (Rossi et al. 2008; Bo et al. 2008, 2009, 2011; Gori et al. 2011a, b; Gori et al. 2012). These studies typically focused on the distribution patterns of one or a few species and did not consider the entire macrobenthic community together with the associated factors affecting species composition along the depth gradient.
For successful conservation of marine biodiversity and resource management, we need to describe the habitats that support unique assemblages and important species. Concurrently developing an understanding of how abiotic variables affect habitat characteristics over meaningful spatial scales will enable scientists to predict where unique assemblages may be found. At local scales, factors thought to influence diversity patterns on reefs include physical disturbance, the number of species in the geographic area ready to colonize the available habitat (Connell 1978), light intensity and growth rates (Huston 1985a). In the marine environment, abiotic factors such as light, water movement, nutrient availability, sedimentation and temperature vary predictably with depth (Garrabou et al. 2002). The depth gradient can therefore be seen as a niche axis on which species occurrence is dependent on its tolerance to the covarying environmental conditions and ability to complete for limiting resources. Over evolutionary scales, species coexistence is regulated by their abilities to compete for resources. For example, suspension feeders belong to the same guild (Woodin and Jackson 1979), which is a group of species that exploit the same set of resources in a similar manner and are therefore expected to show considerable overlap in niche requirements (Root 1967). This niche overlap is thought to drive structural adaptations that allow for slight variations in resource acquisition that ultimately result in resource partitioning (limiting similarity; Macarthur and Levins 1967), enabling coexistence (Blondel 2003; Booth and Murray 2008).
To address the deficits in our knowledge on macrobenthos living beyond SCUBA depths, we investigated reef communities in the center of a large and well-established MPA. Our aims were to characterize the species composition and distribution of the macrobenthos and identify the processes that might be responsible for any differences between shallow (11–25 m) and deep (45–75 m) nearshore reefs. Because the majority of macrobenthic species are sessile, their distribution is largely influenced by the prevailing environmental conditions. Consequently, we hypothesized that species most suited to a particular environment, as determined by the depth gradient, would demonstrate similar morphological adaptations related to resource acquisition and would form depth-related species distribution clusters.
Materials and methods
Study area and sampling strategy
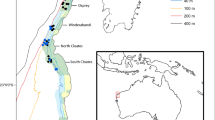
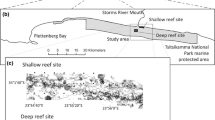
The research was conducted in the Tsitsikamma National Park (TNP) MPA, which is one of Africa’s oldest (established in 1964) and largest (360 km2) no-take MPAs (Tilney et al. 1996; Hanekom et al. 2012). The TNP MPA is situated in the middle of the warm-temperate Agulhas Ecoregion. It protects a 60-km stretch of coastline and extends 5 km offshore to a depth of approximately 100 m (Fig. 1; Tilney et al. 1996). The geology comprises steeply sloped quartzitic sandstone beds that lie parallel to the coastline (Buxton 1987; Cowley et al. 2002). Subtidally, these rocky formations form a series of parallel reef ridges separated by valleys filled with fine-grained sand (Buxton and Smale 1984). Sampling was conducted at shallow (11–25 m) and deep (45–75 m) reef sites situated in the middle of the TNP MPA (Fig. 1c). Both the shallow (area: 1.8 km2) and deep (area: 3.15 km2) sites included large expanses of solid high- and low-profile reefs. The reef sites represent some of the best examples of pre-exploitation subtidal communities in South Africa. Ecological baseline data collected from large, well-established no-take MPAs better reflect natural or pristine conditions (Shears and Babcock 2002) and improve the understanding and knowledge on which management policies are based. Prior to sampling, both reef sites were bathymetrically mapped with a GPS-linked echo sounder and a 300 × 300 m grid was overlaid on the mapped sites (Fig. 1c). The grid size was selected by taking into account the maximum depth of sampling in the present study (75 m), GPS error and boat swing on anchor. Each grid was classified according to profile (high or low), and sampling was conducted between 2009 and 2012 and followed a stratified random approach, with even allocation of sampling effort between reef sites and high- and low-profile reefs.
Location of the study area. a Map of South Africa indicating the position of the Tsitsikamma National Park marine protected area (TNP MPA), b the study area (Tsitsikamma), and c bathymetric maps of the two study sites, with inserts to explain sample strategy employed for each study site. Sample stations were the midpoints of 300 × 300 m grid cells
Assemblage composition
The species compositions of the macrobenthic assemblages were determined by estimating percentage cover from photoquadrats collected at six sample stations within each reef site. Photoquadrats on the shallow reef site were obtained by SCUBA divers. From the midpoint of each station, divers swam 25 meters in eight predefined directions. Eight to ten photographs were then haphazardly taken around the 25-m distance mark using a Canon G9 camera (12.1 megapixels) mounted on a tripod. This strategy was employed to avoid resampling the same area and to maximize sampling effort during dive times. The tripod setup maintained a set distance from the substrate and sampled an area of ca. 0.33 m2. On the deep reef, photoquadrats were obtained with a ROV (Falcon Seaeye: 12177) fitted with a 1Cam (SubC Control; 12.3 megapixel HD camera). The 1Cam, which could be orientated to capture benthic images at a 90° angle, was fitted with two laser pointers, thus permitting size approximation of the sampled area. Due to strong currents and restricted maneuverability, sampling at each deep reef station was conducted along a single 100-m transect, in contrast to the method employed at the shallow reef site. Along each transect, the ROV captured between 100 and 150 photoquadrats within 2 m of either side of the transect line (Fig. 1c). During transects, the ROV followed the depth contour to ensure that a standard depth was sampled at each station and to minimize environmental variability within the transect. According to the recommendations of Deter et al. (2012), 30 photographs were selected randomly from each sample station, amounting to 180 photoquadrats per reef site. Photoquadrats were calibrated in Coral Point Count with Excel extensions (CPCe 4.1; Kohler and Gill 2006), and 56 × 31 cm (0.2 m2) blocks were superimposed onto individual images. A species accumulation curve was plotted to estimate the number of points required to identify 95 % of the macrobenthic species per photoquadrat, which indicated that 54 points were required to analyze each photoquadrat. Under each point, individuals or colonies were identified to the nearest taxon (noting substrate cover where applicable) by referring to Samaai and Gibbons (2005), Jones (2008), Branch et al. (2010), and the invertebrate collection hosted by the South African Institute for Aquatic Biodiversity.
Environmental variables
During November 2011 and February 2012, light intensity (photosynthetically active radiation; 400–700 nm) was measured at three randomly selected sample stations from both the shallow and deep reef sites. Light intensity measurements were taken by employing a LICOR LI-193 Spherical Quantum Sensor. Temperature data were recorded by divers on the shallow reef, and a temperature probe (Onset HOBO Pro v2) was attached to the ROV to obtain temperature data when deep reef photoquadrats were collected. Reef profiles for each sampling station were estimated by divers on the shallow reef and from the ROV footage on the deep reef. The overlay from the ROV provided accurate depth measurements and allowed for estimates of the deep reef profile. Substrate type was estimated as percentage cover obtained from the photoquadrats. Depth was recorded by divers at the beginning and end of each transect. Care was taken to follow a depth contour when conducting all transects with the ROV, thereby standardizing depth during sampling. To summarize, data for temperature, reef profile, depth and substrate type were collected from at each sampling station. In contrast, light intensities were extrapolated according to station depth, from light profiles constructed from data collected during two seasons from three stations per reef, as indicated above.
Data analyses
Assemblage composition
Percentage cover and guild data of the macrobenthos (excluding substrate type) were analyzed using the statistical analysis software PRIMER v6 (Clarke and Warwick 2001; Clarke and Gorley 2006) and the PERMANOVA + add-on package (Anderson et al. 2008). All data were fourth-root-transformed to decrease the importance of highly abundant species, and analyses were performed on Bray–Curtis similarity matrices. Differences between reefs were tested by permutational analysis of variance (PERMANOVA), with ‘reef’ as fixed and ‘station’ as random (nested in reefs) factors using 9999 permutation of residuals under a reduced model. In addition, a two-way analysis of similarity (ANOSIM; sample stations nested in reefs) was performed obtain the R-statistic, which gives an indication of the magnitude of difference between tested factors. R-values close to one indicate completely different communities and a R-value near zero indicates very similar communities (Clarke and Gorley 2006).
Diversity
Shannon diversity (\(H^{\prime }\)) and species richness (S) were calculated in PRIMER 6 (Clarke and Gorley 2006). Following detailed exploratory data analysis (see Zuur et al. 2010), the effect of ‘water depth’ within ‘reef’ on the species richness (S) and Shannon diversity index (\(H^{\prime }\)) data was analyzed with linear mixed-effects models (LMMs). Linear mixed-effects models were selected to accommodate the dependency structure in the dataset caused by having multiple photoquadrats per sampling station.
The LMM with a random effect for station was defined as:
where \(S_{ij} \;{\text{or}}\;H_{ij}^{{\prime }}\) is the species richness or Shannon diversity index, respectively, from photoquadrat j at sampling station i. β 1 and β 2 are the intercept and slope, respectively, for the model. The random part of the model is specified by α i + ɛ ij , where α i is the random intercept and ɛ ij is the error, which follows a normal distribution with a mean of 0 and variance σ 2.
Due to the large gap in depth coverage between the shallow and deep reefs, the data were modeled by looking at the effect of water depth on S and \(H^{\prime }\) within ‘reef.’ This procedure avoided the possibility of erroneous conclusions to be drawn from the depths where no data existed. The LMM analysis was conducted in the R-environment for statistical analysis through the R-Studio 2.15.3 interface (RStudio Team 2015) using the ‘nlme’ package (Pinheiro et al. 2014). The results were plotted using the ‘lattice’ package (Sarkar 2008). The model selection and diagnostics followed the approach specified in Zuur et al. (2013).
Indicator species
Indicator species were determined by following the indicator value index (IndVal) procedure (Dufrêne and Legendre 1997). The IndVal index can be defined by computing two values: specificity (A kj ) and fidelity (B kj ). For each species j in each cluster of k sites, A kj and B kj. can be computed by:
The specificity (A) is based on abundance values and describes the degree to which a species is found only in a group of predefined sites. Fidelity (B) is computed from presence/absence data and describes the degree to which a species is present at all sites of a group (Legendre 2013). Statistical significance of the species–site group associations was determined by a permutation test. Indices were computed using the multipat() function from the ‘indicspecies’ package (De Cáceres and Jansen 2013) in R-Studio 2.15.3 (R Core Team 2013). Indicator species were calculated for the shallow and deep reefs and the clusters produced by the LINKTREE analysis from the average species assemblage data calculated for each sample station.
Environmental variables
To correlate species assemblages with environmental data, the assemblage data were averaged over stations. Following the recommendations of Clarke and Gorley (2006), environmental data with non-normal distributions were log-transformed, after which all environmental data were normalized. Depth was removed from the analysis since it was colinear with most other environmental variables. To clarify how the environmental variables affected species assemblages, the global BEST test, including all combinations (BIOENV), was performed (Clarke and Gorley 2006). Correlations between the environmental data and the species resemblance matrices were determined by calculating Spearman’s rank correlation coefficients (ρ). Environmental variables included depth (m), light intensity (µmol s−1 m2), temperature (°C), reef profile (categorized as low or high) and substrate type [categorized as bare rock, rubble, sand, shells or settled particulate matter (settled PM)]. Reef profile for each transect was estimated by SCUBA divers on the shallow reef and from the ROV footage on the deep reef site. Significance of the correlations was estimated with the global BEST match permutation test. To visualize the effect of the most important environmental variable on the assemblage data (as identified by the BEST test), the values of these variables were superimposed as bubbles on a principle coordinate analysis (PCO) biplot. To determine whether the macrobenthic assemblages clustered according to depth, and to identify the environmental variables that best explained each cluster, a LINKTREE procedure was performed (Clarke and Gorley 2006; Clarke et al. 2008). A Similarity Profiles (SIMPROF) permutation at the 5 % significance level was performed to provide the LINKTREE with objective stopping criteria when there was no further statistical evidence for subdivision of clusters (Clarke et al. 2008).
Guilds
The LINKTREE analysis revealed four clusters, based on macrobenthic species assemblage composition, which separated out along the depth gradient. To determine whether the depth at which these clusters split represents an important changeover in limiting resources (habitat, food and light), the macrobenthic species were assigned to guilds according to structural traits associated with resource exploitation. Species were grouped according to the different strategies used to occupy and gain space as well as the strategies and mechanisms employed to obtain food. These morphological adaptations can be classified into the following categories: (1) height above seafloor [growth form: solitary/colonial and shape; Woodin and Jackson (1979), Jackson (1977)] and (2) size and selection of food particles (feeding apparatus/mechanism; Wildish and Kristmanson (1997)). Algae were separated from the rest of the macrobenthos because food in the form of light affects only autotrophs. For the majority of macrobenthic species found on reefs, food is available in the form of suspended particulate matter; thus, suspension feeders tend to dominate. Suspension feeders can be active, passive, facultatively active or combined passive–active (Wildish and Kristmanson 1997). Additionally, solitary animals can feed by grazing or scavenging. To classify the macrobenthic species into guilds, each species had a combination of the adaptive traits assigned to it that resulted in 21 different trait combinations (Table S1).
A constrained canonical analysis of principal coordinates (CAP) was conducted on the guild data (Anderson and Willis 2003; Anderson et al. 2008). This procedure maximizes separation of multivariate data in reference to an a priori hypothesis (LINKTREE clusters). To establish if the CAP model identified the correct number of principle coordinate (PCO) axes, and how well the PCO axes discriminated among grouping variables, cross-validation tests were performed to determine the misclassification error (Anderson et al. 2008). A high allocation success suggests distinct groups, indicating that particular guild traits are associated with each depth cluster, providing evidence that the changeover in species along the depth gradient is related to resource acquisition.
Results
Assemblage composition
Compared to the deep reef, on which slightly less than half (48 %) of the area was inhabited by macrobenthos, the shallow reef was covered predominantly by macrobenthic species (85 %). The remaining (52 %) of the deep reef surface was composed of different substrate types. Here, the dominant substrate type was settled PM (38 %), whereas the shallow reef was covered by near equal amounts of sand (5 %), settled PM (4 %) and rubble (3 %).
From the photoquadrats, 161 taxa were identified: 111 to genus and 67 to species level (Table S1). The remaining specimens were either grouped to higher-order taxa, identified to genus (but may include several taxa), or recognized as a species (but could not be identified—due to a lack of comprehensive species lists). Similar proportions of unidentified specimens were present on the shallow and deep reefs (23.6 and 18.9 %, respectively).
The multivariate species composition differed significantly between the shallow and deep reefs (PERMANOVA: Pseudo-F 1 = 11.1, p = 0.0013), and according to the ANOSIM, the reefs comprised two very different communities (R-statistic = 1). From the 161 identified species, 78 were exclusive to the shallow and 38 to the deep reef, with 45 species common to both (Fig. 2). Sponges were the most diverse taxonomic group with a total of 43 species, of which 19 species were exclusive to the deep reef. This pattern stood in sharp contrast to that observed for ascidians. Of the 29 identified ascidian species, 20 were exclusive to the shallow reef, and only one ascidian species was unique to the deep reef. Species richness (S; Fig. 3a) and the Shannon diversity index (\(H^{\prime }\); Fig. 3b) showed complementary patterns, with significantly higher richness (F-value = 44.7; p < 0.001) and diversity (F-value = 18.5; p < 0.001) of macrobenthos occurring on the shallow reef (mean ± SD: S = 10.9 ± 3.8; \(H^{\prime }\) = 2.0 ± 0.5) compared to the deep reef (S = 6.0 ± 2.6; \(H^{\prime }\) = 1.5 ± 0.5; Table 1). The LMM indicated that there was a significant interaction effect between ‘reef’ and ‘depth’ (S: F-value = 6.2, p < 0.05; \(H^{\prime }\): F-value = 7.4, p < 0.05), with richness and diversity positively related to depth at the shallow site and negatively related to depth at the deep site (Fig. 3).
Sketch of the fitted values from the linear mixed-effects model illustrating the effect of ‘reef’ and ‘water depth’ within ‘reef’ on species richness (a) and Shannon diversity (b). The dots are the actual observed data, the black line is the predicted species richness and the gray area is the approximate 95 % confidence interval
According to the IndVal analysis, significant indicator species for the shallow reef community included encrusting algae (Hildenbrandia lecanellierii, Leptophytum spp., Mesophyllum spp.), ascidians (Distaplia skoogi, Polyandrocarpa anguinea, Polyandrocarpa sp. 1, Pseudodistoma sp. 1 and Pycnoclavella filamentosa), sponges (Haliclona sp. 1, Isodictya ectofibrosa, Tedania spp.), the purple soft coral (Alcyonium fauri), hydroids (Lytocarpia formosa, Macrohychia filamentosa) and the bryozoan Cryptopolyzoon concretum. All of these taxa were found at all stations of the shallow reef and only on the shallow reef. Significant indicator species for the deep reef included bryozoans (Adeonella sp. 3, Celleporaria sp. 1, Flustramorpha spp.), many of which were fragile calcareous false corals (Adeonella sp. 1, Tennysonia spp., Laminopora sp 1), and the sponge, Haliclona sp. 2. The following sponges were recorded only on the deep reef, but not at all stations; Psammocinia sp. 1, Proteleia sollasi, Clathria (Thalysias) oxitoxa, Isodictya frondosa, Clathria (Clathria) axociona). Taxa present over the entire depth range included cup corals (Balanophyllia bonaespei, Caryphyllia sp. 1), seafans (Eunicella papillosa, Acabaria rubra), the planar hydroid (Sertularella arbuscular) and the sponge, Psammoclema sp. 1.
When considering the contributions of the major taxonomic groups in terms of percentage cover, ascidians, algae and sponges represented similar contributions on the shallow reef (Fig. 4). On the deep reef, sponges and bryozoans were the dominant taxa, and ascidians contributed very little to percentage cover. Although sponges were represented by more species on the deep reef, they contributed slightly less in terms of percentage cover (Fig. 4) owing to the lower overall percentage cover of macrobenthos on the deep reef.
Environmental variables
Depth explained 92.2 % of the variability observed in the macrobenthic assemblage data (BEST test: Spearman’s correlation coefficient ρ = 0.922; p < 0.002). Since an increase in water depth typically corresponds to changes in other physical parameters (e.g., decrease in light intensity), another BEST procedure was run with ‘depth’ excluded, thereby clarifying the importance of the remaining environmental variables on species composition. The results revealed that settled PM on the reefs explained 79.3 % (p < 0.002) of the variability observed in the macrobenthic assemblage data. With an increase in depth, there was a clear gradient from very little settled PM on the shallowest site (13 m; 0.6 % settled PM) to large amounts on the deepest site (73 m), where 57 % of the reef was covered by settled PM (Fig. 5).
Principal coordinate analysis (PCO) of the species assemblage data employing Bray–Curtis distance measures. The percentage cover of settled particulate matter (settled PM) at each site is superimposed as a bubble. Shallow reef sample stations are indicated in light gray and deep reef sample stations in black. Average site depths are provided in meters
The BEST results indicated that depth plays a central role in structuring the macrobenthic assemblages. This finding was further supported by the LINKTREE analysis, which produced four significantly different macrobenthic clusters, each associated with a separate depth range (Fig. 6). The four clusters split into two sets, one confined to the shallow reef, and the other to the deep reef. Each cluster had a set of associated environmental variables that was responsible for the internal structure of that cluster. The first split separated the shallow reef from the deep reef assemblage. The shallow reef was characterized by low settled PM cover and greater light intensity compared to the deep reef (Fig. 6; R-statistic = 1; π = 17.6; p < 0.001). The LINKTREE analysis further split the shallow reef macrobenthic assemblage into clusters A and B, based on higher light intensities and less settled PM cover observed in cluster A compared to B (R-statistic = 0.75; π = 1.78; p < 0.003). Further distinction of the deep reef into two smaller clusters was due to higher light intensities, and less settled PM and sand cover, on the shallower cluster C (52 m) compared to the deeper cluster D (average depth: 64.8 m; R-statistic = 1; π = 3.49; p < 0.001).
LINKTREE analysis. Linkage trees explain the division of each cluster (based on species assemblage data; percentage contribution indicated by pie charts) with a set of environmental variables specific to that group. In the context of LINKTREE analysis, the R-statistic is a measure of the multivariate difference between groups (biotic) and ranges from approximately 0 to 1 (0 indicating ‘no difference’ and 1 signifying ‘completely different’ communities). On the y-axis, B (%) signifies an absolute measure of dissimilarity between the clusters. Depths indicated are averages measured during each station. Pie charts represent the average percentage contributions of the major taxa to each cluster
Indicator species calculated for the LINKTREE clusters revealed three algal species and the low-growing encrusting ascidian Aplidium mernooensis as significant indicator species for cluster A. Indicator species for cluster B included the low-growing encrusting sponge and ascidian species and two false coral species (Bryozoa; Table 2). Moving from the shallow to the deep reef, ascidians were replaced by hydrozoans, bryozoans and hard corals (pie charts, Fig. 6). Only one indicator species (hard coral Dendrophyllia sp. 1) was identified for cluster C. Hydrozoan cover declined from cluster C to D, and on cluster D sponges accounted for half of the macrobenthic cover. Significant indicator species for cluster D were exclusively sponge species, and all except one (Proteleia sollasi) demonstrated upright growth. Sponges were consistently present at all depth ranges, but became the dominant cover at station ≥60 m.
Guilds
The estimation of the misclassification error determined from the leave-one-out procedure of the CAP analysis indicated a high allocation success. A total of 72.4 % photoquadrats were correctly assigned to the LINKTREE clusters, suggesting that the dominant species found in each of the clusters belonged to a common guild type (Fig. 7). Vectors based on Pearson’s correlations >0.35 were superimposed onto the CAP biplot, which provides additional information on the guilds most closely associated with each cluster. In Cluster A, both encrusting and upright algae dominated. Moving slightly deeper, encrusting and massive active suspension feeders associated with cluster B. At the deep study site, upright growing combined active–passive suspension feeders (sponges) proliferated.
Discussion
The aims of this study were to describe the composition and distribution patterns of macrobenthos along a depth gradient and to determine the influential processes responsible for any shifts along the gradient. Specifically, a shallow (11–25 m) reef site and a deep (45–75 m) reef site in a large and well-established MPA were compared in terms of species richness and composition. These macrobenthic communities were further explored to establish if depth-related zonation patterns existed.
The results revealed well-defined changes in the macrobenthic assemblage related to depth. The shallow and deep reefs differed significantly in all measured aspects, and a finer scale depth zonation was apparent within each reef. The shallow reef community was characterized by greater species richness consisting mostly of algal species or low-growing/encrusting colonial species. In contrast, the deep reef macrobenthic community was characterized by lower species richness and upright growth forms were more prevalent.
Patterns of species diversity
Overall, the shallow reef site displayed greater diversity and richness when compared to the deep reef site. However, inspection of the diversity patterns of each reef in isolation revealed that species diversity and richness increased with depth on the shallow reef site and decreased with depth on the deep reef site (Fig. 3). This pattern is in agreement with previous studies conducted on coral reefs, which measured an increase in species diversity and richness up to depths of roughly 30 m (Loya 1972; Porter 1972; Huston 1985b), followed by a progressive reduction in diversity and richness below 30 m (Sheppard 1980; Huston 1985a). Since corals are also sessile suspension feeders, similar forces should drive patterns of diversity in other suspension feeding communities. The relatively low diversity observed at either end of the depth gradient can be explained by the intermediate disturbance hypothesis (Connell 1978). Physical disturbance in the form of wave action, surge and storms are highest at shallow reefs. However, decreases in light and water movement with depth ultimately cause low light levels and high rates of sedimentation (settlement of particles from the water column) at depth. The high physical disturbance concomitant with high light intensities at shallow reefs favors a few fast growing algal species that can cope with harsh conditions and competitively exclude other slow-growing species (Connell 1978; Huston 1985a). With an increase in depth, light intensity and the frequency of storm damage and wave action decreases, negating the competitive advantage of algae, and allowing new species to colonize open space (Huston 1985a). Moving deeper, light diminishes, which results in the complete loss of photosynthetic species, while at the same time there appears to be higher settlement of particles from the water column (Fig. 6). Increased settled PM causes clogging of the feeding apparatus of low-growing suspension feeders and favors only a few upright growing species.
However, water movement is not only important as a physical disturbance. Since colonial macrobenthic species are sessile, they rely on water movement to bring suspended particles into contact with their feeding structures (Okamura and Partridge 1999) and disperse their propagules (Russ 1982; Palardy and Witman 2011). Consequently, water flow is key to determining the distribution of sessile macrobenthic species (Gili and Coma 1998). Several authors have demonstrated, both experimentally and empirically, that flow speed drives both density and diversity (Gili and Coma 1998; Palardy and Witman 2011). Specifically, they showed that a decline in current intensity and speed resulted in a progressive decline in richness and density of suspension-feeder species (Gili and Coma 1998; Palardy and Witman 2011)—and observation which mirrors the overall trend observed in this study. While current speed was not measured during the present study, previous research conducted on the deep reef (Middlebank Reef; TNP MPA) indicated that current velocity gradually decreased with depth, with the lowest velocities measured on the bottom (Roberts and van den Berg 2005; Hancke 2010). This is a seemingly wide-ranging pattern observed on the Agulhas Bank (Boyd et al. 1992) and elsewhere around the South African coast (Roberts et al. 2006). Thus, water movement, which is a combination of tidal, wind and oceanic currents and wave surge, is greater on shallow compared to deep sites and would translate into higher potential recruitment rates and food availability.
Depth-related zonation
In contrast to the patterns of diversity and richness that appear to be affected by local scale processes, the zonation patterns as identified from the LINKTREE (Fig. 6) might be better explained by slower processes working at evolutionary scales. The decrease in bottom water movement with an increase in depth (Boyd et al. 1992; Roberts and van den Berg 2005; Hancke 2010) can explain the observed increase in settled PM with depth at the study area in the TNP MPA (Fig. 5). This logical inference is based on the fact that lower current velocities result in the increased settlement of particles from the water column (Sundborg 1956). As such, food availability and light intensity change predictably with depth and can be incorporated into a niche axis on which sessile macrobenthic species are lost or gained, depending on strategies evolved to obtain limiting resources (habitat, food and light).
Indeed, when taxa were classified according to traits associated with resource acquisition (guilds), 72.4 % were correctly classified by the CAP model (Fig. 7) into the depth range identified by the LINKTREE results (Fig. 6). The results from the CAP analysis provided evidence that the changeover of species on the depth gradient is related to resource acquisition, and on closer inspection, there seems to be a clear taxonomic association with groups that dominate at particular depths.
Because closely related species (congeners) often share similar adaptations, we can correlate the depth zonation, and the prevailing environmental conditions, with the structural adaptations identified in major macrobenthic taxonomic groups. Additionally, because of their niche preferences, indicator species can be used to reflect their ecological preferences (biotic or abiotic states of the environment; De Cáceres et al. 2012) and so provide further insight into zonation patterns.
The first taxonomic groups/guilds lost along the niche axis relative to the depth gradient were primary producers and their associated grazers. Light intensities were highest within the shallowest cluster and explain the dominance of algae and the three algal species that were selected as significant indicator species for this cluster (Table 2). The remaining changes observed in the species composition of the macrobenthos were related to the increase in settled PM, which in turn can be explained by the decrease in water movement with depth. The current speed at which different suspension-feeder function optimally differs between taxonomic groups due to small differences in adaptations necessary to acquire suspended particles (Hentschel and Shimeta 2008).
Active filter-feeders were present on both the shallow and deep reefs; however, ascidians were present almost exclusively on the shallow reef and were progressively replaced by bryozoan species with increased depth. Ascidians obtain food particles by actively filtering water through a mucous net (Petersen 2007), and they grow best in high current speed conditions (Wing and Jack 2012). Bryozoans obtain food particles by actively beating ciliated tentacles (Hentschel and Shimeta 2008), and they grow best where currents are weaker (Eckman and Duggins 1993). The two feeding mechanisms differ in terms of the environmental conditions under which they function optimally. Ascidian growth is slowed at high particle concentrations (due to the risk of clogging their filtering mechanisms, resulting in reduced retention and pumping rates; Kowalke 1999; Petersen 2007; Torre et al. 2012), while bryozoans can discard unwanted particles through selective flicking, or expel their gut contents at unusually high particle concentrations (Riisgård and Manríquez 1997). However, in turbulent conditions, the feeding structures of bryozoans are deformed, thereby reducing growth and causing colony miniaturization (Eckman and Duggins 1993; Okamura and Partridge 1999). These differences in feeding optimization help to explain the absence of bryozoans at the very shallow stations (13–15 m; Fig. 6) and the selection of the low-growing encrusting ascidian, Aplidium mernooensis, as an indicator species for the most shallow cluster. The selection of A. mernooensis as an indicator species for cluster A confirmed the tolerance of this species to more turbulent conditions. In contrast, the selection of fragile calcareous bryozoan species (false corals) as indicators for the conditions in cluster B and the deep reef confirmed their preference for weaker current speeds.
At the other extreme, sponges dominated depths where settled PM cover was high, suggesting low current speeds (cluster D: 59–73 m; Fig. 6). This pattern can be explained by the exceptional morphological plasticity of sponges, which are capable of modifying their shape depending on prevailing flow conditions (Palumbi 1984; Okamura and Partridge 1999; Kaandorp 1999; Bell and Barnes 2000b). When current speeds are low, sponges can modify their growth to form upright tree-like shapes (Okamura and Partridge 1999; Kaandorp 1999), preventing sediment accumulation on sponge surfaces (Bell and Barnes 2000b). Furthermore, sponges are capable of feeding on a large range of particle sizes (Jackson and Winston 1982). This flexibility provides sponges with a competitive advantage at all depths, but in this study sponges were particularly successful on the deep reef, reflected in the higher species richness and relative percentage cover at the deeper stations in Tsitsikamma (Figs. 3, 4, respectively). As a result, three upright sponge species were selected as indicators for the deepest cluster (Table 2). Several studies conducted on sponge communities within different marine biogeographic regions, e.g., temperate reefs in Lough Hyne, a marine lake in County Cork, Ireland (Bell and Barnes 2000a, b) and tropical reefs in southeast Sulawesi, Indonesia (Bell and Smith 2004), demonstrated similar findings regarding the morphological adaptations of sponges and their distribution related to current speed. These studies sampled sponge assemblages down to 30 m at sites that differed in terms of current speed, intensity and direction (Bell and Barnes 2000a, b; Bell and Smith 2004). At very turbulent sites, Bell and Barnes (2000a) found that massive and encrusting forms dominated sponge communities. The shallowest stations at Tsitsikamma were equivalent to the turbulent sites sampled by Bell and Barnes (2000a), and very little upright growth was observed here. Sites with low current speeds sampled by Bell and Barnes (2000a) were marked by high sedimentation, and upright sponges were most abundant. Similarly, cluster D from the deep reef of Tsitsikamma can be regarded as equivalent to the calm sites of Bell and Barnes (2000a), because the increased settled PM (Fig. 5) that suggested low current speeds also explained the prevalence of upright growth in sponges.
Conclusions
The macrobenthic community of Tsitsikamma demonstrated a distinct changeover of species along the depth gradient, with greatest diversity and richness observed at the deep end of the shallow reef. The patterns in diversity seem to support the intermediate disturbance hypothesis, where disturbance operating in different directions resulted in highest diversity at intermediate depths. The changeover in species along the niche axis (depth gradient) revealed a strong taxonomic link, which can be explained by feeding adaptations best suited for particular environmental conditions associated with variable depths. The current MPA network of South Africa mostly affords protection only to shallow nearshore reefs. The very different macrobenthic communities of the shallow and deep reef sites, in combination with increased presence of sensitive calcareous and upright macrobenthic species with depth, point to the importance of considering deep nearshore reefs in future MPA network design. Such vulnerable species would be particularly sensitive to physical damage from activities such as trawling, seabed mining and anchoring. Thus, to effectively conserve biodiversity and distinct habitats, protection must be afforded to reefs across the full depth spectrum covered in this study. We anticipate that the zonation patterns identified in Tsitsikamma could represent specific habitat requirements for fish, and as such suggest that certain fish assemblages are also currently afforded little protection. Determination of fine-scale fish habitat association patterns will provide further insight for the identification of priority habitats that require preferential consideration to ensure effective resource management.
References
Anderson MJ, Willis TJ (2003) Canonical analysis of principal coordinates: a useful method of constrained ordination for ecology. Ecology 84:511–525
Anderson MJ, Gorley RN, Clarke KR (2008) PERMANOVA+ for PRIMER: guide to software and statistical methods. PRIMER-E, Plymouth
Barange M, Gili JM (1988) Feeding cycles and prey capture in Eudendrium racemosum (Cavolini, 1785). J Exp Mar Biol Ecol 115:281–293. doi:10.1016/0022-0981(88)90160-8
Bell J, Barnes D (2000a) The distribution and prevalence of sponges in relation to environmental gradients within a temperate sea lough: vertical cliff surfaces. Divers Distrib 6:283–303
Bell J, Barnes DKA (2000b) The influences of bathymetry and flow regime upon the morphology of sublittoral sponge communities. J Mar Biol Assoc UK 80:707–718
Bell J, Smith D (2004) Ecology of sponge assemblages (Porifera) in the Wakatobi region, south-east Sulawesi, Indonesia: richness and abundance. J Mar Biol Assoc UK 84:581–591. doi:10.1017/S0025315404009580h
Blondel J (2003) Guilds or functional groups: does it matter? Oikos 2:223–231
Bo M, Tazioli S, Spanò N, Bavestrello G (2008) Antipathella subpinnata (Antipatharia, Myriopathidae) in Italian seas. Ital J Zool 75:185–195. doi:10.1080/11250000701882908
Bo M, Bavestrello G, Canese S, Giusti M, Salvati E, Angiolillo M, Greco S (2009) Characteristics of a black coral meadow in the twilight zone of the central Mediterranean Sea. Mar Ecol Prog Ser 397:53–61. doi:10.3354/meps08185
Bo M, Bertolino M, Borghini M, Castellano M, Covazzi Harriague A, Di CamilloCG, Gasparini G, Misic C, Povero P, Pusceddu A, Schroeder K, Bavestrello G (2011) Characteristics of the mesophotic megabenthic assemblages of the Vercelli Seamount (north Tyrrhenian Sea). PLoS ONE 6:e16357
Bongaerts P, Ridgway T, Sampayo EM, Hoegh-Guldberg O (2010) Assessing the “deep reef refugia” hypothesis: focus on Caribbean reefs. Coral Reefs 29:309–327. doi:10.1007/s00338-009-0581-x
Booth DJ, Murray BR (2008) Coexistence. In: Jorgensen S (ed) Encycl. Elsevier, Ecol, pp 664–668
Boyd AJ, Taunton-Clark J, Oberholster GPJ (1992) Spatial features of the near-surface and midwater circulation patterns off western and southern South Africa and their role in the life histories of various commercially fished species. S Afr J Mar Sci 12:189–206
Branch G, Griffiths CL, Branch M, Beckley L (2010) Two oceans: a guide to the marine life of southern Africa. Struik, Cape Town
Brouwer S (2002) Movement patterns of red steenbras Petrus rupestris tagged and released in the Tsitsikamma National Park, South Africa. S Afr J Mar Sci 24:375–378
Brouwer SL, Griffiths MH (2004) Age and growth of Argyrozona argyrozona (Pisces: Sparidae) in a marine protected area: an evaluation of methods based on whole otoliths, sectioned otoliths and mark-recapture. Fish Res 67:1–12. doi:10.1016/j.fishres.2003.08.007
Buxton CD (1987) Life history changes of two reef fish species in exploited and unexploited marine environment in South Africa. Dissertation, Rhodes University, Grahamstown
Buxton C, Smale M (1984) A preliminary investigation of the marine ichthyofauna in the Tsitsikamma Coastal National Park. Koedoe 27:13–24
Cardinale BJ, Palmer MA, Collins SL (2002) Species diversity enhances ecosystem functioning through interspecific facilitation. Nature 415:426–429. doi:10.1038/415426a
Clarke KR, Gorley RN (2006) PRIMER v6: user manual/tutorial. Plymouth Marine Laboratory, Plymouth
Clarke K, Warwick R (2001) Change in marine communities: an approach to statistical analysis and interpretation, 2nd edn. PRIMER-E, Plymouth
Clarke KR, Somerfield PJ, Gorley RN (2008) Testing of null hypotheses in exploratory community analyses: similarity profiles and biota-environment linkage. J Exp Mar Biol Ecol 366:56–69. doi:10.1016/j.jembe.2008.07.009
Coma R, Gili J, Zabala M, Riera T (1994) Feeding and prey capture cycles in the aposymbiotic gorgonian Paramuricea clavata. Mar Ecol Prog Ser 115:257–270
Connell JH (1978) Diversity in tropical rain forests and coral reefs. Science 80(199):1302–1310. doi:10.1126/science.199.4335.1302
Cowley P, Brouwer S, Tilney R (2002) The role of the Tsitsikamma National Park in the management of four shore-angling fish along the south-eastern Cape coast of South Africa. S Afr J Mar Sci 24:37–41
De Cáceres M, Jansen F (2013) Package ‘indicspecies’. [Documentation files] http://cran.r-project.org/web/packages/indicspecies/index.html
De Cáceres M, Legendre P, Wiser SK, Brotons L (2012) Using species combinations in indicator value analyses. Methods Ecol Evol 3:973–982. doi:10.1111/j.2041-210X.2012.00246.x
Deter J, Descamp P, Boissery P, Ballesta L, Holon F (2012) A rapid photographic method detects depth gradient in coralligenous assemblages. J Exp Mar Biol Ecol 418–419:75–82. doi:10.1016/j.jembe.2012.03.006
Dufrêne M, Legendre P (1997) Species assemblages and indicator species: the need for a flexible asymmetrical approach. Ecol Monogr 67:345–366. doi:10.1890/0012-9615(1997)067[0345:SAAIST]2.0.CO;2
Eckman JE, Duggins DO (1993) Effects of flow speed on growth of benthic suspension feeders. Biol Bull 185:28–41
Fitzpatrick BM, Harvey ES, Heyward AJ, et al. (2012) Habitat specialization in tropical continental shelf demersal fish assemblages. PLoS One 7:e39634. doi:10.1371/journal.pone.0039634
Garrabou J, Ballesteros E, Zabala M (2002) Structure and dynamics of north-western Mediterranean rocky benthic communities along a depth gradient. Estuar Coast Shelf S 55:493–508. doi:10.1006/ecss.2001.0920
Gili J, Coma R (1998) Benthic suspension feeders: their paramount role in littoral marine food webs. Trends Ecol Evol 13:316–321. doi:10.1016/S0169-5347(98)01365-2
Gori A, Rossi S, Berganzo E, Pretus JL, Dale MRT, Gili J (2011a) Spatial distribution patterns of the gorgonians Eunicella singularis, Paramuricea clavata, and Leptogorgia sarmentosa (Cape of Creus, northwestern Mediterranean Sea). Mar Biol 158:143–158. doi:10.1007/s00227-010-1548-8
Gori A, Rossi S, Linares C, Berganzo E, Orejas C, Dale MR, Gili J (2011b) Size and spatial structure in deep versus shallow populations of the Mediterranean gorgonian Eunicella singularis (Cap de Creus, northwestern Mediterranean Sea). Mar Biol 158:1721–1732. doi:10.1007/s00227-011-1686-7
Gori A, Viladrich N, Gili J, Kotta M, Cucio C, Magni L, Bramanti L, Rossi S (2012) Reproductive cycle and trophic ecology in deep versus shallow populations of the Mediterranean gorgonian Eunicella singularis (Cap de Creus, northwestern Mediterranean Sea). Coral Reefs 31:823–837. doi:10.1007/s00338-012-0904-1
Griffiths M, Wilke C (2002) Long-term movement patterns of five temperate-reef fishes (Pisces: Sparidae): implications for marine reserves. Mar Freshw Res 53:233–244. doi:10.1071/MF01148
Hancke L (2010) Dynamics of the Tsitsikamma current, with implications for larval transport of chokka squid (Loligo reynaudii) on the eastern Agulhas Bank. Dissertation, Cape Peninsula University of Technology
Hanekom N, Randall R, Bower D, Riley A, Kruger N (2012) Garden Route National Park: The Tsitsikamma SANParks section—state of knowledge. South African National Parks
Hentschel B, Shimeta J (2008) Suspension feeders. In: Jorgensen S (ed) Encyclopedia of Ecology. Elsevier, Amsterdam, pp 3437–3442
Hinderstein LM, Marr JCA, Martinez FA, Dowgiallo M, Puglise KA, Pyle RL, Zawada DG, Appeldoorn R (2010) Theme section on “Mesophotic Coral Ecosystems: characterization, ecology, and management”. Coral Reefs 29:247–251. doi:10.1007/s00338-010-0614-5
Huston M (1985a) Patterns of species diversity on coral reefs. Annu Rev Ecol Syst 16:149–177
Huston M (1985b) Patterns of species diversity in relation to depth at Discovery Bay, Jamaica. Bull Mar Sci 37:928–935
Jackson J (1977) Competition on marine hard substrata: the adaptive significance of solitary and colonial strategies. Am Nat 111:743–767
Jackson J, Winston J (1982) Ecology of cryptic coral reef communities. I. Distribution and abundance of major groups of encrusting organisms. J Exp Mar Biol Ecol 57:135–147. doi:10.1016/0022-0981(82)90188-5
Jones G (2008) Marine animals of the Cape Peninsula. Southern Underwater Research Group Press, Cape Town
Kaandorp JA (1999) Morphological analysis of growth forms of branching marine sessile organisms along environmental gradients. Mar Biol 134:295–306. doi:10.1007/s002270050547
Kahng SE, Garcia-Sais JR, Spalding HL, Brokovich E, Wagner D, Weil E, Hinderstein L, Toonen RJ (2010) Community ecology of mesophotic coral reef ecosystems. Coral Reefs 29:255–275. doi:10.1007/s00338-010-0593-6
Kohler KE, Gill SM (2006) Coral point count with excel extensions (CPCe): a Visual Basic program for the determination of coral and substrate coverage using random point count methodology. Comput Geosci 32:1259–1269. doi:10.1016/j.cageo.2005.11.009
Kowalke J (1999) Filtration in antarctic ascidians–striking a balance. J Exp Mar Biol Ecol 242:233–244. doi:10.1016/S0022-0981(99)00108-2
Legendre P (2013) Indicator species: computation. In: Levin Simon (ed) Encyclopedia of biodiversity, 2nd edn. Academic Press, Waltham, MA, pp 264–268
Lesser MP, Slattery M, Leichter JJ (2009) Ecology of mesophotic coral reefs. J Exp Mar Biol Ecol 375:1–8. doi:10.1016/j.jembe.2009.05.009
Locker SD, Armstrong RA, Battista TA, Rooney JJ, Sherman C, Zawada DG (2010) Geomorphology of mesophotic coral ecosystems: current perspectives on morphology, distribution, and mapping strategies. Coral Reefs 29:329–345. doi:10.1007/s00338-010-0613-6
Loya Y (1972) Community structure and species diversity of hermatypic corals at Eilat, Red Sea. Mar Biol 13:100–123. doi:10.1007/BF00366561
Macarthur R, Levins R (1967) The limiting similarity, convergence, and divergence of coexisting species. Am Nat 101:377–385
Okamura B, Partridge JC (1999) Suspension feeding adaptations to extreme environments in a marine bryozoan. Biol Bull 196:205–215
Palardy JE, Witman JD (2011) Water flow drives biodiversity by mediating rarity in marine benthic communities. Ecol Lett 14:63–68. doi:10.1111/j.1461-0248.2010.01555.x
Palumbi S (1984) Tactics of acclimation: morphological changes of sponges in an unpredictable environment. Science 225:1478–1480
Petersen JK (2007) Ascidian suspension feeding. J Exp Mar Biol Ecol 342:127–137. doi:10.1016/j.jembe.2006.10.023
Pinheiro J, Bates D, DebRoy S, Sarkar D, R Core team (2014) Nlme: linear and nonlinear mixed effects models. R package version 3.1-202. http://cran.r-project.org/package=nlme.pdf
Porter JW (1972) Patterns of species diversity in Caribbean reef corals. Ecology 53:745–748
R Core Team (2013) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
RStudio Team (2015) RStudio: Integrated Development for R. RStudio, Inc., Boston, MA. http://rstudio.com/
Riisgård HU, Manríquez P (1997) Filter-feeding in fifteen marine ectoprocts (Bryozoa): particle capture and water pumping. Mar Ecol Prog Ser 154:223–239. doi:10.3354/meps154223
Roberts MJ, van den Berg M (2005) Currents along the Tsitsikamma coast, South Africa, and potential transport of squid paralarvae and ichthyoplankton. Afr J Mar Sci 27:375–388. doi:10.2989/18142320509504096
Roberts M, Ribbink A, Morris T, van den Berg M, Engelbrecht D, Harding R (2006) Oceanographic environment of the Sodwana Bay coelacanths (Latimeria chalumnae), South Africa. S Afr J Sci 102:435–444
Root R (1967) The niche exploitation pattern of the blue-gray gnatcatcher. Ecol Monogr 37:317–350
Rossi S, Tsounis G, Orejas C, Padrón T, Gili J, Bramanti L, Teixidó N, Gutt J (2008) Survey of deep-dwelling red coral (Corallium rubrum) populations at Cap de Creus (NW Mediterranean). Mar Biol 154:533–545. doi:10.1007/s00227-008-0947-6
Russ GR (1982) Overgrowth in a marine epifaunal community: competitive hierarchies and competitive networks. Oecologia 53:12–19
Samaai T, Gibbons MJ (2005) Demospongiae taxonomy and biodiversity of the Benguela region on the west coast of South Africa. Afr Nat Hist 1:1–96
Sarkar D (2008) Lattice: multivariate data visualization with R. Springer, New York
Seitz RD, Wennhage H, Bergstro U, Lipcius RN, Ysebaert T (2014) Ecological value of coastal habitats for commercially and ecologically important species. ICES J Mar Sci 71:648–665. doi:10.1093/icesjms/fst152
Sheppard C (1980) Coral cover, zonation and diversity on reef slopes of Chagos atolls, and population structures of the major species. Mar Ecol Prog Ser 2:193–205. doi:10.3354/meps002193
Shears N, Babcock R (2002) Marine reserves demonstrate top-down control on temperate reefs. Oecologia 132:131–142
Sherman C, Nemeth M, Ruíz H, Bejarano I, Appeldoorn R, Pagán F, Schärer M, Weil E (2010) Geomorphology and benthic cover of mesophotic coral ecosystems of the upper insular slope of southwest Puerto Rico. Coral Reefs 29:347–360. doi:10.1007/s00338-010-0607-4
Sink KJ, Boshoff W, Samaai T, Timm PG, Kerwath S (2006) Observations of the habitats and biodiversity of the submarine canyons at Sodwana Bay. S Afr J Sci 102:466–474
Sundborg Å (1956) The River Klarälven: a study of fluvial processes. Geogr Ann 38:125–237
Tilney R, Nelson G, Radloff S, Buxton C (1996) Ichthyoplankton distribution and dispersal in the Tsitsikamma National Park Marine Reserve, South Africa. S Afr J Mar Sci 17:1–14
Torre L, Servetto N, Leonel Eory M, Momo F, Titian M, Abele D, Sahade R (2012) Respiratory responses of three Antarctic ascidians and a sea pen to increased sediment concentrations. Polar Biol. doi:10.1007/s00300-012-1208-1
Virgilio M, Airoldi L, Abbiati M (2006) Spatial and temporal variations of assemblages in a Mediterranean coralligenous reef and relationships with surface orientation. Coral Reefs 25:265–272. doi:10.1007/s00338-006-0100-2
Wildish D, Kristmanson D (1997) Benthic suspension feeders and flow. Cambridge University Press, Cambridge
Wing S, Jack L (2012) Resource specialisation among suspension-feeding invertebrates on rock walls in Fiordland, New Zealand, is driven by water column structure and feeding mode. Mar Ecol Prog Ser 452:109–118. doi:10.3354/meps09588
Woodin S, Jackson J (1979) Interphyletic competition among marine benthos. Am Zool 19:1029–1043. doi:10.1093/icb/19.4.1029
Zuur A, Ieno EN, Elphick CS (2010) A protocol for data exploration to avoid common statistical problems. Methods Ecol Evol 1:3–14. doi:10.1111/j.2041-210X.2009.00001.x
Zuur A, Hilbe J, Ieno E (2013) A beginner’s guide to GLM and GLMM with R. A frequentist and bayesian perspective for ecologists. Highland Statistics Ltd., Newburgh
Acknowledgments
Funding for this project was provided by the National Research Foundation of South Africa, the Elwandle Node of the South African Environmental Observation Network, the South African Institute for Aquatic Biodiversity, the African Coelacanth Ecosystem Programme and the British Ecological Society. We thank SANParks, in particular Kyle Smith and CapeNature for their assistance in the field, Toufiek Samaai, Kerry Sink, Shirley Parker-Nance, Lara Atkinson and Wayne Florence for assistance in identification of invertebrates and Angus Paterson and Clinton Veale for their constructive comments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: J.-M. Gili.
Reviewed by G. Gonzalez-Mirelis and undisclosed experts.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Heyns, E.R., Bernard, A.T.F., Richoux, N.B. et al. Depth-related distribution patterns of subtidal macrobenthos in a well-established marine protected area. Mar Biol 163, 39 (2016). https://doi.org/10.1007/s00227-016-2816-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-016-2816-z