Abstract
Despite the widespread perception that non-native species threaten biodiversity, there are few documented cases of non-native species displacing rare or specialized native species. Here, I examined changes in plant species composition over 5 years during patch expansion of a non-native grass, Imperata cylindrica, in longleaf pine flatwoods in Mississippi, USA. I used a multivariate approach to quantify the degree of habitat specialization and geographic range of all species encountered. I examined losses of species collectively as a function of plant height (controlling for initial frequency) and then the relationship between height and the degree of association with longleaf pine flatwoods, disturbed habitats, and the outer Gulf Coastal Plain of the southeastern USA. Patch expansion resulted in dramatic declines in species richness and increases in ground-level shade at both sites in just 3 years. Most tall saplings, shrubs, and vines were not endemic to longleaf pine communities and were less likely to be displaced than short herbs, most of which were indicative of longleaf pine communities. These results suggest that invasion of longleaf pine communities by I. cylindrica will likely cause significant losses of short, habitat-specialists and reduce the distinctiveness of the native flora of these threatened ecosystems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite the alarming rates at which some non-native species invade and dominate some habitats and expand their geographic ranges, there are very few documented cases of losses or extinctions of native species caused by non-native species (Simberloff 1981; Davis 2003; Gurevitch and Padilla 2004; Bruno et al. 2005). In addition, relatively few studies have directly examined the impact of non-native species on rare or threatened native species (Huenneke and Thompson 1995). Most species vulnerable to extinction tend to be local endemics and habitat specialists (Lawton and May 1995), but such species are not necessarily more vulnerable to competitive displacement than common species within sites (Rabinowitz et al. 1989). The local abundance of many rare plant species may be limited more by low reproductive output (which is not necessarily the result of competition; Rabinowitz et al. 1989) or soil-mediated negative feedbacks (Klironomos 2002). Nevertheless, if these species are inferior competitors to invasive species and are habitat specialists, then regional losses may be particularly likely in a landscape characterized by habitat destruction and unchecked range expansion of invasive species (Nee and May 1992). Conversely, life-history traits of ruderal or fugitive species often make them more vulnerable to competition or less well adapted to local environmental conditions than specialists (Platt and Weiss 1977; Campbell and Grime 1992). They typically are in little danger of regional extirpation, however, because of efficient dispersal and their ability to colonize a wide range of habitats (including disturbed habitats; Alverson et al. 1990). Careful consideration of the habitat requirements and distribution of species vulnerable to competitive displacement is crucial for assessing the impact of non-native species in a way that is relevant to biological conservation.
In this study, I determine whether species indicative of mesic longleaf pine (Pinus palustris) communities of the outer Gulf Coastal Plain of the USA are being locally displaced by an invasive species that is rapidly expanding its range (Imperata cylindrica). Furthermore, by examining traits potentially related to the competitive ability of these species (e.g., height; Leach and Givnish 1996), I reveal which if any characteristics shared by endemic or specialized species make them vulnerable to displacement by non-native species.
Methods
Description of Imperata cylindrica and its invasion of longleaf pine ecosystems
Imperata cylindrica (cogongrass, Japanese bloodgrass, alang-alang) is a long-lived, rhizomatous perennial C4 grass originating from east Asia and introduced to the Mobile Bay area of Alabama (USA) in the mid-1900s. It has since spread throughout much of the outer coastal plain region of the southeastern United States, with no indication of slowing its range expansion (Bryson and Carter 1993; Dozier et al. 1998). It has been described as one of the world’s 10 worst weeds (Holm et al. 1977), largely due to its effects on commercial forestry, agriculture, and fire regimes within natural ecosystems in the southeastern United States and tropical and subtropical areas of Asia and Africa (Bryson and Carter 1993; Kuusipalo et al. 1995; Premalal et al. 1995; Dozier et al. 1998; Lippincott 2000). Its effects on individual non-commercial species are not as well understood (NatureServe database; I-rank for I. cylindrica). One natural ecosystem in the southeastern USA that may be particularly vulnerable to invasion by I. cylindrica is the longleaf pine savanna (King and Grace 2000). This fire-dependent ecosystem, which hosts a variety of rare and endemic species (Walker 1993), once dominated upland portions of the outer coastal plain of the southeastern USA, but is now relatively rare and threatened by a variety of human activities (Frost 1993). I. cylindrica is significantly taller and recovers from fire more rapidly than most native herbs in longleaf pine savannas and therefore has the potential to displace many of these species via aboveground competition (Lippincott 1997, 2000). Hence, it is critical that we precisely document and contrast its impact on all native species. If the only species that are negatively affected are common, widespread species, then perhaps local eradication and control of I. cylindrica, though still important, should not be the highest conservation priority in longleaf pine savannas. Conversely, if habitat- and range-restricted species are displaced by I. cylindrica, then targeted eradication of I. cylindrica (especially along dispersal corridors within natural areas) might be a high priority, if for no other reason than to reduce its rate of spread to other sites.
Field study of post-invasion changes in composition and the light environment
Post-invasion changes in species composition were monitored over a 5-year period (2000–2005) at two mesic flatwoods sites within De Soto National Forest in southeastern Mississippi. Sites were chosen because both contained 100-year-old second-growth Pinus palustris stands and diverse groundcover plant communities typical of mesic flatwoods along the Gulf Coast (Peet and Allard 1993). Both also contained colonies of I. cylindrica invading from the edge. Both sites occurred on sandy, nutrient-poor soils and contained a relatively open overstory (<40% canopy density) with a minimal midstory, and diverse groundcover vegetation dominated by perennial grasses (e.g., Andropogon virginicus and Schizachyrium scoparium) and forbs (see Brewer and Cralle 2003 for more details).
Another criterion for selection was that the two flatwoods sites examined here differed with respect to prescribed burning history. One site, Henley Park, had been prescribed burned nearly every year during the winter or early spring between from 1980 to 2003. The other site, Wolf Branch, had been burned approximately once every 3 years during the winter or early spring between 1980 and 1996. It was also burned in April 2004. These differences in fire histories could have explained the higher species diversity at Henley Park at the beginning of the study (Glitzenstein et al. 2003). In addition, the different fire regimes could have influenced the impact of I. cylindrica on plant species (Lippincott 2000). Because fire effects were not replicated in this study, however, I can only suggest possible effects of fire on composition.
Within each site, a large (greater than 50 m at the widest section) contiguous patch of I. cylindrica was located at a roadside edge of the longleaf pine savanna (see Brewer and Cralle 2003 for a diagram of the shape and configuration of one of these patches). Sampling plots (0.5 × 0.5 m) were established at the border of each patch and were identical to the control plots in Brewer and Cralle (2003) in 1999. In that study, 60 plots (two treatments, one control, n = 20) were located at what appeared to be the advancing border of each patch. The plots were established far enough from one another to avoid edge effects but otherwise randomly. Each plot contained an average of three ramets of I. cylindrica in 1999; lower densities occurred farther from the road, whereas higher densities occurred nearer the road. Twenty of these plots were randomly assigned to be controls. By July 2000, ramet densities increased to a mean of 11 ramets, but at that time, most native species were still present (Brewer and Cralle 2003). The presence or absence of all species less than 2 m tall and ramet density of I. cylindrica was determined in July 2000. In addition, I measured photosynthetically-active photon flux density (PPFD) at each plot above and below the I. cylindrica canopy. Measurements were taken between 1,100 and 1,300 on a cloudless day in July 2000 at 1.5 m and 0.1 m above the ground in each plot using a Li-Cor optical sensor. The first time a species was encountered in a border plot, the height of its highest leaf was measured (hereafter, maximum height). The average of these heights was taken for each species at each site. Follow-up measurements of species composition were made in July 2003 and 2005. Follow-up measurements of ramet density and PPFD were taken in July 2003. No obvious differences in ramet density between 2003 and 2005 were apparent.
Although there was no control for the effect of patch expansion in this study, I did take measurements (presence-absence) of species composition in 20 plots in 2005 just outside (2 m from) the border of the patch of I. cylindrica at Henley Park. Each non-invaded plot was paired with the closest border plot. Species composition in these plots was compared to that of the border plots in 2000 (excluding I. cylindrica) and in 2005 (including I. cylindrica) to get an idea of background changes in species composition between 2000 and 2005 at this site.
Characterization of habitat and range of each species
To objectively integrate the responses of multiple species to invasion by I. cylindrica, I first quantified habitat associations for each species by generating a binary habitat × species matrix for all species encountered at either site. Habitats and their associated descriptions were derived from Clewell’s (1985) Guide to the Vascular Plants of the Florida Panhandle. Nomenclature and habitat types followed Clewell (1985).
Community similarity between each of the tabulated habitats and the focal habitat (longleaf pine flatwoods) was quantified using the following equation:
where S is the community similarity between flatwoods and a given habitat, x (hereafter, flatwoods similarity) and p indicates the presence or absence (1 for presence, 0 for absence) of species i in flatwoods or habitat x. The resulting scores potentially ranged from 1 (for flatwoods) to 0 (a community that shared no species with flatwoods). Even though all species encountered occurred in at least one of the two flatwoods studied here, a flatwoods similarity of 0 was possible because Clewell (1985) did not report all of the species as occurring in flatwoods.
To determine the degree to which each species was indicative of longleaf pine flatwoods, I calculated the flatwoods indicator score for each species as being the mean flatwoods similarity score for the habitat types in which it was reported to occur. Scores were similarly calculated for similarity of each habitat to disturbed habitats (disturbed habitat similarity score) and for how indicative each species was of disturbed habitats (disturbance indicator score) and of the Gulf Outer Coastal Plain (GOCP indicator score). The last score was based on presence in each of five physiographic regions (GOCP, Atlantic Outer Coastal Plain, Atlantic Interior Coastal Plain, Piedmont, and Mountains), following Radford et al. (1968) or NatureServe and other databases for species not included in that work. The Gulf region was defined as Mississippi, Alabama, and Florida, and the Atlantic as Georgia, the Carolinas, and Virginia.
Fifty species including I. cylindrica were identified in the border plots at the two sites combined (list available from the author). Fourteen more species found only in the non-invaded plots at Henley Park and five unidentified species were excluded from the habitat and range analysis. Scores for sets of species that could not be distinguished in the vegetative state (Andropogon virginicus, A. tenarius, and Schizachyrium scoparium; and Pityopsis graminifolia and P. adenolepis) were averaged before analysis of change in frequency.
Statistical analyses
Changes in species richness (number of species other than I. cylindrica per plot) in border plots at both sites were quantified using univariate repeated-measures analysis of variance (ANOVA). The relationship between the relative rate of increase in ramet density (invasion) of I. cylindrica (ln(density2003) − ln(density2000)) and initial native species richness and reduction in PPFD in 2003 each were examined separately using simple linear regression. I used one-tailed t-tests (assuming unequal variances) to test the hypothesis that plots containing no native species in 2003 (following invasion by I. cylindrica) would be shadier at ground level than those that contained at least one native species. Separate analyses were done for each of the two sites.
Changes in species composition were examined separately at each site using nonparametric multivariate analysis of variance (npMANOVA; Anderson 2001), with year as a fixed grouping variable and plot as a blocking variable. Species composition at Henley Park was similarly compared between border plots in 2000 and non-invaded plots in 2005 and between border plots in 2005 and non-invaded plots in 2005. To increase detection of differences in native species composition between border plots in 2000 and the adjacent non-invaded plots, I. cylindrica was scored as absent from the border plots in 2000 (but not in 2005). Because I. cylindrica was frequently the only species present in border plots in 2005, removing it from the 2005 border plot data would have precluded a contrast of species composition in border and non-invaded plots in 2005.
I used stepwise multiple regression to determine the relationship between the relative rate of change in frequency of a species between 2000 and 2005 and its initial frequency, height, and three indicator scores. The net rate of change in frequency was quantified as ln(frequency2005 + 1) − ln(frequency2000 + 1); wherein frequency was the number of plots (out of a total of 20) in which the species occurred. To examine the relationship between maximum height and indicator scores, I regressed maximum height against flatwoods indicator score, disturbance indicator score, or GOCP indicator score. Separate analyses were done for each site. Univariate ANOVAs and linear regressions were done using JMP 5.0.1. Non-parametric MANOVA was done using PC-Ord version 5.
Results
Post-invasion changes in species richness, light environment, and overall species composition
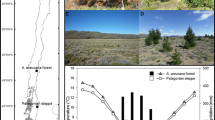
Patch expansion resulted in highly significant declines in species richness at both sites between 2000 and 2003 (F 1,76 = 315, Greenhouse-Geiser P < 0.0001; Fig. 1). No further decline was observed between 2003 and 2005 (F 1,76 = 0.12, G-G P = 0.56; Fig. 1). The declines in species richness between 2000 and 2003 were greater at Henley Park (which had higher species richness at the beginning of the study) than at Wolf Branch (Site × Year F 1,76 = 13.7, G-G P = 0.0005; Fig. 1). The rate of invasion of plots by I. cylindrica between 2000 and 2003 was greater in those plots with higher species richness (r = 0.51, P = 0.02 for Henley Park; r = 0.54, P = 0.01 for Wolf Branch). The increase in the amount of shade (reduction in light) between 2000 and 2003 was greater in those plots with higher rates of invasion by I. cylindrica (r = 0.56, P = 0.01 for Henley Park; r = 0.51, P = 0.02 for Wolf Branch). Ramet density of I. cylindrica and ground-level shade were lower and more variable at both sites in 2000 than in 2003 (average ramet density = 10.8 ± 0.7 s.e. vs. 26.4 ± 0.5 s.e.; average percent reduction in light = 77.4 ± 0.02 s.e. vs. 98.7 ± 0.002 s.e.) At Henley Park, plots that lacked any native species in 2003 were associated with higher levels of shade than were plots that contained at least one native species (99.5% vs. 97.9%; t (df = 11.2) = 2.91; P = 0.007). The differences at Wolf Branch were not as pronounced (99.2% vs. 98.6%; t (df = 14.2) = 1.68; P = 0.058).
Species composition shifted significantly between 2000 and 2003 at both sites, as determined by a significant effect of year (npMANOVA t = 3.36, P < 0.0002 for Henley Park; npMANOVA t = 3.14, P < 0.0002 for Wolf Branch). Changes in species composition between 2003 and 2005 were not statistically significant (npMANOVA t < 0.0001, P = 0.998 for Henley Park; npMANOVA t = 0.097, P = 0.997 for Wolf Branch). Species composition in plots outside of the patch of I. cylindrica at Henley Park was not significantly different from that in the border plots in 2000 (npMANOVA t = 0.926, P = 0.638).
Post-invasion changes in species occurrence in relation to height, habitat, and range
Stepwise multiple regression retained two explanatory variables, initial frequency and maximum height, which together explained 74% and 82% of the variation in changes in frequency between 2000 and 2005 at Henley Park and Wolf Branch, respectively (P < 0.0001). Declines in frequency were greatest among the most common species at both sites. The partial regression coefficients for the slope of the relationship between change in frequency and initial frequency were −0.69 and −0.8 for Henley Park and Wolf Branch, respectively (t = −6.5 and −5.2; P < 0.0001). As a group (and after controlling for differences in initial frequency), short herbaceous species tended to decline to a greater extent than tall species at both sites (Fig. 2). The partial regression coefficients for the slope of the relationship between change in frequency and maximum height were 0.52 and 0.44 for Henley Park and Wolf Branch, respectively (t = 5.3 and 3.1, respectively; P < 0.005). All species shorter than the maximum height per plot of I. cylindrica (1.2 m) and one taller species (Carphephorus odoratissimus) declined at Henley Park. None of the remaining tall species declined at either site. Tall species were primarily animal-dispersed trees, shrubs, and vines, none of which were indicative of flatwoods or similar longleaf pine communities (flatwoods indicator scores <0.58, with most <0.38). As a result, maximum height was negatively correlated with flatwoods indicator scores at both sites (Fig. 3; r = −0.4; P = 0.007 for Henley Park; r = −0.5; P = 0.002 at Wolf Branch). Maximum height was not significantly correlated with disturbance indicator scores at either site (r = 0.22 and 0.1 at Henley Park and Wolf Branch, respectively; P > 0.14), and marginally correlated with GOCP indicator scores at both sites (r = −0.24 and P = 0.12 at Henley Park; r = −0.35 and P = 0.06 at Wolf Branch).
Discussion
The results of this study show that the invasion of longleaf pine communities by I. cylindrica can cause significant losses of herbaceous plants indicative of longleaf pine savannas and reduce the distinctiveness of the native flora of these ecosystems. Any plant shorter than I. cylindrica is at risk of displacement, and most groundcover herbs indicative to longleaf pine savannas are shorter than I. cylindrica. In contrast, the taller species (e.g., tall shrubs, saplings, climbing vines) that avoided displacement were not indicative of longleaf pine savannas. These results strongly suggest that I. cylindrica displaced most native species in this community, at least in part or indirectly, by increasing shade. I. cylindrica dramatically increased shade (up to 99% at ground level) at these sites. Given the threatened status of the longleaf pine ecosystem (Noss 1988) and the rapid range expansion of I. cylindrica and its propensity to invade and spread rapidly through longleaf pine savannas and similar habitats, at least some short longleaf pine flatwoods indicator species could eventually become rare enough to warrant listing.
The results of this study are relevant to a recent debate over whether species invasions represent a crisis for conservation of native biodiversity (Davis 2003; Brown and Sax 2004, 2005; Cassey et al. 2005; Gurevitsch and Padilla 2004; Bruno et al. 2005). Compared to the effects of most non-native plants on plant species diversity, the dramatic loss of species following the expansion of the I. cylindrica may be somewhat unusual (Davis 2003; Gurevitch and Padilla 2004; Bruno et al. 2005). One possible reason is the local and patchy nature of competition (Bruno et al. 2005). In this study, the displacement of a large number of plant species by a single species in the current study required the combination of several factors. Unlike the majority of resident species, I. cylindrica is a tall, rapidly-resprouting grass that produces long rhizomes, and thus has the potential to shade the resident herbs and spread rapidly through a site. No other species at these sites exhibited this combination of characteristics. Many of the taller native species that were not affected by I. cylindrica (e.g., Ilex vomitoria, Myrica cerifera, Diospyros virginiana) also have the potential to displace native herbs in longleaf pine communities, especially after prolonged periods of fire suppression (Glitzenstein et al. 2003). These species lack I. cylindrica’s ability to spread rapidly by rhizomes, however, and likely do not recover from fire as quickly (Lippincott 1997; Glitzenstein et al. 2003). These results contradict the hypothesis that communities growing on nutrient-poor soils tend to resist invasion (see Funk and Vitousek 2007 for additional evidence) and are consistent with the hypothesis that invaders with large impacts possess novel competition-related traits not represented within the resident community (Callaway and Aschehoug 2000). They are also consistent with the hypothesis that species rich plant communities offer very little resistance to invasion by superior competitors with novel traits (Levine and D’Antonio 1999). In fact, plots with lower initial species richness were invaded less rapidly, perhaps because they were dominated by tall shrubs (e.g., Ilex vomitoria). Despite differences in fire frequencies between the two sites studied here, the expansion of I. cylindrica caused catastrophic declines at both sites. The greater decline at Henley Park (the more frequently burned site) resulted from the fact that border plots at this site had greater species richness at the beginning of the study. By 2003, border plots at both sites had low species richness. Hence, the competitive effects of this species on plant diversity may be of more immediate conservation concern than the effects of this species on fire regimes in longleaf pine ecosystems.
References
Alverson WS, Kuhlmann W, Waller DM (1990) Wild forests: conservation biology and public policy. Island Press, Washington, DC
Anderson MJ (2001) A new method for non-parametric multivariate analysis of variance. Austral Ecol 26:32–46
Brewer JS, Cralle SP (2003) Phosphorus addition reduces invasion of a longleaf pine savanna (Southeastern USA) by a non-indigenous grass (Imperata cylindrica). Plant Ecol 167:237–245
Brown JH, Sax DF (2004) An essay on some topics concerning invasive species. Austral Ecol 29:530–536
Brown JH, Sax DF (2005) Biological invasions and scientific objectivity: reply to Cassey et al. (2005). Austral Ecol 30:481–483
Bruno JF, Fridley JD, Bromberg KD, Bertness MD (2005) Insights into biotic interactions from studies of species invasions. In: Sax DF, Stachowicz JJ, Gaines SD (eds) Species invasions: insights into ecology, evolution, and biogeography. Sinauer Associates, Inc., Sunderland, Massachusetts, pp 13–40
Bryson CT, Carter R (1993) Cogongrass, Imperata cylindrica, in the United States. Weed Technol 7:1005–1009
Callaway RM, Aschehoug ET (2000) Invasive plants versus their new and old neighbors: a mechanism for exotic invasion. Science 290:521–523
Campbell BD, Grime JP (1992) An experimental test of plant strategy theory. Ecology 73:15–29
Cassey P, Blackburn TM, Duncan RP, Chown SL (2005) Concerning invasive species: reply to Brown and Sax. Austral Ecol 30:475–480
Clewell AF (1985) Guide to the vascular plants of the Florida panhandle. The Florida State University Press, Tallahassee, Florida
Davis MA (2003) Biotic globalization: does competition from introduced species threaten biodiversity? Bioscience 53:481–489
Dozier H, Gaffney JF, McDonald SK, Johnson ER, Shilling DG (1998) Cogongrass in the United States: history, ecology, impacts, and management. Weed Technol 12:737–743
Funk JL, Vitousek PM (2007) Resource-use efficiency and plant invasion in low-resource systems. Nature 446:1079–1081
Frost CC (1993) Four centuries of changing landscape patterns in the longleaf pine ecosystem. Proc Tall Timbers Fire Ecol Conf 18:17–43
Glitzenstein JS, Streng DR, Wade DD (2003) Fire frequency effects on longleaf pine (Pinus palustris P. Miller) vegetation in South Carolina and northeast Florida, USA. Nat Areas J 23:22–37
Gurevitch J, Padilla DK (2004) Are invasive species a major cause of extinctions? Trends Ecol Evol 19:470–474
Holm LG, Plucknett DL, Pancho JV, Herberger JP (1977) The world’s worst weeds: distribution and biology. The University of Hawaii Press, Honolulu, HI, USA, 609 pp
Huenneke LF, Thompson JK (1995) Potential interference between a threatened endemic thistle and an invasive nonnative plant. Conserv Biol 9:416–425
King SM, Grace JB (2000) The effects of gap size and disturbance type on invasion of wet pine savanna by cogongrass, Imperata cylindrica (Poaceae). Am J Bot 87:1279–1286
Klironomos JN (2002) Feedback with soil biota contributes to plant rarity and invasiveness in communities. Nature 417:67–70
Kuusipalo J, Ådjers G, Jafarsidik Y, Otsamo A, Tuomela K, Vuokko R (1995) Restoration of natural vegetation in degraded Imperata cylindrica grassland: understory development in forest plantations. J Veg Sci 6:205–210
Lawton JH, May RM (eds) (1995) Extinction rates. Oxford University Press, Oxford, UK
Leach MK, Givnish TJ (1996) Ecological determinants of species loss in remnant prairies. Science 273:1555–1558
Levine JM, D’Antonio CM (1999) Elton revisited: a review of evidence linking diversity and invasibility. Oikos 87:15–26
Lippincott CL (1997) Ecological consequences of Imperata cylindrica (cogongrass) invasion in Florida sandhill. Ph.D. dissertation, University of Florida, Gainesville, Florida, USA
Lippincott CL (2000) Effects of Imperata cylindrica (L.) Beauv. (cogongrass) invasion on fire regime in Florida sandhill. Nat Areas J 20:140–149
Nee S, May RM (1992) Dynamics of metapopulations: habitat destruction and competitive coexistence. J Anim Ecol 61:37–40
Noss RF (1988) The longleaf pine landscape of the southeast: almost gone and almost forgotten. Endangered Spec Update 5:16
Peet RK, Allard DJ (1993) Longleaf pine vegetation of the southern Atlantic and eastern Gulf Coast regions: a preliminary classification. Proc Tall Timbers Fire Ecol Conf 18:45–81
Platt WJ, Weiss IM (1977) Resource partitioning and competition within a guild of fugitive prairie plants. Am Nat 111:479–513
Premalal KPSB, Meylemans B, Sangakkara UR, Van Damme P (1995) Depression of growth of spear grass (Imperata cylindrica) by velvet bean (Mucuna pruriens). Mededelingen Faculteit Landbouwkundige en Toegepaste Biologische Wetenschappen Universiteit Gent 60:213–216
Rabinowitz D, Rapp JK, Cairns S, Mayer M (1989) The persistence of rare prairie grasses in Missouri: environmental variation buffered by reproductive output of sparse species. Am Nat 134:525–544
Radford AR, Ahles HA, Bell CR (1968) Manual of the vascular plants of the Carolinas. University of North Carolina Press, Chapel Hill, North Carolina, USA
Simberloff D (1981) Community effects of introduced species. In: Nitecki MH (ed) Biotic crises in ecological and evolutionary time. Academic Press, New York, pp 53–81
Walker J (1993) Rare vascular plant taxa associated with the longleaf pine ecosystems: patterns in taxonomy and ecology. Proc Tall Timbers Fire Ecol Conf 18:105–125
Acknowledgments
I thank Gomez Parker of the University of Mississippi Forest Lands and the Fire Crew at Desoto National Forest for technical assistance and access to the sites, and Ashley Cain, Allison Grisham, Alex Pabst, and Sean Cralle for assistance in the field, and two anonymous reviewers and associate editor P. Alpert. Support for this project was provided by a grant from the University of Mississippi Faculty Summer Support Program.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Brewer, S. Declines in plant species richness and endemic plant species in longleaf pine savannas invaded by Imperata cylindrica . Biol Invasions 10, 1257–1264 (2008). https://doi.org/10.1007/s10530-007-9200-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-007-9200-3