Abstract
Conifers, which are widely planted as fast growing tree crops, are invading forested and treeless environments across the globe, causing important changes in biodiversity. However, how small-scale impacts on plant diversity differ according to pine size and habitat context remains unclear. We assessed the effects of different stages of pine invasion on plant communities in forest and steppe sites located in southern Chile. In each site, we sampled plant diversity under and outside the canopy of Pinus contorta individuals, using paired plots. We assessed the relative impact of pine invasion on plant species richness and cover. In both sites, richness and cover beneath pine canopy decreased with increasing pine size (i.e. height and canopy area). A significant negative impact of pines on species richness and plant cover was detected for pines over 4 m in height. The impact of pines on plant richness and cover depended on pine size (i.e. canopy area) and habitat type. Larger pines had more negative impacts than smaller pines in both sites, with a greater impact for a given pine size in the Patagonian steppe compared to the A. araucaria forest. Species composition changed between under and outside canopy plots when pines were 4 m or taller. Pine presence reduced cover of most species. The impacts of pine invasions are becoming evident in forested and treeless ecosystems of southern Chile. Our results suggest that the magnitude of pine invasion impacts could be related to how adapted the invaded community is to tree cover, with the treeless environment more impacted by the invasion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tree species belonging to the genus Pinus are the most widely used plantation species and many of them have escaped from plantations, becoming invasive globally (Richardson et al. 1994; Richardson and Higgins 1998). The high propagule pressure caused by large-scale industrial pine plantations, and the intrinsic ecological capacity of pines to colonize new areas are thought to be the main drivers of invasions in the pine’s introduced range (Richardson 1998). Once pines are able to establish in an area, they quickly increase their density and biomass causing important impacts on biodiversity and ecosystem processes. For example, in ca. 30-year-old stands of the non-native Pinus nigra in dune environments of the United States, a reduction in cover and richness of native plants was recorded, which was associated with a reduction in light levels (Leege and Murphy 2001). In tropical systems, Pinus elliottii is transforming the Brazilian savanna by causing the local loss of several native plant species (Abreu and Durigan 2011), and affecting wetlands where pine occurrence decreased macrophyte richness and altered species composition (Rolon et al. 2011). Although evidence of the invasiveness of pines has mounted in recent decades, the comparison of quantitative data about the impacts of different stages of invasion on structure and composition of plant communities among distinct natural habitats is rather scarce. Understanding how context-dependency may shape the effects of invasion may improve our ability to make predictions or generalizations about the ecological impacts caused by invasive species on natural habitats (Pyšek et al. 2012; Hulme et al. 2013; Kumschick et al. 2015).
Replacement of open native treeless vegetation with dense, closed, even-aged forests is by far the most striking impact of pine invasions (Richardson et al. 1994; Richardson and Higgins 1998). Pinaceae species may convert entire shrubland and grassland communities to conifer forests, with several native species in danger of at least local extirpation (Harding 2001), even in high elevation environments (Pauchard et al. 2009). For instance, in the Cape floristic region of South Africa, invasive pines (especially Pinus pinaster and Pinus radiata) have converted large areas of native fynbos shrublands to pine forests, with the local disappearance of many shade intolerant native plants (Richardson et al. 1994). Similarly, in New Zealand Pinus contorta grows 200 m above the treeline established by native species, thus invading high-elevation shrublands (Wardle 1985). Also in New Zealand, introduced conifers invade tussock grasslands and herbaceous communities, suppressing native vegetation (Richardson et al. 1994; Ledgard 2001; Williams and Wardle 2007; Froude 2011). Pine invasions and plantations have also been associated with impacts on different habitat components or characteristics such as invertebrate diversity (Pawson et al. 2010), soil biota and nutrient cycling (Dehlin et al. 2008; Dickie et al. 2011), and streamflow (Fahey and Jackson 1997; Farley et al. 2005).
To understand the potential impacts of pine invasion, the tendency has been to study the effects of pine plantations on the diversity of native species (Cowling et al. 1976; Richardson and van Wilgen 1986; Williams and Wardle 2007; Paritsis and Aizen 2008). However, the differences between pine invasions and pine plantations are obvious, as plantations usually have a low tree density to increase productivity and reduce costs, they are even-aged with a single dominant canopy layer, and trees have uniform heights, ages and trunk diameters (Richardson and Higgins 1998). Also, plantations are commonly established after the suppression of natural vegetation (Richardson 1993). In contrast, invaded sites tend to resemble natural pine forests, where there could be more than one cover layer, different tree heights, ages and trunk diameters, and where density is highly variable (Ledgard 2001). Additionally, pine invasion impacts on biodiversity and soil processes have been found to vary considerably with invasion density and the impacts of a final closed canopy stage (such as a plantation) will not fully represent the impacts that occur throughout the invasion process (Dickie et al. 2011; Taylor et al. 2016a). Therefore, predicting the impacts of pine invasions should not be based exclusively on evidence from plantations but should incorporate finer-scale effects of the invasions’ initial stages, including the effects of variable stand structure and density.
To advance the understanding of the various components of invasion impact (i.e., distribution, abundance and ecological effects, sensu Parker et al. 1999) it is desirable to assess a species that causes profound changes in the environment of the invaded ecosystem (Gundale et al. 2014). Because of its impacts and broad invasive range (described below), Pinus contorta has been proposed as a model species to study several processes in invasion biology (Gundale et al. 2014). Although potentially significant impacts on various aspects of the ecosystem can occur where this species invades (Ledgard 2001; Langdon et al. 2010; Dickie et al. 2014; Taylor et al. 2016a); it is relatively unknown if impacts on surrounding plant communities can develop early in invasion under isolated pines, or if they only occur in dense stands once the canopy has closed. Our aim is to determine the impacts of P. contorta invasion on vegetation diversity in two contrasting Patagonian ecosystems: an open Araucaria araucana—Nothofagus antarctica forest, and a treeless grassland steppe, both located in the Chilean Patagonia. We hypothesize that P. contorta affects the diversity, abundance and composition of the surrounding vegetation depending on pine size, where smaller trees should have a limited effect on the vegetation, and larger trees may completely alter the community. Additionally, we expected greater negative effects in treeless communities such as the steppe, where pines represent a completely novel life-form, than in ecosystems with natural tree cover (Rundel et al. 2014). By looking at the small-scale effects of isolated invasive pines (Williams and Wardle 2007) and how they vary with pine size, we may be able to detect how pine invasions can shape local impacts and elucidate potential trajectories of plant community change. Also, by assessing if the magnitude of pine impact depends on the habitat type being invaded, we can contribute to disentangling the importance of context-dependence for pine invasions.
Methods
Pinus contorta distribution, ecology, and impacts
Pinus contorta’s native range includes the Rocky Mountains and the Pacific West of the United States and Canada, under a wide variety of climates (Despain 2001). Within its broad distributional range, this species experiences minimum temperatures between 7 and −57 °C, maximum temperatures between 27 and 38 °C, and annual precipitation varying from 250 mm to 500 mm. Viable seed production starts at ages as young as 5–10 years old and this prolific species can have mass seed crops every one to three years. Cone production varies from a few hundred to a few thousand cones per tree (Despain 2001). Germination occurs best under full sunlight and on mineral soil (Despain 2001). Seedlings are resistant to frost damage and are very shade intolerant. Stand density can reach thousands or even a hundred thousand trees per hectare (Lotan and Critchfield 1990).
Pinus contorta invasion can have a variety of impacts on native ecosystems ranging from modification of vegetation structure and composition to alteration of belowground processes to changes in wildfire fuel accumulation. Vegetation structure is most often altered by the addition of a dense tree layer to open or treeless environments, such as grasslands or shrublands (Ledgard 2001; Langdon et al. 2010). Where this occurs, P. contorta invasions can cause dramatic declines in native species richness and diversity (Ledgard and Paul 2008). Due to differences in shade cover, litter abundance and chemistry, and mycorrhizal associations, P. contorta invasions can cause significant changes to soil nutrient cycling that persist even after the trees are removed (Dickie et al. 2014). Recent reports of P. contorta invasion in the Argentinean and Chilean Patagonia (Sarasola et al. 2006; Peña et al. 2008; Langdon et al. 2010; Gundale et al. 2014; Taylor et al. 2016b) highlight the preponderance of early invasion stages (i.e. wildings less than 8 years old) in different habitat types. In this region, the risk of invasion has increased in recent decades since P. contorta is still considered a forest crop species, especially in areas where no other trees can establish because of the harsh weather conditions. Pinus contorta invasion impacts have been observed in Patagonia where Cóbar-Carranza et al. (2014) reported changes in the fuel structure of native Araucaria araucana forests in Chile due to the addition of P. contorta, which could result in a higher risk of crown fires, and Taylor et al. (2016a) found a decline in plant species richness and cover associated with increasing P. contorta canopy cover.
Study area
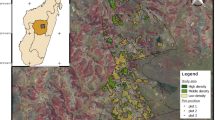
The forest site is located on the southern slope of Lonquimay volcano, in the Malalcahuello National Reserve (S38°30′–W71°35′; 1420 masl), La Araucanía Region, Chile (Fig. 1). Mean annual precipitation is 3083 mm (mainly snow), and mean annual temperature is 8.5 °C (Barros et al. 1979, Fig. 1). Topographic characteristics of the area have been mainly influenced by glaciation and volcanic activity (Peralta 1980). The site is dominated by a timberline forest type of Araucaria araucana ((Mol.) C. Koch.; Araucariaceae) and Festuca scabriuscula (Phil.; Poaceae) (Oberdorfer 1960; Gajardo 1994). There are also small and dense patches dominated by Nothofagus antarctica (G. Forst. Oerst.; Nothofagaceae) and Chusquea culeou (E. Desv.; Poaceae), that along with the forest, conferred heterogeneity to the landscape. In 1970, several native and exotic species were introduced in the Reserve as trial plots by the “Instituto Forestal Chileno” (INFOR), in order to evaluate the possibility of diversifying forestry production (Loewe and Murillo 2001). Although many exotic Pinaceae species (i.e. Pinus contorta, Pinus sylvestris, Pinus ponderosa and Pseudotsuga menziesii) were introduced in the area (Loewe and Murillo 2001), the only one that became invasive (i.e. that spread significantly from the trial plots and increased notably in population abundance) is P. contorta (Peña et al. 2008; Urrutia et al. 2013), which is invading 78 ha of the Reserve, and reaches a mean density of 6600 plants ha−1 in the more heavily invaded sectors (Peña et al. 2008).
Location of the study sites in Chile (left), pictures of invaded and uninvaded areas from the Araucaria araucana forest in the Malalcahuello National Reserve (a, b), and from the Patagonian steppe in the Province of Coyhaique (c, d) (upper right), and climograph for each site (lower right). Meteorological data were obtained from the Worldclim dataset (Hijmans et al. 2005)
The Patagonian steppe site is located in the Province of Coyhaique (S45°33′–W72°04′; 730 masl), Aysen Region, Chile (Fig. 1). The climate is cold and dry, characterized by several months during the growing season with a precipitation of less than 80 mm (Fig. 1). Most of the precipitation falls in autumn and winter (Langdon et al. 2010). The mean annual temperature ranges from 6–9 °C. The vegetation is dominated by native species, mainly by the perennial grass Festuca pallescens ((St. Yves) Parodi; Poaceae) and the cushion plants Baccharis magellanica ((Lam.) Pers.; Asteraceae), Acaena integerrima (Gillies ex Hook. & Arn.; Rosaceae), and Mulinum spinosum [(Cav.) Pers.; Apiaceae]. In 1970 pines were introduced by the Chilean government with the aim of evaluating the potential of these species for soil erosion control (Langdon et al. 2010). However, the plantation directly adjacent to this site was established in 1984 and therefore was 25 years old at the time of sampling. Currently, the invasion of P. contorta is in an early stage, with the greatest natural regeneration within the first 60 m from the original seed source, registering a maximum mean density of 13,222 pines ha−1 (Langdon et al. 2010). Grazing pressure in the steppe by domestic sheep and invasive European hare is high, shaping vegetation and producing important disturbance to the soil cover (Langdon et al. 2010). As it can be deduced from the age of pines located in the core of the invaded area in each habitat type (steppe: 8 years old, forest: 18 years old), the invasion started much later in the steppe than in the forest (Aníbal Pauchard unpublished data).
Sampling design
We sampled plant diversity under and outside the canopy of Pinus contorta individuals in invaded areas of Malalcahuello (January, 2010) and Coyhaique Alto (January, 2011). In each site, we selected individuals of P. contorta in three height categories (n = 15 per category): 1–3 m; 4–6 m, and 7–9 m. Pines were selected in the invasion front, avoiding the presence of other pines in the surroundings. We recorded height of the selected pines, and estimated their canopy area by measuring the canopy diameter. The tallest pines (7–9 m) were absent from the steppe site as this site is in an early invasion stage compared to the forest site (Langdon et al. 2010; Peña et al. 2008). For each selected pine (n = 45 for the forest, n = 30 for the steppe), we setup 1 m2 paired plots located two meters apart: one under the pine canopy and the other outside the canopy. In each plot, we recorded presence and percentage cover of all vascular plants species. Each plot was subdivided into four 50 × 50 cm quadrats, to increase measurement accuracy. Data was compiled at the 1 m2 resolution.
Data analysis
Dependent variables were total species richness and plant cover (excluding P. contorta presence and cover; according to Thomsen et al. 2016). We also calculated the mean percentage reduction of both variables in ‘Under canopy’ plots as compared to ‘Outside canopy’ plots. Species richness under and outside the pine canopy for each height category was estimated through sample-based rarefaction curves. This sampling model for species richness accounts for undersampling bias by adjusting or controlling for differences in abundance and / or sampling effort (Gotelli and Colwell 2010). For each rarefaction, 100 resamples were randomly drawn without replacement (EstimateS 9.1, Colwell 2013). To assess statistical differences between rarefaction curves, 95% confidence intervals were estimated and significant differences were assumed when confidence intervals did not overlap. Although species accumulation curves did not approach to an asymptote, we considered them valid to use to compare relative differences in species accumulation between the two plot types.
To assess the impact of pine invasion on richness (S) and cover for each pine height category, we calculated the mean percentage reduction of richness in ‘Under canopy’ (SU) compared to ‘Outside canopy’ (SO) plots (Hejda et al. 2009): [(SU − SO)/SO] × 100. In this case, a negative value indicates a lower richness in under canopy plots (i.e. negative pine effect), while a positive value indicates the opposite (i.e. positive pine effect). We performed multiple linear regression to model the influence of pine size (quantitative), habitat type (categorical with two levels), and their interaction on the impact of P. contorta on total richness and total cover (lm package, R software; R Development Core Team 2006). In these analyses, canopy area (a variable highly correlated to height: Spearman’s r = 0.93, p < 0.0001) was use as a proxy of pine size. Model assumptions were checked by the inspection of residual graphs and the use of the Kolmogorov–Smirnov normality test.
We assessed differences in species composition between plots under and outside of the canopy using the Bray-Curtis distance between plots within each height class and a Permutational Analysis of Variance (PERMANOVA; Anderson 2001; Oksanen et al. 2013). We also tested for differences in dispersion between groups (betadisper; Oksanen et al. 2013). Similarity percentages analysis (SIMPER) was used to assess the contribution of individual species to dissimilarities between under-canopy and outside-canopy plots in each site. SIMPER analyses were performed using the software Primer v6 (Clarke and Gorley 2006). Significance level was 0.05. Standard errors (SE) were used to calculate variation.
Results
We registered a total of 26 species (11% exotic species) for the steppe and 40 species for the forest (15% exotic species) (“Appendix”). At both sites, species richness beneath pines was lower under larger pines compared to smaller pines (Rarefaction curves; Fig. 2). On the contrary, no difference was detected in plots outside the pine canopy. In the steppe, a significant reduction in species richness occurred at the 4–6 m height category, while in the A. araucaria forest it only occurred at 7–9 m.
Rarefaction curves for ‘Under canopy’ and ‘Outside canopy’ plots of pines 1–3 m, 4–6 m, and 7–9 m high (black, white and grey squares respectively) in the Patagonian steppe and the Araucaria araucana forest sites. Bars indicate 95% confidence intervals, and significant differences were assumed when confidence intervals did not overlap
The impact of pines on plant richness and cover depended on pine size (i.e. canopy area) and habitat type (Table 1; Fig. 3). Larger pines had more negative impacts than smaller pines in both sites, while the magnitude of these impacts for a given pine size was greater in the Patagonian steppe compared to the A. araucaria forest (Fig. 3a, b).
Relationships between pine canopy area and (a) impact on richness, and (b) impact on cover for the Patagonian steppe (white circles) and the Araucaria araucana forest (grey circles) sites. Impact is expressed as the mean percentage reduction or increase in richness or cover in ‘Under canopy’ plots compared to ‘Outside canopy’ plots (100%). Negative values indicate a lower species number (or cover) in under canopy plots, and positive values indicate the opposite. The coefficient of determination (R2) and the regression formula are reported
Species composition, at both sites, changed between under and outside canopy plots for pines of more than 4 m in height (PERMANOVA, steppe: 4–6 m: R2= 0.119, p < 0.01; forest: 4–6: R2= 0.064, p < 0.05, and 7–9 m: R2= 0.095, p < 0.01; Fig. 4). For small pines (1–3 m), we found no differences in species composition between plots under and outside the canopy (PERMANOVA, steppe: p = 0.39; forest: p = 0.70). Plot position explained between 6.4% to 11.9% of the variation in species composition based on Bray-Curtis dissimilarity. Dispersion did not differ between the groups (betadisper p > 0.1). Dominant species of the community were the biggest contributors to dissimilarity between plots in each site (SIMPER; Fig. 4; Appendix).
Mean cover values (±SE) of species in the Patagonian steppe (upper) and the A. araucana forest (lower) that made a cumulative contribution of 50% to dissimilarity between ‘under’ and ‘outside canopy’ of pines (a) 1–3 m, (b) 4–6 m, and (c) 7–9 m high. Species are shown in decreasing order (left to right) in terms of their individual contribution to the dissimilarity. Species code name: Aca int: Acaena integerrima; Bac con: Baccharis concava; Bac mag: Baccharis magellanica; Cal lag: Calceolaria lagunae-blancae; Chu cul: Chusquea culeou; Dis cha: Discaria chacaye; Emp rub: Empetrum rubrum; Fes pal: Festuca pallescens; Fes sca: Festuca scabriuscula; Gau poe: Gaultheria poeppigii; Lat sp.: Lathyrus sp.; Mul spi: Mulinum spinosum; Ore gla: Oreopulus glacialis; Qui chi: Quinchamalium chilense; Rum ace: Rumex acetosella
Discussion
Our results indicate that Pinus contorta invasion is having a negative impact on vascular plant diversity in natural habitats of southern Chile. Pines in our study sites are rapidly reducing plant species richness and cover as they grow and increase their canopies. The effects on biodiversity beneath pine canopies are evident early in the invasion when trees have not reached their maximum density and the canopy has not yet closed. Significant pine effects were registered for trees with canopy areas starting at ca. 5 m2, while the maximum individual pine canopy area was 13 m2 for the steppe and 40 m2 for the forest. Based on the fact that similar sized-pines had different impacts on plant diversity depending on the invaded plant community type, context-dependency (i.e. composition and structure of the recipient community) seems to have a greater importance than pine size in determining the magnitude of the invasion impact. Thus, the magnitude of the negative impact of pine invasions is likely greater in communities not adapted to tree cover, such as the Patagonian steppe.
The addition of new functional traits (Levine et al. 2003), such as the incorporation of a new life form or a plant stratum that increases total vegetation height, may explain the greater impact of pines in the steppe. Our results highlight the role of the invaded community in modulating the impact of a particular plant invasion. It has been hypothesized that invasive species with novel traits will have a greater impact on the invaded community than species without novel traits (Rundel et al. 2014), because the functional distinctiveness of the introduced species may convey an advantage to the invader over ecologically naïve residents (Kumschick et al. 2015). However, evidence of such phenomenon remains scarce. Our study, by contrasting two very different invaded communities, was able to show strong differences in how species diversity and abundance is reduced by pine invasions, as seen with other woody invaders (Mason and French 2008). In the treeless Patagonian steppe most of the species are adapted to open and sometimes extreme conditions (e.g. extremely windy conditions, very low winter temperature, and low precipitations; Conti 1998). Some species may be more sensitive and could be filtered out earlier by the pine invasion (Bravo-Monasterio et al. 2016). Our results show that in the steppe this ecological filtering occurs at an earlier, less advanced stage of invasion, while in the Araucaria forest species can better withstand low pine densities. Similarly, in New Zealand some native forest understory species have been found to establish in Pinus radiata plantations (Brockerhoff et al. 2003), while in open grassland habitat all 26 native species originally present went locally extinct after 32 years of Pinus contorta invasion (Ledgard and Paul 2008). In treeless environments, pine invasions may be having an effect both because they are a novel life-form for those ecosystems, similarly to what a native tree would do by colonizing new habitat, but also because they can bring a completely new set of traits and establish novel biological interactions (e.g. litter composition, allelopathy, soil-biota interactions) (Saure et al. 2014). This would explain why the impacts of increasing P. contorta tree cover differ between the native and the invasive range, even when P. contorta invades treeless environments in the native range (Taylor et al. 2016a).
The dominant species in the steppe and the forest were the greatest contributors to the differences in composition between plots under and outside the canopy of Pinus contorta individuals. Dominant native species such as tussock grasses (Festuca spp.) and cushion plants (e.g. Baccharis magellanica) can play a key role in the community by providing shelter to diverse plant species under unfavorable environmental conditions (Gibson 2009), conferring high community resilience after disturbances (Gonzalez et al. 2015), and affecting species germination through the differential effect of litter (Ruprecht et al. 2010), among others. Therefore, the reduction of certain native species due to pine presence could result in cascading effects on habitat structure and functioning. This has been observed for pines in New Zealand where the reduction in high quality grass litter and change in dominant species from grasses to pines altered belowground biotic communities, nutrient cycling, soil pH, and soil carbon levels (Dickie et al. 2011, 2014).
Other studies finding similar declines in plant richness or diversity with pine invasion support our results (Ledgard and Paul 2008; Dickie et al. 2011; Steers et al. 2013; Urrutia et al. 2013; Taylor et al. 2016a). However, the dominant mechanism by which pines reduce diversity in the invaded community remains unclear. In their introduced ranges, pines can change adjacent plant community composition by multiple mechanisms including direct competition for light and water, changes in litter accumulation, allelopathic components, changes in soil chemistry and changes in the mycorrhizal community and other components of the soil biota (Raffaele and Schlichter 2000; Dehlin et al. 2008; Sartor et al. 2009; Salomón et al. 2011; Steers et al. 2013; Hess and Austin 2014; de Oliveira et al. 2014). The decrease in species richness and abundance under the canopy of adult pines may be promoted by the characteristic morphology of P. contorta, with dense lower branches close to the ground level (Van Gelderen and Van Hoey 1996). Moreover, the decline of plant diversity could also result from the arrival of “novel weapons” (sensu Callaway and Ridenour 2004). An allelopathic potential has been documented for other pines, such as Pinus halepensis (Fernandez et al. 2006), P. taeda L. (Sartor et al. 2009), and P. sylvestris (Kaligaričč et al. 2011). In addition, pines may reduce native plant cover by the accumulation of a thick litter layer (Steers et al. 2013) that probably decomposes slowly in cold environments such as the ones studied here. In fact, needle litter accumulation in pine stands could create a physical and/or chemical barrier for plant establishment (Bueno and Baruch 2011; Valera-Burgos et al. 2012) and has been found to alter species interactions in potentially unpredictable ways (Metlen et al. 2013; Metlen and Callaway 2015). New studies, observational and experimental, should address the specific mechanisms by which pines are able to outcompete resident species and modify the invaded community in the studied habitats.
The large-scale influx of pines in Chile began in 1970, as the forestry industry expanded. Now, the impacts of pine invasions are becoming evident in forests and treeless ecosystems of southern Chile. Specifically for P. contorta, in order to conserve biodiversity and ecosystem functions, and reduce unnecessary economic costs, actions should be taken 1) to halt new P. contorta plantations and gradually remove existing plantations and 2) to actively control ongoing P. contorta invasions. As pointed out by Steers et al. (2013), the removal of pines in early growth stages can minimize their impact on plant diversity. The removal of pines while they are still young can also reduce the potential legacy effects on soil biota and nutrient cycling (Dickie et al. 2014). According to our results, there would be a considerable time window (until pines reach 3 m in height) before invasive pines begin to have a significant impact on resident plant composition. Management options should be particularly considered for temperate grasslands, since these ecosystems are poorly represented in national and worldwide protected area systems (Hoekstra et al. 2005; Paruelo et al. 2005), and show high vulnerability to invasive pine impacts.
Our results highlight the importance of quantifying tree invasion impacts in multiple ecosystems to understand the interaction between the invader and the invaded ecosystem in defining the magnitude and direction of the impacts. Standardized protocols that study tree invasion impacts and legacies after removal across multiple regions, such as the Global Invader Impact Network (Barney et al. 2015), will help to increase our understanding of the differential effects of one invasive species across multiple ecosystems. By contrasting a treeless and an open forest ecosystem, we can suggest that the magnitude of pine invasion impacts is related to how adapted the invaded community is to tree cover. However, more studies are needed to detect whether these responses persist across a larger biogeographical context (i.e. cross regional and continental comparisons) or across climatic gradients (e.g. latitudinal, elevation), and whether this response is due to the biogeographic origin of the species or just to the novelty of the life form in the invaded community.
References
Abreu RCR, Durigan G (2011) Changes in the plant community of a Brazilian grassland savannah after 22 years of invasion by Pinus elliottii Engelm. Plant Ecol Divers 4:269–278
Anderson MJ (2001) Permutation tests for univariate or multivariate analysis of variance and regression. Can J Fish Aquat Sci 58:626–639
Barney JN, Tekiela DR, Barrios-Garcia MN, Dimarco RD, Hufbauer RA, Leipzig-Scott P, Nuñez MA, Pauchard A, Pyšek P, Vítková M, Maxwell BD (2015) Global Invader Impact Network (GIIN): towards standardized methods to evaluate the ecological impacts of invasive plants. Ecol Evol 5:2878–2889
Barros S, Barros D, Cogollor G, Navia P, Rojas P, Toro J, Vita A (1979) Informe I: Situación actual de los programas de introducción de especies forestales en Chile. INFOR, Santiago
Bravo-Monasterio P, Pauchard A, Fajardo A (2016) Pinus contorta invasion into treeless steppe reduces species richness and alters life-history traits of the local community. Biol Invasions. doi:10.1007/s10530-016-1131-4
Brockerhoff EG, Ecroyd CE, Leckie AC, Kimberley MO (2003) Diversity and succession of adventive and indigenous vascular understorey plants in Pinus radiata plantation forests in New Zealand. For Ecol Manage 185:307–326
Bueno A, Baruch Z (2011) Soil seed bank and the effect of needle litter layer on seedling emergence in a tropical pine plantation. Rev Biol Trop 59:1071–1079
Callaway RM, Ridenour WM (2004) Novel weapons: Invasive success and the evolution of increased competitive ability. Front Ecol Environ 2:436–443
Clarke KR, Gorley RN (2006) Plymouth routines in multivariate ecological research. Primer-E, Plymouth Marine Laboratory, London, England
Cóbar-Carranza AJ, García RA, Pauchard A, Peña E (2014) Effect of Pinus contorta invasion on forest fuel properties and its potential implications on the fire regime of Araucaria araucana and Nothofagus antarctica forests. Biol Invasions 16:2273–2291
Colwell RK (2013) EstimateS: Statistical estimation of species richness and shared species from samples. Version 9. Persistent URL <purl.oclc.org/estimates>
Conti HA (1998) Características climáticas de la Patagonia. In: Correa MN (ed) Flora Patagónica, VIII, Parte I. Instituto Nacional de Tecnología Agropecuaria (INTA), Buenos Aires, pp 31–47
Cowling RM, Moll EJ, Campbell BM (1976) The ecological status of the understorey communities of pine forests on Table Mountain. South African For J 99:13–23
de Oliveira SM, Boll PK, Baptista VD, Leal-Zanchet AM (2014) Effects of pine invasion on land planarian communities in an area covered by Araucaria moist forest. Zool Stud 53:19
Dehlin H, Peltzer DA, Allison VJ, Yeates GW, Nilsson MC, Wardle DA (2008) Tree seedling performance and below-ground properties in stands of invasive and native tree species. NZ J Ecol 32:67–79
Despain DG (2001) Dispersal ecology of lodgepole pine (Pinus contorta Dougl.) in its native environment as related to Swedish forestry. For Ecol Manage 141:59–68
Dickie IA, Yeates GW, St John MG, Stevenson BA, Scott JT, Rillig MC, Peltzer DA, Orwin KH, Kirschbaum MUF, Hunt JE, Burrows LE, Barbour MM, Aislabie J (2011) Ecosystem service and biodiversity trade-offs in two woody successions. J Appl Ecol 48:926–934
Dickie IA, St John MG, Yeates GW, Morse CW, Bonner KI, Orwin K, Peltzer DA (2014) Belowground legacies of Pinus contorta invasion and removal result in multiple mechanisms of invasional meltdown. AoB Plants. doi:10.1093/aobpla/plu056
Fahey B, Jackson R (1997) Hydrological impacts of converting native forests and grasslands to pine plantations, South island, New Zealand. Agric For Meteorol 84:69–82
Farley KA, Jobbagy EG, Jackson RB (2005) Effects of afforestation on water yield: a global synthesis with implications for policy. Glob Chang Biol 11:1565–1576
Fernandez C, Lelong B, Vila B, Mévy JP, Robles C, Greff S, Dupouyet S, Bousquet-Mélou A (2006) Potential allelopathic effect of Pinus halepensis in the secondary succession: an experimental approach. Chemoecology 16:97–105
Froude VA (2011) Wilding conifers in New Zealand: Status report. Report prepared for the Ministry of Agriculture and Forestry. Pacific Eco-Logic, Bay of Islands, New Zealand
Gajardo R (1994) La vegetación natural de Chile. Editorial Universitaria, Santiago, Clasificación y distribución geográfica
Gibson DJ (2009) Grasses and grassland ecology. Oxford University Press, New York
Gonzalez SL, Ghermandi L, Peláez DV (2015) Fire temperature effects on perennial grasses from northwestern Patagonian grasslands. Ecol Res 30:67–74
Gotelli NJ, Colwell RK (2010) Estimating species richness. In: Magurran AE, McGill BJ (eds) Biological diversity: frontiers in measurement and assessment. Oxford University Press, Oxford, pp 39–54
Gundale MJ, Pauchard A, Langdon B, Peltzer DA, Maxwell BD, Núñez MA (2014) Can model species be used to advance the field of invasion ecology? Biol Invasions 16:591–607
Harding M (2001) South Island Wilding Conifer Strategy. Department of Conservation, Christchurch. http://www.doc.govt.nz/publications/conservation/threats-and-impacts/weeds/south-island-wilding-conifer-strategy/. Accessed 25 Feb 2016
Hejda M, Pyšek P, Jarošík V (2009) Impact of invasive plants on the species richness, diversity and composition of invaded communities. J Ecol 97:393–403
Hess JT, Austin AT (2014) Pinus ponderosa alters nitrogen dynamics and diminishes the climate footprint in natural ecosystems of Patagonia. J Ecol 102:610–621
Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A (2005) Very high resolution interpolated climate surfaces for global land areas. Int J Climatol 25:1965–1978
Hoekstra JM, Boucher TM, Ricketts TH, Roberts C (2005) Confronting a biome crisis: global disparities of habitat loss and protection. Ecol Lett 8:23–29
Hulme PE, Pyšek P, Jarošík V, Pergl J, Schaffner U, Vilà M (2013) Bias and error in current knowledge of plant invasions impacts. Trends Ecol Evol 28:212–218
Kaligaričč M, Meister MH, ŠŠkornik S, ŠŠajna N, Kramberger B, Bolhààr-Nordenkampf HR (2011) Grassland succession is mediated by umbelliferous colonizers showing allelopathic potential. Plant Biosyst 145:688–698
Kumschick S, Gaertner M, Vilà M, Essl F, Jeschke JM, Pyšek P, Ricciardi A, Bacher S, Blackburn TM, Dick JTA, Evans T, Hulme PE, Kühn I, Mrugała A, Pergl J, Rabitsch W, Richardson DM, Sendek A, Winter M (2015) Ecological impacts of alien species: quantification, scope, caveats and recommendations. Bioscience 65:55–63
Langdon B, Pauchard A, Aguayo M (2010) Pinus contorta invasion in the Chilean Patagonia: local patterns in a global context. Biol Invasions 12:3961–3971
Ledgard NJ (2001) The spread of lodgepole pine (Pinus contorta Dougl.) in New Zealand. For Ecol Manage 141:43–57
Ledgard NJ, PaulTSH (2008) Vegetation successions over 30 years of high country grassland invasion by Pinus contortaN Z Plant Prot 6198104
Leege LM, Murphy PG (2001) Ecological effects of the non-native Pinus nigra on sand dune communities. Can J Bot 79:429–437
Levine JM, Vilà M, D’Antonio CM, Dukes JS, Grigulis K, Lavorel S (2003) Mechanisms underlying the impacts of exotic plant invasions. Proc R Soc Lond 270:775–781
Loewe V, Murillo P (2001) Estudio de ensayos de introducción de especies. INFOR, Santiago
Lotan JE, Critchfield WB (1990) Pinus contorta Dougl. ex. Loud. Lodgepole Pine. In: Burns RM, Honkala BH (eds). Silvics of North America.Volume 1.Conifers.Handbook 654. USDA Forest Service and Agriculture, Washington DC, pp 302–315
Mason TJ, French K (2008) Impacts of a woody invader vary in different vegetation communities. Divers Distrib 14:829–838
Metlen KL, Callaway RM (2015) Native North American pine attenuates the competitive effects of a European invader on native grasses. Biol Invasions 17:1227–1237
Metlen KL, Aschehoug ET, Callaway RM (2013) Competitive outcomes between two exotic invaders are modified by direct and indirect effects of a native conifer. Oikos 122:632–640
Oberdorfer E (1960) Pflanzensoziologische Studien in Chile Ein Vergleich mit Europa. Flora et Vegetatio Mundi 2:1–208
Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Stevens MHH, Wagner H (2013) vegan: Community Ecology Package. R package version 2.0-10.http://CRAN.R-project.org/package=vegan
Paritsis J, Aizen MA (2008) Effects of exotic conifer plantations on the biodiversity of understory plants, epigeal beetles and birds in Nothofagus dombeyi forests. For Ecol Manage 255:1575–1583
Parker IM, Simberloff D, Lonsdale WM, Goodell K, Wonham M, Kareiva PM, Williamson MH, von Holle B, Moyle PB, Byers JE, Goldwasser L (1999) Impact: toward a framework for understanding the ecological effects of invader. Biol Invasions 1:3–19
Paruelo JM, Golluscio RA, Jobbagy EG, Canevari M, Aguiar MR (2005) Situación ambiental en la estepa patagónica. In: BrownA, Martinez Ortiz U, Acerbi M, Corcuera J (eds) La situación ambiental argentina. Fundación Vida Silvestre Argentina, Buenos Aires, pp 303–313
Pauchard A, Kueffer C, Dietz H, Daehler CC, Alexander J, Edwards PJ, Arévalo JR, Cavieres LA, Guisan A, Haider S, Jakobs G, McDougall K, Millar CI, Naylor BJ, Parks CG, Rew LJ, Seipel T (2009) Ain’t no mountain high enough: plant invasions reaching high elevations. Front Ecol Environ 7:479–486
Pawson SM, McCarthy JK, Ledgard NJ, Didham RK (2010) Density-dependent impacts of exotic conifer invasion on grassland invertebrate assemblages. J Appl Ecol 47:1053–1062
Peña E, Hidalgo M, Langdon B, Pauchard A (2008) Patterns of spread of Pinus contorta Dougl.ex Loud. invasion in a natural reserve in southern South America. For Ecol Manage 256:1049–1054
Peralta M (1980) Geomorfología, clima y suelos del Tipo Forestal Araucaria en Lonquimay. Universidad de Chile, Santiago
Pyšek P, Jarošík V, Hulme PE, Pergl J, Hejda M, Schaffner U, Vilà M (2012) A global assessment of invasive plant impacts on resident species, communities and ecosystems: the interaction of impact measures, invading species’ traits and environment. Glob Chang Biol 18:1725–1737
R Development Core Team (2006) R: a language and environment for statistical computing, R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org.http://www.R-project.org
Raffaele E, Schlichter T (2000) Efectos de las plantaciones de pino ponderosa sobre la heterogeneidad de micrositios en estepas del noroeste patagónico. Ecología Austral 10:151–158
Richardson B (1993) Vegetation management practices in plantation forest of Australia and New Zealand. Can J For Res 23:1989–2005
Richardson DM (1998) Forestry trees as invasive aliens. Conserv Biol 12:18–26
Richardson DM, Higgins S (1998) Pines as invaders in the southern hemisphere. In: Richardson DM (ed) Ecology and biogeography of Pinus, 1st edn. Cambridge University Press, Cambridge, pp 450–473
Richardson DM, van Wilgen BW (1986) Effects of thirty-five years of afforestation with Pinus radiata on the composition of mesic mountain fynbos near Stellenbosch. SAfr J Bot 52:309–315
Richardson DM, Williams PA, Hobbs RJ (1994) Pine invasions in the Southern Hemisphere: determinants of spread and invadability. J Biogeogr 21:511–527
Rolon AS, Rocha O, Maltchik L (2011) Does pine occurrence influence the macrophyte assemblage in Southern Brazil ponds? Hydrobiologia 675:157–165
Rundel PW, Dickie IA, Richardson DM (2014) Tree invasions into treeless areas: mechanisms and ecosystem processes. Biol Invasions 16:663–675
Ruprecht E, Józsa J, O¨lvedi TB, Simon J (2010) Differential effects of several ‘‘litter’’ types on the germination of dry grassland species. J Veg Sci 21:1069–1081
Salomón MES, Barroetaveña C, Rajchenberg M (2011) Do pine plantations provide mycorrhizal inocula for seedlings establishment in grasslands from Patagonia, Argentina? New Forest 21:191–205
Sarasola MM, Rusch VE, Schlichter TM, Ghersa CM (2006) Invasión de coníferas forestales en áreas de estepa y bosques de ciprés de la cordillera en la Región Andino Patagónica. Ecologia Austral 16:143–156
Sartor LR, Adami PF, Chini N, Martin TN, Marchese JA, Soares AB (2009) Alelopatia de acículas de Pinus taeda na germinação e no desenvolvimento de plântulas de Avena strigosa. Ciência Rural 39:1653–1659
Saure HI, Vandvik V, Hassel K, Vetaas OR (2014) Do vascular plants and bryophytes respond differently to coniferous invasion of coastal heathlands? Biol Invasions 16:775–791
Steers RJ, Fritzke SL, Rogers JJ, Cartanand J, Hacker K (2013) Invasive pine tree effects on northern coastal scrub structure and composition. Invasive Plant Sci Manag 6:231–242
Taylor KT, Maxwell BD, Pauchard A, Nuñez MA, Rew LJ (2016a) Native versus non-native invasions: similarities and differences in the biodiversity impacts of Pinus contorta in introduced and native ranges. Divers Distrib. doi:10.1111/ddi.12419
Taylor KT, Maxwell BD, Pauchard A, Nuñez MA, Peltzer DA, Terwei A, Rew LJ (2016b) Drivers of plant invasion vary globally: evidence from pine invasions within six ecoregions. Glob Ecol Biogeogr 25:96–106
Thomsen MS, Wernberg T, South PM, Schiel DR (2016) To include or not to include (the invader in community analysis)? That is the question. Biol Invasions 18:1515–1521
Urrutia J, Pauchard A, García RA (2013) Diferencias en la composición vegetal de un bosque de Araucaria araucana (Molina) K. Koch y Nothofagus antarctica (G. Forst.) Oerst. asociadas a un gradiente de invasión de Pinus contorta Douglas ex Loudon. Gayana Botanica 70:92–100
Valera-Burgos J, Díaz-Barradas MC, Zunzunegui M (2012) Effects of Pinus pinea litter on seed germination and seedling performance of three Mediterranean shrub species. Plant Growth Regul 66:285–292
Van Gelderen D, Van Hoey J (1996) Conifers: The illustrated encyclopedia. Timber Press, Oregon
Wardle P (1985) New Zealand timberlines: growth and survival of native and introduced trees in the Craigieburn Range, Canterbury. N Z J Bot 23:219–234
Williams MC, Wardle GM (2007) Pinus radiata invasion in Australia: identifying key knowledge gaps and research directions. Austral Ecol 32:721–739
Acknowledgements
We are grateful to Paul Alaback for useful comments on earlier versions of the manuscript. We acknowledge constructive comments of three anonymous referees and the Associate Editor, David Richardson. The study was funded by Fondecyt grant 1140485, and the Institute of Ecology and Biodiversity with the grants by the Ministry of the Economy ICM P05-002 and CONICYT PFB-23.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
See Table 2.
Rights and permissions
About this article
Cite this article
Franzese, J., Urrutia, J., García, R.A. et al. Pine invasion impacts on plant diversity in Patagonia: invader size and invaded habitat matter. Biol Invasions 19, 1015–1027 (2017). https://doi.org/10.1007/s10530-016-1344-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-016-1344-6