Abstract
The introduced mussel Mytilus galloprovincialis is progressively increasing in abundance along the south coast of South Africa. Quantitative 0.1 m2 samples were collected in the mid-zone of an indigenous Perna perna mussel bed in the 1980s prior to the arrival M. galloprovincialis (12) and in the 2000s during the M. galloprovincialis invasion (16). In addition, in situ counts of M. galloprovincialis were done on eight occasions between 1993 and 2005, and in the low- and high-zones on four occasions. In the mid-zone M. galloprovincialis was absent until 1987, its mean densities were low (< 15 individuals/0.1 m2) between 1993 and 1996, but thereafter increased steadily, peaking in 2004 (at 721 individuals/0.1 m2), before declining in 2005 (331 individuals/0.1 m2). The greatest densities of M. galloprovincialis were recorded at the high-zone (1121 individuals/0.1 m2) and the smallest in the low-zone. As M. galloprovincialis numbers increased, there was an associated, but smaller decline in P. perna numbers and the overall density of mussels increased significantly (P < 0.05). No major change was recorded in the size composition of P. perna. The density of associated fauna differed significantly (P < 0.01) between sampling dates with the lowest and highest values being recorded near the ‘beginning’ (2001) and ‘end’ (2005) of the invasion period respectively. These differences were largely due to variations in the density of barnacles, and the toothed barnacle Chthamalus dentatus appeared to be the only associated faunal species that was directly affected by the M. galloprovincialis invasion, experiencing a significantly (P ≤ 0.05), but temporary decline in density and biomass values.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
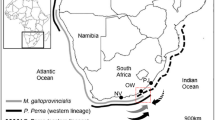
Historically three indigenous mussel species formed extensive beds on rocky intertidal and shallow subtidal reefs of the South African coastline (Brown and Jarman 1978). The black mussel Choromytilus meridionalis and the ribbed mussel Aulacomya ater attained their greatest densities in the cooler waters of the west coast, whereas the brown mussel Perna perna was abundant on the warmer south- and east coasts (Van Erkom Schurink and Griffiths 1990). The Mediterranean mussel Mytilus galloprovincialis was accidentally introduced on the west coast of South Africa between the late 1970s and early 1980s (Grant and Cherry 1985; Griffiths et al. 1992), and later (in 1988) a small population was intentionally established at Port Elizabeth on the south–east coast for mariculture purposes (McQuaid and Phillips 2000). Mytilus galloprovincialis originated in the Mediterranean Sea, and has been introduced to the coastlines of California, Japan, China, Korea, Hawaii, Australia and South Africa (Seed 1992). It exhibits several characteristics of an invasive species, namely rapid growth rate, high fecundity, substantial tolerance to desiccation (Hockey and Van Erkom Schurink 1992), and resistance to parasites (Calvo-Ugarteburu and McQuaid, 1998). On the rocky shores of the west coast of South Africa M. galloprovincialis has proved to be a superior competitor for primary space relative to the ribbed mussel Aulacomya ater, adults of the granular limpet Scutellastra granularis and, at sites with intermediate to high wave exposure, Argenville’s limpet S. argenvillei (Hockey and Van Erkom Schurink 1992; Steffani and Branch 2003a, b; Branch and Steffani 2004). The biomass, growth rates and recruitment of M. galloprovincialis on this coast all peaked at localities with intermediate wave exposure, declining towards sheltered and extremely exposed sites (Steffani and Branch 2003c; Hammond and Griffith 2004). By 1990 M. galloprovincialis comprised over 70% of intertidal mussel biomass on the west coast, compared to only 1% on the warmer (ca. 3–5°C, van der Elst 1981; F.A. Duncan, personal communication) south and south–east coasts (Cape Agulhas and East London, Fig. 1), where P. perna was the dominant mussel (Van Erkom Schurink and Griffiths 1990). Its abundance on the south and south–east coasts has continued to increase, but it is variable and site specific (McQuaid and Phillips 2000; Rius 2004; Robinson et al. 2005). At sites where M. galloprovincialis is plentiful, it dominates the high-shore and P. perna the low-shore, with a mixed zone at the mid-tide level (Bownes and McQuaid 2006). Mytilus galloprovincialis has a faster growth rate and a higher tolerance to desiccation and siltation than P. perna, and it has therefore the potential to increase the standing stock and intertidal extent of the mussel beds on the south coast (Hockey and Van Erkom Schurink 1992).
This paper documents the increase in abundance of M. galloprovincialis in a densely populated P. perna bed on the south coast, and changes in the density, biomass, diversity and species richness of the associated faunal community over the study period. It complements more comprehensive studies done on the changes that occurred on the west coast following the invasion by M. galloprovincialis (Hockey and Van Erkom Schurink 1992; Griffiths et al. 1992; Steffani and Branch 2003a, b, c; Robinson et al. 2005).
Materials and methods
The study area, Tsitsikamma National Park, is situated on the south coast (Fig. 1), where the temperature regime appears to be near the edge of the optimal range of P. perna and M. galloprovincialis, which generally have subtropical and temperate distributions respectively (Bownes 2005). The shoreline of the park is narrow and rocky, running along the base of a precipitous coastal escarpment. The sampling site has a mono-layered mussel bed and is located approximately midway along the length of a marine reserve where bait collecting and fishing are prohibited.
The profile of the mussel bed at Site 1 was divided into three zones, namely the low-zone (0.86–1.25 m above low water of spring tide), mid-zone (1.26–1.55 m) and high-zone (1.56–1.86 m), while sea surface temperature was measured daily at 08h00 to the nearest 0.1°C by park personnel using either a thermometer or a thermistor at Site 2. In the 1980s, prior to the arrival of M. galloprovincialis, a total of twelve 0.32 × 0.32 m quadrats were quantitatively sampled in the mid-zone of Site 1 (Table 1). All organisms within the quadrat were removed using a hammer and paint-scraper, stored in plastic bags and frozen until analyzed. The samples were sorted, but small organisms, such as amphipods and tiny polychaetes, were ineffectively captured, because of the large volume of shells and debris in the samples and the coarse 1 mm retaining mesh used. The organisms collected were counted and identified to species level when possible, under a dissecting microscope. Shell lengths of P. perna were measured using a vernier caliper, and a single length mass relationship was determined for this species. For every millimetre size class (e.g. 20.0–20.9 mm) measured between 10 and 15 individuals were pooled together. The flesh component of the mussels in each class was manually removed, oven dried (100°C for 24 h), cooled in desiccators, weighed to the nearest 0.01 g, and then ashed (500°C for 6 h) to determine ash free dry mass (Crisp 1971). The length mass data were used to calculate the biomass values of mussels in all the samples. Samples of the other species were individually weighed. In this instance shells of molluscs were manually removed before ashing, but barnacles and echinoderms were ashed intact, as weight loss due to combustion was low (<12% and <27% respectively).
Following the detection of M. galloprovincialis, in situ counts were done in the mid-zone at Site 1 during spring/summer between 1993 and 2005. A 0.32 × 0.32 m quadrat was laid contiguous to itself 100 times along of the mussel bed, with adjustments being made to exclude steep crevices, protrusions and experimentally cleared areas. Counts of the numbers of M. galloprovincialis per quadrat were done in every alternate one, except in 2002 and 2005 when only 40 quadrats and 20 quadrats were evaluated respectively. In the low- and high-zones (sometimes 1–2 months later than those in the mid-zone) counts were done in 10 quadrats, spaced approximately 1 m (or 3 quadrats) apart. Only mussels on which a distinctive blue-black tinge could be discerned were classed and counted as M. galloprovincialis (Van Erkom Schurink and Griffiths 1990; Branch et al. 2005). The accuracy of the counts and the associated faunal (all organism beside P. perna and M. galloprovincialis) composition of the mussel bed were determined by removing the contents of four of the evaluated quadrats (mussel cover >70%) in both the mid- and high-zones on each sampling date between 2001 and 2005, and in the low-zone in 2003 (Table 1). The samples were processed in a manner described above.
In processing the samples of the 1980s, which all had >75% mussel cover, some small or encrusting species were inadequately recorded. As a result I excluded them from all analyses that compared the associated fauna from pre-invasion (1980s) samples with those from during the invasion (2000s) samples. The density, biomass, Shannon-Weaver diversity index (H′ = −∑ (Pi) (Log (Pi)), where Pi = proportion of total sample belonging to the ith species) and Margalef’s richness index (d = (S−1)/log (N)), where S = number of species, and N = number of individuals) of the associated fauna were determined for each sampling date (Odum 1971).
Comparisons of the above values between sampling dates were done using Kruskal–Wallis (nonparametric analysis of variance) test and the software program STATGRAPHICS (Statistical Graphic Corporation 1989). Where significant differences (P ≤ 0.05) were recorded, post-hoc comparisons were done using Dunn test (Zar 1984). Classification of sampling dates was based on the Bray-Curtis similarity analysis done on fourth-root transformed density and biomass values of the associated fauna (Gauch 1995). Linear regression analyses were used to examine relationships between the density of associated faunal species and that of mussels (Zar 1984).
Results
Comparative sampling indicated that on average the in situ counts underestimated the density of M. galloprovincialis in the quadrats by a factor of 0.47 (SD = 0.15, n = 32), and the values from the counts (Fig. 2) have been adjusted accordingly. In the mid-zone the mean densities of M. galloprovincialis were initially low (<15 individuals/0.1 m2) between 1993 and 1996, a period when sea temperatures were abnormally cold (Fig. 2). Thereafter the mean densities of M. galloprovincialis increased steadily, peaking at 721 individuals/0.1 m2 in 2004, and then declining sharply in 2005 (331 individuals/0.1 m2). Similar trends were noted in the low- and high-zones. In 2004 the highest densities of M. galloprovincialis were recorded in the high-zone (1121 individuals/0.1 m2) and the smallest in the low-zone (338 individuals/0.1 m2), where the lowest proportional contribution of M. galloprovincialis to the overall mussel population (<65%) was encountered (Fig. 2).
Variation in (a) monthly surface sea temperatures above and below the mean monthly values for the study period, (b) mean adjusted density (±SD) of M. galloprovincialis recorded from in situ counts, and (c) mean proportions (±SD) of M. galloprovincialis relative to the overall mussel density determined from quantitative samples
Data from the harvested samples indicated that the mean density and biomass values of both M. galloprovincialis and P. perna varied significantly between sampling dates (Kruskal–Wallis test X 2 6, H > 18, P < 0.01, Table 2). The mean values for the pre-invasion period were similar (Dunn test Q < 2.7, P > 0.05) and differed significantly from those recorded in 2004 and 2005 (Dunn test Q > 6.7; P < 0.01, Table 2). As the numbers and biomass of M. galloprovincialis increased in the 2000s, there was an associated, but smaller decline in those of P. perna (Fig. 3). Consequently, the mean densities of mussels in 2004 and 2005 were significantly higher than those in the 1980s (Kruskal–Wallis test X 2 6, H > 14, Dunn test, Q = 4.4, P < 0.05, Table 2). In the high-zone the density and biomass values fluctuated in a similar manner to those of the mid-zone (Fig. 3). The decline in overall mussel densities noted in the 2005 samples (Fig. 3) was accompanied by the presence of bare patches in the mussel bed (pers. obs.), which was apparently caused by wave-driven dislodgement of mussels. A similar situation was noted in mussel beds at some 46 km west of Site 1 (Erlandsson et al. 2006; Zardi et al. 2006).
The pooled P. perna sample from the 1980s showed a slightly higher proportion of large individuals (shell length > 40 mm) than that from the 2000s (40 vs. 35%, Fig. 4), but otherwise the size compositions were similar. The size distribution of M. galloprovincialis individuals varied with tidal elevation, with the modal length being smallest in the high-zone, and largest in the low-zone (Fig. 5).
A total of 34 associated faunal species were effectively recorded throughout the study period, and use in the comparative analyses. The total density of associated fauna differed significantly between sampling dates (Kruskal–Wallis test X 2 6, H = 18.1, P < 0.01) with the largest differences occuring during the invasion period between 2001 and 2005 (Dunn test, Q = 13.6, Fig. 6). The density of associated fauna steadily increased from a low in 2001 to a high in 2005, and this change was largely due to an increase in the number of barnacles (Chthamalus dentatus and Octomeris angulosa) present (Table 2). The associated faunal biomass varied in a similar manner, but the changes were not significant (X 2 6, H = 10.0, P > 0.05). Species richness fluctuated substantially between sampling dates (H = 15.3, P < 0.02), with 1984 value differing significantly from those of 2001, 2003, 2004 and 1987 (Q > 8.5, P < 0.05), and both high and low values were noted in the pre-invasion period (Fig. 6, Table 2). In the density and biomass dendrograms (Bray Curtis analyses) 2001, 2003 and 2004 were >84% similar to each other, but 2005 showed the least affinity to the other sampling dates (Fig. 7). Unusually high densities of the barnacle O. angulosa, isopod Cirolana venusticauda and polychaete Pseudonereis variegata were recorded in the 2005 samples (Table 2). Both C. venusticauda and P. variegata are mobile predators associated with mussel beds and the loss of mussel cover elsewhere on the rocks may have concentrated these species in intact mussel areas. Regression analyses of samples collected over the entire study period gave a significant, but poor correlation between densities of P. variegata and those of mussels (r = 0.55, n = 28, P < 0.01). No significant correlation was found between densities of C. venusticauda and those of mussels, the barnacle O. angulosa or the limpet Scutellastra granularis (r < 0.32, n = 28, P > 0.1).
The densities and biomass values of four of the more abundant (mean density > 5 individuals/0.1 m2) associated faunal species varied significantly (P≤ 0.05) between sampling dates (Table 2). However, it was only in the case of the barnacle C. dentatus that, in terms of both density and biomass, the pre-invasion values were similar to each other (Q < 2.2, P > 0.05) and differed significantly from the majority of the during invasion values (Q > 4.6, P ≤ 0.05, Table 2). The lowest density of C. dentatus was recorded in 2001, shortly after the first major influx of M. galloprovincialis (Fig. 8). Thereafter the numbers increased steadily. The biomass values of C. dentatus reflected a similar trend. The vast majority ( \( \overline{x} \) ± SD = 96 ± 13%) of C. dentatus recorded from nine 0.1 m2 samples investigated were attached to the valves of mussels rather than to the rock face. Therefore, it is postulated that the decline in C. dentatus numbers in the early 2000s was associated with the displacement of mature P. perna that were densely colonized by C. dentatus. As the invading M. galloprovincialis population aged, so C. dentatus was able to colonize the valves of these mussels and increase in abundance.
The density values of another barnacle O. angulosa showed similar fluctuations to those of C. dentatus, but no significant change in biomass was recorded (X 2 6, H = 9.5, P > 0.10). Moreover, O. angulosa is a pioneer colonizer of the bare rock face, and the reasons for the variations in densities are unknown.
Discussion
The mean density (expressed per square metre) of P. perna recorded in the mid-zone of Site 1 in the 1980s (\( \overline{x} \) ± SD = 4,790 ± 982 mussels/m2) was moderately high. It was greater than the mean (3,819 ± 6,062 mussels/m2) recorded at nine sites along the Tsitsikamma coast by Crawford and Bower (1983), but about 16% lower than that (ca. 5,700 mussels/m2) determined for 18 exposed- and sheltered low-shore sites along the south–east coast of South Africa (McQuaid et al. 2000). The latter data, which were collected from randomly placed quadrats that were not restricted to areas of 100% mussel cover, showed no significant relationship between either biomass or adult densities of P. perna and the degree of wave exposure experienced (McQuaid et al. 2000). In contrast M. galloprovincialis on the west coast of South Africa attained the greatest cover, biomass, and recruitment at sites with moderate wave exposure, with lower values being noted at sheltered and extremely exposed sites (Branch and Steffani 2004; Hammond and Griffith 2004).
On the south coast M. galloprovincialis appears to have a summer and autumn/winter spawning peak (Van Erkom Schurink and Griffiths 1991; Bownes 2005). Following the introduction of M. galloprovincialis to Algoa Bay (ca. 160 km west of the Storms River) for mariculture purposes in 1988, the larvae of M. galloprovincialis dispersed along this coast like passive particles, matching the speed and direction of surface water currents generated by wind (McQuaid and Phillips 2000). The yearly average increase in distributional range of M. galloprovincialis along this coast from 1988 to 1992 was 42 km eastwards compared to only 19 km westwards (McQuaid and Phillips 2000). Acoustic Doppler Current Profiler measurements taken over 12 months in 1998–1999 at Tsitsikamma indicated that surface water (5 m deep) flow was also predominantly in an easterly (or long-shore) direction, particularly during spring and summer (Roberts and Van den Berg 2005). The eastward water flow was generally slightly onshore and at times associated with warmer sea temperatures (Attwood et al. 2002; Roberts and Van der Berg 2005). It is, therefore, noteworthy that substantial increases in the population density of M. galloprovincialis at Site 1 were first recorded in 1999, following the relaxation of a series of cold seawater events of between 1993 and 1996 (Fig. 2), and a spring–summer season when surface seawater flow was predominantly eastwards (Roberts and Van der Berg 2005). Water circulation patterns can be important in determining larval recruitment patterns (Connolly and Roughgarden 1998; Archambault and Bourget 1999), and between 1994 and 2000 the geographical distributional limit of M. galloprovincialis steadily extended eastwards along the south and south–east coast (Rius 2004). Conversely, the higher sea temperatures experienced in the late 1990s were likely to adversely affect recruitment of M. galloprovincialis at Site 1, as this species occurs primarily in temperate areas (Bownes 2005).
On the south coast settlement and recruitment of M. galloprovincialis generally decreases with increasing tidal height, and post-settlement mortality of juveniles is low in both the low- and high-zones (Bownes and McQuaid 2006). Despite this trend, the densities and proportional contributions of M. galloprovincialis to the overall mussel population (>5 mm shell length) at Site 1 increased with increasing tidal height (Fig. 2–3). These results are in agreement with the findings that at sites where the two mussel species co-occur, M. galloprovincialis dominates on the high-shore and P. perna the low-shore, with a mixed zone at the mid-shore level (Bownes 2005). Mytilus galloprovincialis is apparently able to maintain high densities on the high-shore through persistence of successive settlement of individuals, even though the growth rate of juvenile M. galloprovincialis decreases upshore (Bownes and McQuaid 2006). In the mid-zone at Site 1 the population density of P. perna declined by approximately 66% over the study period, while the contribution of M. galloprovincialis to the overall mussel population increased to more than 70%. The latter value is marginally higher than the mean M. galloprovincialis contribution (ca. 67%) recorded for two mid-shore sites at Plettenberg Bay in 2004 (Bownes 2005). Near the end of this study (2004–2005) the density and biomass values of M. galloprovincialis at the mid-zone of Site 1 declined substantially, while those of P. perna changed only slightly (Fig. 3). The presence of bare patches and loose byssus threads within mussel bed suggests that wave action had dislodged portions of the mussel population. The attachment strength of M. galloprovincialis to the substratum is approximately 25% lower than that of P. perna and its broader shell is subject to higher hydrodynamic loads than P. perna, making this species more susceptible to dislodgement by wave action (Zardi et al. 2006). Despite this, in the mid-zone of two mixed-species mussel beds at Plettenberg Bay, wave-driven dislodgement was highest for species that initially dominated at each site, irrespective of whether it was M. galloprovincialis or P. perna (Erlandsson et al. 2006). The weaker attachment, higher hydrodynamic resistance and dominant presence of M. galloprovincialis probably all contributed to the substantial loss of mussels in the mid 2000s at Site 1 (Fig. 3). The re-colonization and recovery rates of M. galloprovincialis in wave-disturbed areas at Plettenberg Bay were substantially greater than that of P. perna (Erlandsson et al. 2006). This rapid recovery rate and weak attachment strength of M. galloprovincialis is likely to result in more frequent and pronounced fluctuations in mussel densities than that which occurred in the P. perna dominated, pre-invasion population. Mussels are prone to removal by waves (Denny 1995; Erlandsson et al. 2006; Rius and McQuaid 2006; Zardi et al. 2006), and episodic, storm-driven declines in M. galloprovincialis abundance may allow P. perna to persist in the mid-zone at Site 1, but at low densities.
The modal size of the adult (>20 mm) cohort of M. galloprovincialis decreased with increasing tidal elevation at Site 1 (Fig. 4), as well as at many of M. galloprovincialis sites studied on the west coast (Leeb 1995; Hammond 2001). A similar decline in modal size has been observed for P. perna on the south–east coast (McQuaid et al. 2000) and C. meridionalis on the west coast of South Africa (Griffiths and Buffenstein 1981), as well as M. edulis in England (Seed 1969a, b). Mussels may be smaller higher up the shore as a result of slower growth rates due to a reduction in the available feeding time (Seed, 1969a, b; Griffiths and Buffenstein 1981; Van Erkom Schurink and Griffiths 1993) and/or increased mortality of larger mussels on the high shore by predation. Experiments have shown that growth rates of both M. galloprovincialis and P. perna are retarded by a decline in feeding time (Van Erkom Schurink and Griffiths 1993) and that they decrease higher up the shore in summer (Bownes 2005). Conversely, Griffiths and Hockey (1987), evaluating factors influencing the structure of mussel populations on the west coast, did not consider predation on mussels to increase with shore height.
Mussel beds often support a diverse assemblage of associated fauna (Suchanek 1985; Seed 1996; Hammond and Griffiths 2004), with recorded species richness ranging from 23 for Modiolus modiolus (Witman 1984 in Hammond and Griffiths 2004) to 303 for Mytilus californianus (Suchanek 1980, 1985). In this study a total of 38 species (excluding amphipods) was obtained from the more thoroughly processed M. galloprovincialis dominated samples (1.2 m2) of the 2000s. This total was similar to those recorded in mid-shore zones of P. perna (32 species/0.09 m2) and M. galloprovincialis (33 species/0.09 m2) at Gansbaai (Hammond 2001) and two (mid-shore) P. perna sites (ca. 37 species) at De Hoop Nature Reserve (Coetzee and Zoutendyk 1993), as well as the total (35 species/0.27 m2) from nine M. galloprovincialis sites near Groenrivier (Fig. 1; Hammond and Griffiths 2004). However, it was substantially lower than the 69 species/0.2 m2 noted among A. ater and 68 species/0.17 m2 among M. galloprovincialis at Marcus Island on the west coast (Griffith et al. 1992). The number of species recorded would have been affected by the number, size and seasonal spread of samples taken, as well as the resolution in terms of minimum size of organism and taxonomic expertise to which the species were sorted and identified. Nevertheless the above values suggest that the associated faunal richness in both P. perna and M. galloprovincialis on the west and south coasts is generally low.
Species richness of the associated fauna at Site 1 differed significantly (P < 0.05) between sampling dates. However, some of the greatest differences occurred within pre-invasion sampling periods and changes resulting from the M. galloprovincialis invasion could not be discerned. At Gansbaai, where associated faunal communities from co-occurring intertidal mussel species were apparently sampled in the same month, significantly (P < 0.05) higher species richness was recorded in M. galloprovincialis than in P. perna beds, but no difference (P > 0.05) was noted in density, biomass and species diversity values (Hammond 2001). Likewise, Iwasaki (1995) found that mussel beds of two different mussel species (Septifer virgatus and Hormomya mutabilis) supported associated faunal assemblages with similar species diversity, but different faunal composition.
Densities of the associated fauna at Site 1 varied significantly (P < 0.01) between sampling dates, showing a marked decline in 2001 followed by a steady recovery (Fig. 6). Faunal biomass values showed a similar trend, but the changes were not significant (P > 0.05). These variations were largely due to changes in the densities of the barnacles C. dentatus and O. angulosa, and the variations in the abundance of former species could be explained by the M. galloprovincialis invasion.
Associated faunal communities are often dominated by a few abundant species (Seed 1996). In both the pre-invasion and during invasion samples at Site 1 O. angulosa was the dominant associated faunal species, constituting approximately 33–44% of the total numbers and 77–90% of the biomass, followed by C. dentatus (29– 41% of numbers and 3–12% of biomass), and the granular limpet S. granularis (4–9% of numbers and 1–4% of biomass). These two barnacle species were less abundant in the mussel beds studied at Gansbaai and near Groenrivier (Hammond 2001; Hammond and Griffiths 2004). At Gansbaai the most important associated faunal species in P. perna were the amphipod Ceradocus rubromaculatus (28% of numbers) and the polychaete P. variegata (74% of biomass), while in M. galloprovincialis they were the isopod D. huttoni (30% of numbers) and S. granularis (44% of biomass) (Hammond 2001). Scutellastra granularis also dominated the infaunal biomass at two-thirds of the M. galloprovincialis beds studied near the Groenrivier (Hammond and Griffiths 2004). On the west coast M. galloprovincialis competes successfully against adult S. granularis for primary space, but M. galloprovincialis beds also provide a favoured settlement site for juvenile S. granularis (Hockey and Van Erkom Schurink 1992). The net result is that adult S. granularis become spatially contained by encroaching M. galloprovincialis, while the density of limpet recruits increases markedly. The impact of M. galloprovincialis on the adult S. granularis population at Tsitsikamma was less discernible than that observed on the west coast, because at Tsitsikamma the size and growth rate of the indigenous- and invasive alien mussel species are more comparable, and the adult size of S. granularis is smaller than that on the west coast (Van Erkom Schurink and Griffiths 1990; Hockey and Van Erkom Schurink 1992; Bustamante et al. 1995).
In conclusion the invasion by M. galloprovincialis reduced P. perna densities, but increased the overall mussel density in both the mid- and high-zone at Site 1. By the mid 2000s M. galloprovincialis constituted most of the mussels in the mid-zone (>70%) and more especially the high-zone (>85%). Large, episodic declines in M. galloprovincialis numbers, resulting from wave-driven dislodgement, will probably allow the more securely attached P. perna to persist at low densities in the mid-zone at Site 1. Variations in density and species richness values during both the pre- and during-invasion period made it difficult to assess the full impact of M. galloprovincialis on the associated fauna. The toothed barnacle Chthamalus dentatus appeared to be the only faunal species that was directly affected by the M. galloprovincialis invasion, experiencing a significantly (P ≤ 0.05), but temporary decline in density and biomass values. These changes in the mussel bed at Site 1 are substantially less than that reported for the west coast, where M. galloprovincialis displaced the smaller, slowing growing ribbed mussel A. ater (Griffiths et al. 1992; Hockey and Van Erkom Schurink 1992).
References
Archambault P, Bourget E (1999) Influence of shoreline configuration on spatial variation of meroplanktonic larvae, recruitment and diversity of benthic subtidal communities. J Exp Mar Biol Ecol 238:161–184
Attwood CG, Allen J, Claassen PJ (2002) Nearshore surface current patterns in the Tsitsikamma National Park, South Africa. S Afr J Mar Sci 24:151–160
Bownes S (2005) Habitat segregation in competing species of intertidal mussels in South Africa. PhD Thesis, Department of Zoology and Entomology, Rhodes University, South Africa, 284 pp
Bownes S, McQuaid CD (2006) Will the invasive mussel Mytilus galloprovincialis Lamarck replace the indigenous Perna perna L. on the south coast of South Africa? J Exp Mar Biol Ecol 338:140–151
Branch GM, Steffani CN (2004) Can we predict the effects of alien species? A case-history of the invasion of South Africa by Mytilus galloprovincialis (Lamarck). J Exp Mar Biol Ecol 300:189–215
Branch GM, Griffiths CL, Branch ML, Beckley LB (2005) Two oceans. A guide to the marine life of Southern Africa. David Philip, Cape Town, 360 pp
Brown AC, Jarman N (1978) Coastal marine habitats. In: Werger MJA, van Bruggen AC (eds) Biogeography and ecology of Southern Africa W Junk, The Hague pp 1241–1277
Bustamante RH, Branch GM, Eekhout S, Robertson B, Zoutendyk P, Schleyer M, Dye A, Keats D, Jurd M, McQuaid CD (1995) Gradients of intertidal productivity around the coast of South Africa and their relationship with consumer biomass. Oecologia 102:189–201
Calvo-Ugarteburu G, McQuaid CD (1998) Parasitism and introduced species: epidemiology of trematodes in the intertidal mussels Perna perna and Mytilus galloprovincialis. J Exp Mar Biol Ecol 220:47–65
Coetzee DJ, Zoutendyk P (1993) Species composition, distribution and standing stock of rocky-shore intertidal communities in De Hoop Marine Reserve. Bontebok 8:1–12
Connolly SR, Roughgarden J (1998) A latitudinal gradient in Northeast Pacific intertidal community structure: evidence for an oceanographically based synthesis of marine community theory. Am Nat 151:311–326
Crawford RJM, Bower DF (1983) Aspects of growth, recruitment and observation of the brown mussel Perna perna along the Tsitsikamma coast. Koedoe 26:123–133
Crisp DJ (1971) Energy flow measurements. In: Holmes NA, Mc Intyre AD (eds) IBP Handbook No 16. Methods for the study of marine benthos pp 197–279 Blackwell Scientific Publications, Oxford
Denny M (1995) Predicting physical disturbance: mechanistic approaches to the study of survivorship on wave-swept shores. Ecol Monogr 65:371–418
Erlandsson J, Pal P, McQuaid CD (2006) Re-colonization rate differs between co-existing indigenous and invasive intertidal mussels following major disturbance. Mar Ecol Prog Ser 320:169–176
Gauch HG (1995) Multivariate analysis in community ecology. Cambridge University Press, Cambridge
Grant WS, Cherry MI (1985) Mytilus galloprovincialis Lmk. in southern Africa. J Exp Mar Biol Ecol 90(2):179–191
Griffiths RJ, Buffenstein (1981) Aerial exposure and energy input in the bivalve Choromytilus meridionalis (Kr.). J Exp Mar Biol Ecol 52:219–229
Griffiths CL, Hockey PAR (1987) A model describing the interactive roles of predation, competition and tidal elevation in structuring mussel populations. In: Payne AIL, Gulland JA, Brink KH (eds) The Benguela and comparable ecosystems, S Afr J Mar Sci 5:547–556
Griffiths CL, Hockey PAR, Van Erkom Schurink C, Le Roux PJ (1992) Marine invasive aliens on South African shores: implications for community structure and trophic functioning. In: Payne AIL, Brink KH, Mann KH, Hilborn R (eds) Benguela Trophic Functioning, S Afr J Mar Sci 12:713–722
Hammond W (2001) Factors affecting the infauna associated with mussel beds. MSc Thesis, Department of Zoology, University of Cape Town, South Africa, pp 104
Hammond W, Griffiths CL (2004) Influence of wave exposure on South African mussel beds and their associated infaunal communities. Mar Biol 144:547–552
Hockey PAR, Van Erkom Schurink C (1992) The invasive biology of the mussel Mytilus galloprovincialis on the southern African coast. Trans Roy Soc S Afr 48(1):123–139
Iwasaki K (1995) Comparison of mussel-bed community between two intertidal mytilid Septifer virgatus and Hormomya mutabilis. Mar Biol 123:109–119
Leeb A (1995) Patterns of recruitment, growth and mortality of the mussel, Mytilus galloprovincialis in relation to wave exposure and tidal elevation. MSc Thesis, University of Cape Town, South Africa, 99 pp
McQuaid CD, Phillips TE (2000) Limited wind-driven dispersal of intertidal mussel larvae: in situ evidence from the plankton and the spread of the invasive species Mytilus galloprovincialis in South Africa. Mar Ecol Prog Ser 201:211–220
McQuaid CD, Lindsay JR, Lindsay TL (2000) Interactive effects of wave exposure and tidal height on population structure of the mussel Perna perna. Mar Biol 137:925–932
Odum EP (1971) Fundamentals of ecology, 3rd edn. Saunders College publishing, Philadelphia, USA pp 574
Rius VM (2004) The effect of the invasive mussel Mytilus galloprovincialis and human exploitation on the indigenous mussel Perna perna on the South Coast of South Africa. MSc Thesis, Department of Zoology and Entomology, Rhodes University, South Africa, pp 203
Rius M, McQuaid CD (2006) Wave action and competitive interaction between the invasive mussel Mytilus galloprovincialis and the indigenous Perna perna in South Africa. Mar Biol 150(1):69–78
Roberts MJ, Van den Berg M (2005) Currents along the Tsitsikamma coast, South Africa, and potential transport of squid paralarvae and ichthyoplankton. Afr J Mar Sci 27(2):375–388
Robinson TB, Griffiths CL, McQuaid CM, Rius M (2005) Marine alien species of South Africa–status and impacts. Afr J Mar Sci 27(1):297–306
Seed R (1969a) The ecology of Mytilus edulis L. (Lammellibranchiata) on exposed rocky shores. I. Breeding and settlement. Oecologia 3:277–316
Seed R (1969b) The ecology of Mytilus edulis L. (Lamellibranchiata) on exposed rocky shores. II. Growth and mortality. Oecologia 3:317–350
Seed R (1992) Systematic evolution and distribution of mussels belonging to the genus Mytilus: an overview. Am Malacol Bull 9(2):123–137
Seed R (1996) Patterns of biodiversity in the macro-invertebrate fauna associated with mussel patches on rocky shores. J Mar Biolog Assoc UK 76:203–210
Steffani CN, Branch GM (2003a) Spatial comparisons of populations of an indigenous limpet Scutellastra argenvillei and the alien mussel Mytilus galloprovincialis along a gradient of wave energy. Afr J Mar Sci 25:195–212
Steffani CN, Branch GM (2003b) Temporal changes in an interaction between an indigenous limpet Scutellastra argenvillei and an alien mussel Mytilus galloprovincialis along a gradient of wave energy. Afr J Mar Sci 25:213–229
Steffani CN, Branch GM (2003c) Growth rate, condition, and shell shape of Mytilus galloprovincialis: response to wave exposure. Mar Ecol Prog Ser 246:197–209
Statistical Graphics Corporation (1989) Statistical Graphics System, Version 4.0. Statistical Graphics Corporation. USA
Suchanek TH (1980) Diversity in natural and artificial mussel communities of Mytilus californianus. Am Zool 20:807
Suchanek TH (1985) Mussels and their role in structuring rocky shore communities. In: Moore PG, Seed R (eds) The ecology of rocky coasts Columbia University Press, New York pp 70–96
Van der Elst R (1981) A guide to the common sea fishes of southern Africa C Struik, Cape Town, p 12
Van Erkom Schurink C, Griffiths CL (1990) Marine mussels of southern Africa—their distribution patterns, standing stocks, exploitation and culture. J Shellfish Res 9(1):75–85
Van Erkom Schurink C, Griffiths CL (1991) A comparison of reproductive cycles and reproductive output in four southern African mussel species. Mar Ecol Prog Ser 76:123–134
Van Erkom Schurink C, Griffiths CL (1993) Factors affecting relative rates of growth in four South African mussel species. Aquaculture 109:257–273
Witman JD (1984) Ecology of rocky subtidal communities: the role of Modiolus modiolus (L) and the influence of disturbance and mutualism. PhD Thesis, University of New Hampshire, Durham
Zar JH (1984) Biostatistical Analysis, 2nd edn. Prentice Hall, Englewood Cliffs, New Jersey, pp 718
Zardi GI, Nicastro KR, McQuaid CD, Rius M, Porri K (2006) Hydrodynamic stress and habitat partitioning between indigenous (Perna perna) and invasive (Mytilus galloprovincialis) mussels: constraints of an evolutionary strategy. Mar Biol 150:79–88
Acknowledgements
South African National Parks (SANParks) are thanked for financial and logistical support. I also gratefully acknowledge contributions of the following persons. R. M. Randall, for commenting on the draft manuscript, A. K. Riley for administrative help, D. Colenbrander, E. Kivett, L. Munyai and D. Joubert for field and laboratory assistance, and R. M. Fisher for help with the artwork. I am greatly indebted to the referees who provided detailed and constructive suggestions for improving of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hanekom, N. Invasion of an indigenous Perna perna mussel bed on the south coast of South Africa by an alien mussel Mytilus galloprovincialis and its effect on the associated fauna. Biol Invasions 10, 233–244 (2008). https://doi.org/10.1007/s10530-007-9125-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-007-9125-x