Abstract
CD37 is a member of tetra-spanning superfamily (characterized by their four transmembrane domains). It is one of the specific proteins for normal and malignant mature B cells. Anti CD37 monoclonal antibodies are reported to improve the overall survival in CLL. These therapeutics will increase the efficacy and reduce the toxicity in patients with both newly diagnosed and relapsed and refractory disease. Recent clinical trials have shown promising outcomes for these agents, administered both as monotherapy and in combination with standard chemotherapeutics. Long-term follow-up of combination regimens has even raised the question of whether the patients with CLL could be treated with intensive chemo-immunotherapy. In the present study, CD37 is introduced as an appealing target to treat B cell malignancies. The anti-CD37 antibodies as one of the most successful therapeutics against CD37 are introduced and the clinical outcomes of their exploitation are explained.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Monoclonal antibodies (mAbs) are the most widely used and effective biological drugs for “targeted therapy” of cancer (Payandeh et al. 2018a, b; Sliwkowski and Mellman 2013). These molecules can also serve as relevant diagnostic and biotechnological tools. Based on the latest reports from Food and Drug Administration (FDA) of USA (Reichert 2012), there are currently 46 approved therapeutic mAbs in the market and over 100 antibody candidates are in clinical development (Reichert 2014). Antibodies can act as ‘magic bullets’ in cancer treatment due to their special ability in identifying specific epitopes and their high-affinity binding to different types of antigens (Walsh 2014). The high specificity and binding affinity of antibodies against antigens enable them to be used as therapeutic agents to treat different diseases (Eccles 2001). Therapeutic antibodies have certain advantages over small molecules or other protein therapeutics including longer serum half-live, higher avidity and selectivity and the ability to invoke desired immune responses (Nelson et al. 2010). Antibodies are also organized into distinct structural and functional domains, which have facilitated their engineering. The significance of mAbs is increasingly rising in various areas including industry, medicine, biosensor design and basic research (Caravella and Lugovskoy 2010).
B-CLL is one of the life-threatening disease and the second most common blood cancer in adults. Therefore, developing new therapeutic strategies to treat B-CLL is particularly important. In this regard, monoclonal antibodies against CD37 has demonstrated high efficiency in elimination of Cancer B cells (Carter 2006). This article is dedicated to review different antibodies developed for CD37-targeted cancer therapy in various stages of clinical trials. Moreover, an overview of CD37 and its known mechanisms in cancer-related processes is presented. We believe that getting a good grasp of the limitation encountered in the ongoing immunotherapeutic fight against B cell malignancies would pave the way to develop more efficient antibodies.
An overview of CD37
CD37 is a member of the tetra-spanning superfamily (characterized by their four transmembrane domains) and one of specific proteins for normal and malignant mature B cells. It is specified by the presence of four conserved transmembrane domains. The involvement of CD37 (TSPAN26) protein in various biological processes including cell adhesion, motility, differentiation, proliferation, metastasis, growth, survival, trafficking, intercellular communication via exosomes and immune responses is well recognized. Dysregulation of TCR signaling, enhanced proliferation and altered cytokine production in CD37-deficient (CD37−/−) mice declare regulatory roles for CD37 in the context of T cells (Knobeloch et al. 2004). Moreover, this protein is shown to be involved in T-cell–B-cell interaction and immunoglobulin G (IgG)/IgA production. CD37 is reported to be widely expressed on malignant mature B cells including B-cell chronic lymphocytic leukemia (B-CLL), B-cell non-Hodgkin lymphoma (NHL) and Burkitt lymphoma cell lines (Xu-Monette et al. 2016). This protein can act as a death receptor in B cells upon cross-ligation with its ligand. The resulting complex becomes associated with LYN, SHP1 and SYK which could recruit PI3 Kδ and transduce a signaling cascade favoring cellular death. Moreover, CD37 might act as a “molecular facilitator” especially for antibody-dependent cellular cytotoxicity, apoptosis induction and long-term T-cell responses (Rosenwald et al. 2002). Although the intricacies of CD37 role in these processes are not fully described, CD37 targeting antibodies are demonstrated to be efficiently used as therapeutic agents against B cell malignancies.
Overview of CD37 gene and glycoprotein structure
CD37 was originally defined as a B lymphocyte antigen with high expression on mature B cells and no expression on plasma cells (Tomlinson and Wright 1996). In humans, CD37 gene is located on chromosome 19q (13) and includes 8 exons, while it is located on chromosome 7 in mice including 281 amino acids encoded by 9 exons (Tarrant et al. 2003). The human CD 37 is a member of transmembrane 4 superfamily (TM4SF) of glycoprotein. It weighs 31 kDa with three N-glycosylation sites (Tomlinson and Wright 1996). CD37 has a major N-glycosylated extracellular loop. Both N and C-termini of the polypeptide are localized within the cytoplasm (Virtaneva et al. 1993). The molecule (CD37) does not contain O-linked carbohydrate chains (Hemler et al. 1996). It has been shown that the N-terminal cytoplasmic tail (8–14 aa) of CD37 contains a weak immuno-tyrosine inhibitory motif (ITIM) which binds to the SH2 domains of SHP-1. Scrutiny approaches demonstrated that CD37 is tyrosine phosphorylated and most of its phosphorylation occurs on the N-terminal ITIM motif. This phosphorylation induces apoptosis in primary CLL cells (Schwartz-Albiez et al. 1988). The first insights about the crucial and non-redundant functions of these molecules in the immune system have been provided by mice with null mutations for CD81, CD37 or TSSC6 (tumor sub-transferable candidate) (Gartlan et al. 2013). To understand the functions of CD37, its gene was cloned for the first time in 1996. CD37 associates with CD53, CD81 and CD82 to form multiprotein complexes on cell surfaces (the so-called tetraspanin web). It also interacts with important proteins such as integrins, immune-receptors and signaling molecules which act as regulators for leukocyte activation, motility and antigen presentation (Rosenwald et al. 2002).
Tissue expression profile of CD37
CD37 is expressed on a wide variety of cell types including B-cell chronic lymphocytic leukemia (B-CLL), B-cell non-Hodgkin lymphoma (NHL), Burkitt lymphoma cell lines (Xu-Monette et al. 2016) and mature B cells. On the other hands, it is not expressed on plasma cells, platelets, erythrocytes, T cells and monocytes. Natural killer (NK) cells express CD37 at a low density (Lapalombella et al. 2012). The expression of CD37 has been detected in B-cell malignancies including B-cell non-Hodgkin lymphoma (NHL), B-cell chronic lymphocytic leukemia (B-CLL) and in 60% of Burkitt lymphoma cell lines. Many tetraspanins (CD9, CD37, CD53, CD63, CD81 and CD82) have been reported to associate with MHC class II molecules. The complexes of tetraspanins MHC class II molecules were shown to be enriched with the CD86 co-stimulatory molecule and the HLA-DM peptide editor. CD37 (and probably Tssc6) is thought to regulate peptide/MHC presentation. Taken together, it could be deduced that tetraspanins regulate T cell activation either positively or negatively by controlling the antigen presentation process in APCs. This regulatory role could bear important implications for anti-tumor immunity (Veenbergen and van Spriel 2011).
Targeting of CD37 by monoclonal antibodies
Targeting the CD37 molecule is currently under investigation in various clinical phases for patients with B cell malignancies. However, the involving molecular mechanisms have not yet been fully resolved. During the last decade, advances in recombinant technology, bioinformatics, protein engineering and peptide design have led to development of various therapeutic antibodies and peptides against a broad range of antigens. The first attempts to develop a tetraspanin-targeted therapy predate (nearly a decade) to the approval of rituximab, when 131 I-labeled murine antiCD37 antibody was tested in a small cohort of non-Hodgkin lymphoma (NHL) patients. The following sections are devoted to describing the applications of anti-CD37 MAbs which have been undergoing different stages of clinical development (Zhao et al. 2007).
SMIP-016
Small modular immuno-pharmaceuticals (SMIP) are disulfide-linked single-chain polypeptides comprised of one antigen binding domain (VH/VL), a connecting hinge domain and a Fc domain of human IgG1 (CH2–CH3). SMIPs are designed with specific targeting and effector functions for specific diseases. Due to their smaller sizes, SMIPs are intended to have better tissue penetration while retaining similar pharmacokinetics and activity as traditional IgG1. SMIP-016 is a dimeric recombinant single chain polypeptide engineered to exhibit the full binding activity of an anti-CD37 mAb at one-third of the regular antibody size. Previous studies have shown that SMIP-016 is a potent inducer of caspase-independent apoptosis and antibody dependent cellular cytotoxicity in B-cell leukemia/lymphoma cell lines and primary chronic lymphocytic leukemia (CLL) cells (Gopal et al. 2014). They have been shown to be superior to the therapeutic antibodies used to treat these diseases. It have been demonstrated that SMIP-016 could have superior NK cell-mediated antibody dependent cellular cytotoxicity (ADCC) compared to anti-CD20 rituximab.
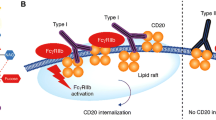
CD37 has intrinsic tyrosine-based signaling capacity that is important for SMIP-016 induced apoptosis in primary CLL cells. SMIP-016-induced apoptosis in samples from CLL patients depends on tyrosine phosphorylation (Zhao et al. 2007). To understand the mechanism underlying this phenomena, the tyrosine phosphorylated proteins associated with CD37 upon SMIP-016 stimulation were primarily identified. SH-2 containing tyrosine phosphatase SHP-1 was among the identified molecules (Fig. 1). SHP-1 downregulates the signaling pathways that promote proliferation, and it is considered a tumor suppressor (Wu et al. 2003). It has been shown that reducing SHP-1 expression could dramatically decrease SMIP-016 induced apoptosis in CLL samples. The authors then explored how SHP-1 is recruited to CD37. The N-terminal cytoplasmic tail of CD37 recruits SHP-1 to CD37. This tail contains a weak S/I/V/LxYxxI/V/L immuno-tyrosine inhibitory motif (ITIM) that is known to bind the SH2 domains of SHP-1. The results obtained from biochemistry, mass spectrometry and mutagenesis approaches have demonstrate that CD37 itself is tyrosine phosphorylated upon SMIP-016 stimulation. Furthermore, most of this phosphorylation occurs on the N-terminal ITIM motif. Mutating the Y13 amino acid to F13 amino acid forms a CD37 ITIM mutant incapable of SHP-1 binding. The cells expressing this mutant form are less susceptible to SMIP-016 based therapies. SHP-1 is reported to be abundantly expressed in hematopoietic cells (Jin and Cambier 2012). Intracellular signaling transmembrane receptors which are down regulated by SHP-1 include Cytokine receptors (e.g. Epo-R, IFNa/b-R, IL-3R and IL-2R), receptors involved in the immune response such as the TCR complex and CD5 and the growth factor receptors with an intrinsic tyrosine kinase activity (e.g. c-kit, CSF-1, TrkA and EGF). Although the molecular intricacies of SHP-1 regulatory mechanism are still under intense investigation, its SH2 domains are introduced to be responsible for binding to inhibitory receptors and dephosphorylating the downstream signal molecules. Members of the Janus activated kinases (JAK) family are the other SHP-1 binding molecules. The interaction between these molecules regulates the activity of both JAK kinase and ‘signal transduction and activators of transcription’ (STATs). Loss of SHP-1 expression or activity could lead to enhanced JAK kinase activity which in turn could trigger abnormal cell growth. Furthermore, termination of proliferation signals is suggested to be the other molecular role played by SHP-1. Plas et al. have confirmed this role by observing prolonged JAK activity and signaling as a consequence of deleting receptor tyrosine phosphorylation motifs (Plas et al. 1996). These motifs are responsible for recruiting SHP-1 to the erythropoietin receptor. This role was further analyzed by experiments on PC-3 cells in which SHP-1, or empty vector, was stably expressed (Zapata et al. 2002). Two clones expressing high levels of SHP-1 also were found to proliferate more slowly than control cells or cells transfected with empty vector. Moreover, the proliferation of these two clones with over-expressed SHP-1 was reduced by 35 and 45% after 6 days of culture, respectively (Wu et al. 2003).
Mode of action for SMIP-016 in CLL. After the ligation between CD37 and SMIP-016, it induces apoptosis in a tyrosine phosphorylation manner in samples form CLL patients. The SH-2 containing tyrosine phosphatase SHP-1 is among the identified tyrosine phosphorylated proteins associated with CD37 upon SMIP-016 stimulation. SHP-1 downregulates the signaling pathways that promote proliferation, and it is considered as a tumor suppressor
TRU-016
The ADAPTIR™ platform was employed to develop a humanized variant of SMIP-016 known as TRU-016 or Otlertuzumab. This anti-CD37 antibody is currently involve in a clinical trial for patients with CLL. It is capable of inducing both pro-apoptotic Akt inactivation and (to a lesser extent) pro-survival PI3 Kδ activation (Lapalombella et al. 2012). The ability of SMIP-016 for simultaneous activation of these opposing signaling pathways makes it an ideal candidate for combination strategies for anti-CD37 therapies. Indeed, Lapalombella et al. showed that adding either a pan-PI3 K inhibitor (LY294002) or the PI3 Kδ-selective CAL-101 (idelalisib) could enhance the SMIP-016 cytotoxicity against CLL B-cells. Binding with SMIP-016 leads to phosphorylation of Tyr13 from CD37 which is located at ITIM-like motif found in the N-terminal cytoplasmic tail (Lapalombella et al. 2012). This phosphorylation is followed by the association and phosphorylation of a complex of proteins including Syk, Lyn and SHP1. An ITAM-like motif (containing Tyr 274 and Tyr 280) located at the C-terminal cytoplasmic tail of the CD37 is also phosphorylated by SMIP-016 binding which consequently enables PI3 Kδ recruitment. Data attained from mutational studies suggested that the N-terminal ITIM motif is involved in apoptosis events, while the C-terminal tail has a role in promoting cell survival. It has been suggested that anti-CD37 antibodies induce cellular death by establishing a balance between these signals with preferential SHP-1 activation. SHP1 is capable of inactivating both PI3 K and Akt signaling. SMIP-016 prevents FoxO3a phosphorylation (and promoting retention in the nucleus) and allows transcription of pro-apoptotic BIM by decreasing the nuclear localization of Akt. The PI3 Kδ molecule binds to the C-terminal ITAM motif which could transduce an opposing signal. This signal activates Akt and results in the downstream phosphorylation of GSK3β (which permits nuclear translocation of pro-survival Β-catenin). However, deletion of the ITAM-containing C-terminal domain from CD37 or combination with a PI3 K inhibitor could diminish the contribution of PI3 Kδ to cell survival. Although binding with anti-CD37 SMIP-016 activates both pro-survival and pro-apoptotic signaling pathways, cellular death promoting signaling is more predominant. Several other CD37 targeting antibodies are reported to promote leukemia cell death. Most likely their pro-apoptotic affects are similar to SMIP-016/TRU-016 mechanism of action. However, unlike the SMIP-016 case, they do not need additional receptor crosslinking. Otlertuzumab has recently been tested in clinical trials involving CLL and NHL patients (Robak and Robak 2014). Modest single-agent activity and well tolerability was observed in Phase I study of CLL patients. The overall response rate (ORR) was reported to be 23% which could experience dramatic improvement by combination with chemotherapy. Similarly, based on a report from a randomized Phase II trial, the efficacy of Otlertuzumab in relapsed CLL patients could be improved in combination with bendamustine (NCT01188681). The ORR of this combination therapy was reported to be 80%. (B. Phase 1/2 (NCT01188681) B (Gopal et al. 2014; Hellman et al. 2013).
IMGN529
Covalently linked cytotoxic agents to tumor-targeting antibodies, also known as Antibody–drug conjugates (ADCs), have recently been developed to enhance antitumor potency. ADC binding, internalization and intracellular payload release would allow for specific delivery of cytotoxic compounds to cells expressing the target antigen. The potential of ADCs as highly effective targeted cancer therapies have been demonstrated considering obtained clinical data. IMGN529 is a CD37-targeting ADC composed of an anti-CD37 antibody component (responsible for intrinsic pro-apoptotic and immune effector activities of ADC) and a DM1 maytansinoid payload (responsible for cytotoxic potency of the ADC). Promising results were attained form evaluation of both in vitro activity and in vivo efficacy of IMGN529 against CD37-positive lymphoma cells (Deckert et al. 2015). These results provided the ground for its clinical development for treatment of B-cell NHL and CLL. An ongoing clinical trial under the ID of NCT01534715 is currently evaluating the efficacy of IMGN529 in NHL treatment. Several patients (participated in this clinical trial) have encountered difficulties like grade III/IV neutropenia. The problem could be largely avoided with the addition of corticosteroids and G-CSF is reported to resolve the condition to a large extent (Stathis et al. 2014a). This condition could be the consequence of the low level of CD37 expression on neutrophils which results in their direct elimination or cell redistribution (Deckert et al. 2015). 4 of 10 relapsed/refractory diffuse large B-cell lymphoma patients have been reported to respond well to the therapy (1 CR, 3 PR), testing relatively low doses of IMGN529. The success rate is expected to improve by escalation of the employed doses, while the necessary prophylaxis is addressed regarding neutropenia (Stathis et al. 2014b).
mAb 37.1
mAb 37.1 (BI 836826) is an IgG1 monoclonal antibody with certain mutations in its CH2 domain within the Fc region. These mutations could improve mAb 37.1 binding to human Fcγ receptors and augment its antibody-dependent cell-mediated cytotoxicity (ADCC) mediated by effectors such as NK cells and macrophages (Heider et al. 2011; Krause et al. 2012). mAb 37.1 directly induces high intrinsic proapoptotic activity on malignant B cells accompanied by homotypic aggregation without the need for an anti-Fc cross linker. Furthermore, mAb 37.1 is shown to deplete normal and malignant B cells in blood samples from healthy donors as well as CLL patients (Heider et al. 2011). Due to the remarkable preclinical pharmacodynamic and antitumor effects of mAb 37.1, clinical trials evaluating the humanized version of this antibody are anticipated in both Europe and the United States for B-cell malignancies. Betrian et al. (2016) have designed an ex vivo study to evaluate the efficiency of BI 836826 antibody as a potential new therapeutic option against B-cell malignant diseases. Their study included CLL patients resistant to chemo therapy. Their study have showed that, BI 836826 could be much more cytotoxic when is given in combination with idelalisib (a PI3 K inhibitor) compared to the treatments with its singular administration (Betrian et al. 2016).
AGS67E
AGS67E was introduced by Pereira et al. (2015). It is the first fully human anti-CD37 monoclonal IgG2 antibody. It is conjugated to the potent microtubule-disrupting agent monomethyl-auristatin-E (MMAE), via reduced cysteines and the protease-cleavable linker. Apoptosis, cell-cycle alterations, in vitro cytotoxicity and in vivo anticancer potential are reported to be among the AGS67E activities. Compared to other anti-CD37 monoclonal antibodies, AGS67E has showed similar or superior efficacies in similar models of B-cell malignant diseases. This antibody has high affinity for CD37 and rapidly internalizes it. It has also been observed that AGS67E inhibits the growth of several xenografts representing NHL and CLL (Pereira et al. 2015). Furthermore, Heider et al. found that AGS67E could induce antibody-dependent cell-mediated cytotoxicity (ADCC) and pro-apoptotic effects on BL cell line. These remarkable preclinical pharmacodynamic and antitumor effects make AGS67E an amenable choice to treat B cell malignancies.
Lu Betalutin
Although higher propensity of CD37 to be internalized may make it a superior therapeutic target compared to CD20, antibodies targeting CD20 managed to get FDA approval to treat cancer patients. The internalization of 177 Lu conjugated to anti-CD37 tetulomab accurse ten times faster than antiCD20 rituximab (Kolstad 2016). A 177 Lu-conjugated anti-CD37 antibody is also evaluated in a phase I/II trial in Europe. Thus far promising results have been attained which highlighted the amenability of anti-CD37 therapies particularly in combination with other agents. Potential anti-CD37 based therapies for leukemia/lymphoma are listed in Table 1.
Conclusion
CD37 is highly expressed on the surface of the majority of CLL and NHL cases (Deckert et al. 2013). This property makes the CD37 molecule to be an attractive target for immunotherapy. Given these circumstances, several CD37-targeting antibodies have been developed and are evaluated in various clinical trials (Deckert et al. 2013; Heider et al. 2011; Zhao et al. 2007). Various reports indicated that conjugation between the anti-CD37 antibodies and chemical agents could enhance the potency of these antibodies in fight against B cell malignancies. Taken together anti-CD37 therapy could be considered as a promising therapeutic approach. Further analyses of its clinical effects and its combination with other therapeutics would bring about new insights about the mechanism of its action and strategies to improve its effects.
References
Betrian S, Ysebaert L, Heider K, Delord J, Fournié J, Quillet-Mary A (2016) Idelalisib improves CD37 antibody BI 836826 cytotoxicity against chemo-resistant/relapse-initiating CLL cells: a rationale for combination treatment. Blood Cancer J 6:e496
Caravella J, Lugovskoy A (2010) Design of next-generation protein therapeutics. Curr Opin Chem Biol 14:520–528
Carter PJ (2006) Potent antibody therapeutics by design. Nat Rev Immunol 6:343
Deckert J et al (2013) A novel anti-CD37 antibody-drug conjugate with multiple anti-tumor mechanisms for the treatment of B-cell malignancies. Blood 122:3500–3510
Deckert J et al (2015) IMGN529, a novel antibody-drug conjugate (ADC) targeting CD37 shows synergistic activity with rituximab in non-Hodgkin lymphoma (NHL) models. Blood 126:1548
Eccles SA (2001) Monoclonal antibodies targeting cancer: magic bullets or just the trigger? Breast Cancer Res 3:86
Gartlan KH et al (2013) Tetraspanin CD37 contributes to the initiation of cellular immunity by promoting dendritic cell migration. Eur J Immunol 43:1208–1219
Gopal AK et al (2014) Phase 1b study of otlertuzumab (TRU-016), an anti-CD37 monospecific ADAPTIR™ therapeutic protein, in combination with rituximab and bendamustine in relapsed indolent lymphoma patients. Invest New Drugs 32:1213–1225
Heider K-H et al (2011) A novel Fc-engineered monoclonal antibody to CD37 with enhanced ADCC and high proapoptotic activity for treatment of B-cell malignancies. Blood 118:4159–4168
Hellman A et al (2013) Phase 2 study of otlertuzumab (TRU-016), an anti-CD37 ADAPTIRTM protein, in combination with bendamustine vs bendamustine alone in patients with relapsed chronic lymphocytic leukemia (CLL). Blood 122:2860
Hemler ME, Mannion BA, Barditchevski F (1996) Association of TM4SF proteins with integrins: relevance to cancer. Biochim Biophys Acta 1287:67–71
Jin L, Cambier JC (2012) SMIP-016 in action: CD37 as a death receptor. Cancer Cell 21:597–598
Knobeloch K-P et al (2004) A regulatory role for CD37 in T cell. J Immunol 172:2953–2961
Kolstad A (2016) Efficacy and safety results of Betalutin®(177 Lu-DOTA-HH1) in a phase 1/2 study of patients with non-hodgkin B-cell lymphoma (NHL). Platelets 10:100
Krause G et al (2012) Action of novel CD37 antibodies on chronic lymphocytic leukemia cells. Leukemia 26:546
Lapalombella R et al (2012) Tetraspanin CD37 directly mediates transduction of survival and apoptotic signals. Cancer Cell 21:694–708
Nelson AL, Dhimolea E, Reichert JM (2010) Development trends for human monoclonal antibody therapeutics. Nat Rev Drug Discov 9:767
Payandeh Z, Rajabibazl M, Mortazavi Y, Rahimpour A, Taromchi AH (2018a) Ofatumumab monoclonal antibody affinity maturation through in silico modeling. Iran Biomed J 22:180
Payandeh Z, Rajabibazl M, Mortazavi Y, Rahimpour A, Taromchi AH, Dastmalchi S (2018b) Affinity maturation and characterization of the ofatumumab monoclonal antibody. J Cell Biochem. https://doi.org/10.1002/jcb.27457
Pereira DS et al (2015) AGS67E, an anti-CD37 monomethyl auristatin E antibody drug conjugate as a potential therapeutic for B/T-cell malignancies and AML: a new role for CD37 in AML. Mol Cancer Ther. https://doi.org/10.1158/1535-7163.MCT-15-0067
Plas DR et al (1996) Direct regulation of ZAP-70 by SHP-1 in T cell antigen receptor signaling. Science 272:1173–1176
Reichert JM (2012) Marketed therapeutic antibodies compendium, vol 3. Taylor & Francis, Abingdon, pp 413–415
Reichert JM (2014) Antibodies to watch in 2014: mid-year update, vol 4. Taylor & Francis, Abingdon, pp 799–802
Robak T, Robak P (2014) Anti-CD37 antibodies for chronic lymphocytic leukemia. Expert Opin Biol Therapy 14:651–661
Rosenwald A et al (2002) The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. N Engl J Med 346:1937–1947
Schwartz-Albiez R, Dörken B, Hofmann W, Moldenhauer G (1988) The B cell-associated CD37 antigen (gp40-52). Structure and subcellular expression of an extensively glycosylated glycoprotein. J Immunol 140:905–914
Sliwkowski MX, Mellman I (2013) Antibody therapeutics in cancer. Science 341:1192–1198
Stathis A et al (2014a) A phase I study of IMGN529, an antibody-drug conjugate (ADC) targeting CD37, in adult patients with relapsed or refractory b-cell non-hodgkin’s lymphoma (NHL). Blood 124:1760
Stathis A et al (2014b) Preliminary findings from a phase I, multicenter, open-label study of the anti-CD37 antibody-drug conjugate (ADC), IMGN529, in adult patients with relapsed or refractory non-Hodgkin lymphoma (NHL). J Clin Oncol 35(15):8526
Tarrant JM, Robb L, van Spriel AB, Wright MD (2003) Tetraspanins: molecular organisers of the leukocyte surface. Trends Immunol 24:610–617
Tomlinson MG, Wright MD (1996) Characterisation of mouse CD37: cDNA and genomic cloning. Mol Immunol 33:867–872
Veenbergen S, van Spriel AB (2011) Tetraspanins in the immune response against cancer. Immunol Lett 138:129–136
Virtaneva KI, Angelisová P, Baumruker T, Hořejší V, Nevanlinna H, Schröder J (1993) The genes for CD37, CD53, and R2, all members of a novel gene family, are located on different chromosomes. Immunogenetics 37:461–465
Walsh G (2014) Biopharmaceutical benchmarks 2014. Nat Biotechnol 32:992
Wu C, Sun M, Liu L, Zhou GW (2003) The function of the protein tyrosine phosphatase SHP-1 in cancer. Gene 306:1–12
Xu-Monette ZY et al (2016) Assessment of CD37 B-cell antigen and cell-of-origin significantly improves risk prediction in diffuse large B-cell lymphoma. Blood. https://doi.org/10.1182/blood-2016-05-715094
Zapata PDO et al (2002) Autocrine regulation of human prostate carcinoma cell proliferation by somatostatin through the modulation of the SH2 domain containing protein tyrosine phosphatase (SHP)-1. J Clin Endocrinol Metab 87:915–926
Zhao X et al (2007) Targeting CD37-positive lymphoid malignancies with a novel engineered small modular immunopharmaceutical. Blood 110:2569–2577
Acknowledgements
The authors thank Shahid Rajaee Teacher Training University and Zanjan University of Medical Sciences for support to conduct this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Payandeh, Z., Noori, E., Khalesi, B. et al. Anti-CD37 targeted immunotherapy of B-Cell malignancies. Biotechnol Lett 40, 1459–1466 (2018). https://doi.org/10.1007/s10529-018-2612-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-018-2612-6