Abstract
Porous microspherical carriers have great promise for cell culture and tissue engineering. Dynamic cultures enable more uniform cell population and effective differentiation than static cultures. Here we applied dynamic spinner flask culture for the loading and multiplication of cells onto porous biopolymer microcarriers. The abilities of the microcarriers to populate cells and to induce osteogenic differentiation were examined and the feasibility of in vivo delivery of the constructs was addressed. Over time, the porous microcarriers enabled cell adhesion and expansion under proper dynamic culture conditions. Osteogenic markers were substantially expressed by the dynamic cell cultures. The cell-cultured microcarriers implanted in the mouse subcutaneous tissue for 4 weeks showed excellent tissue compatibility, with minimal inflammatory signs and significant induction of bone tissues. This first report on dynamic culture of porous biopolymer microcarriers providing an effective tool for bone tissue engineering.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Compared with a two-dimensional culture dish, three-dimensional (3D) scaffolds are often preferred for the culture of cells to enable effective adhesion, multiplication, and tissue-specific differentiation (Cukierman et al. 2001; Maeno et al. 2005; Seo et al. 2006; Bang et al. 2011; Dorj et al. 2012). To perform the role of scaffolds, materials should be non-toxic and stimulate cellular interactions such as cell anchorage and spreading with tailored compositions and surface topology. Furthermore, scaffolds should be developed to have 3D porous structure with an open-channeled network that allows for nutrients to be supplied and cellular migration (Macchetta et al. 2009).
In addition to the scaffold structure, the scaffold type is one of the important parameters in the tissue engineering application. For example, injectable forms are among the most desirable types for the delivery of cells in a minimally invasive manner (Lee and Mooney 2001). Among the possible candidates for injectable carriers, microspherical forms have been an intriguing choice due to the larger surface area relative to the volume, which provides more chances for cell anchorage (Chun et al. 2004; Frauenschuh et al. 2007). Microspherical carriers prepared from either biopolymers or bioceramics have thus been developed to populate tissue cells, expand the cells to large quantity, and ultimately for use in injectable tissue engineering (Drury and Mooney 2003; Hoffman 2012). We have developed an elegant microcarrier form with a highly macroporous structure (Hong et al. 2009b; Jin et al. 2012; Park et al. 2013b). Pores formed throughout the microspheres take up more cells and provide them space and surface area to anchor, spread, and multiply.
Although the porous microcarriers in static cell cultures are dominantly used, their inherent limitations accompanied by the static culture are being constantly addressed, such as limited cell expansion relating to the viability and cell functions. Thus, a dynamic culture method including a spinner flask, rotating wall vessel, and flow perfusion system has been proposed as a promising approach to circumvent the limitations (Frith et al. 2009) with the aim of achieving better efficiencies in uniform cell population within scaffolds, as well as stimulating cellular proliferation and differentiation through the mechanical shear force involved.
Therefore, in this study we introduce porous microcarriers including poly(ε-caprolactone) (PCL) and its blend with poly(d,l-lactide) (PLPC) for effective cell expansion under the spinner flask culture. We investigate the loading, proliferation and differentiation behaviors of pre-osteoblastic cells onto the two types of microcarriers during the dynamic culture, and address the in vivo applicability of the cell-microcarrier construct in a mouse subcutaneous model for 4 weeks.
Materials and methods
Microsphere preparation
Polycaprolactone (~80 kDa, Sigma-Aldrich) blended with poly(d,l-lactide) (PLDLA, l-lactide: d,l-lactide = 70:30, Sigma-Aldrich, USA) was fabricated into porous microspheres as described in our previous report (Hong et al. 2009b). Briefly, PCL and PLDLA were dissolved separately in chloroform within a (w/v) at 10 wt%. As an oligomeric ester porogen, camphene (C10H16) was dissolved in a vial at 60 % (w/v). PLDLA/PCL solutions comprising PCL ratios of 1 and 3 % (w/w) relative to PLDLA, called PLPC1 and PLPC3, respectively, were mixed with the camphene solution and stirred for 3 h. The solution was dropped into a water pool containing 2 % (w/v) of poly(vinyl alcohol) (PVA), gently stirred at 450 rpm at 4 °C, and additionally stirred for 4 h. Then, porous microspheres were obtained through filtration with a Millipore filter paper after washing with ice-cooled distilled water, and kept at 4 °C for further characterization and used after freeze-drying.
The macro-morphology and microstructure of the microspheres were examined with scanning electron microscopy. Optical microscope images of the microspheres were taken, and their diameter was measured from the images on three arbitrarily selected areas.
Water contact angle measurements
The hydrophilic surface of samples was assessed by measuring water contact angles using a contact angle analyzer (Phoenix 300, USA). The measurements were carried out at room temperature in air with deionized water as a probe liquid. To ensure consistency, each dispensing volume of water droplets was maintained at 6 μl, and each measurement was taken 5 s after dispensing. All presented data were the mean values of ten independent measurements.
Cell culture and seeding
MC3T3-E1 cells, a mouse calvaria-derived preosteoblast cell line, were cultured in α-modified minimum essential medium (α-MEM) supplemented with 10 % (v/v) fetal bovine serum (FBS), 100 U penicillin/ml, and 100 mg streptomycin/ml. The cells were maintained in a humidified atmosphere of 5 % (v/v) CO2 at 37 °C. Before seeding cells, microspheres were sterilized with 70 % (v/v) ethanol for 2 h and washed with phosphate buffer saline (PBS) solution three times. One milliliter of 5 × 106 suspended cells were added to the pre-wetted microspheres of 60 mg with culture medium for 12 h, and cell/microsphere constructs were incubated under shaking with a sway of 45° at 3 rpm for 6 h using MyLab SLRM-3 Intelli-Mixer (SLRM-3, SeouLin Bioscience, South Korea).
Cell/microsphere constructs cultured in the spinner flask system
The cell/microsphere constructs were cultured in a spinner flask (S-flask 4500-1L, TAITEC, Japan) containing 70 ml α-MEM with 10 % (v/v) FBS culture medium at 37 °C in a 5 % CO2 incubator. The stirring speed was at 30 rpm. The experiments were carried out for 14 days, and sampling was done once every 7 days.
Cell growth analyses
The cell distribution image onto the microspheres was observed by Alexa Fluor 546-conjugated phalloidin (Invitrogen, USA) staining using an inverted fluorescence microscope. Cells grown on each group were fixed with 4 % (v/v) paraformaldehyde for 30 min, treated with 0.2 % Triton X-100 for 5 min, blocked with PBS containing 1 % (w/v) bovine serum albumin for 30 min, and then incubated with 20 nM Alexa Fluor 546-conjugated phalloidin diluted in PBS for 30 min. Fluorescence images were obtained using an inverted fluorescence microscope equipped with a DP-72 digital camera.
The cell growth level was assessed by means of a cell counting kit-8 (CCK-8) according to the manufacturer’s protocols (Dojindo Molecular Technologies, Japan). Briefly, at each time of culturing (1, 7, and 14 days), the CCK-8 reagent was added to each sample and incubated for 3 h at 37 °C. The absorbance was measured at a wavelength of 450 nM using a microplate reader.
The cell morphology was observed by SEM at an accelerated voltage of 15 kV after fixation with 2.5 % (v/v) glutaraldehyde, dehydration with a graded series of ethanol (75, 90, 95 and 100 %), treatment with a hexamethyldisilazane solution, and gold coating.
Alkaline phosphatase (ALP) assay
The osteoblastic differentiation of MC3T3-E1 cells constructed with the microspheres was assessed by ALP staining. At each time of culturing (7 and 14 days), the constructs were fixed, and ALP staining was performed with an ALP color development kit (Takara, Japan) for 1 h. The samples stained in violet were observed by an optical microscope.
Quantitative real-time PCR
Quantitative assay of bone-associated genes expressed on day 7 and 14, including collagen type I (Col I) and ALP, was analyzed by quantitative real-time PCR. The first strand cDNA was synthesized from the total RNA (1 μg) using a SuperScript first strand synthesis system for reverse transcription-PCR (RT-PCR, Invitrogen, USA) according to the manufacturer’s instructions. Real-time PCR was performed using SYBR GreenER qPCR SuperMix reagents (Invitrogen). The relative transcript quantities were calculated using a ΔΔCt method with GAPDH, as an endogenous reference gene amplified from the samples. The primer sequences of the genes are as follows: Col I: forward 5′-GCAACTCTGAAATCTCTCAA-3′; reverse 5′-GATCCATAGTACATCCT-TG-3′. ALP: forward 5′-ACACCTTGACTGTGGTTACT-3′; reverse 5′-CCTTGTAGCCAG-GCCCGTTA-3′. GAPDH: forward 5′-TGTTCCAGTATGACTCCACT-3′; reverse 5′-TGGT-GAAGACACCAGTAGAC-3′.
In vivo animal study
Experimental procedures for the in vivo animal study were approved by the Dankook University Institutional Animal Care Committee. Ten-week-old male C57BL/6 mice were randomly divided into two groups (three mice per group). The animals were anesthetized by intramuscular injection of a mixture of xylazine (10 mg/kg; Rompun, Bayer Korea, Korea) and ketamine (80 mg/kg; Ketara, Yuhan, Korea). Small incisions were made on the dorsal skin. Two pouches per animal were created by blunt dissection of subcutaneous sites, and the cell-microsphere constructs were immediately implanted into each pouch. The grafts were harvested at 4 weeks after implantation.
Histological analysis of in vivo grafts
The grafts were fixed in a 10 % (v/v) formaldehyde for histological analyses. The specimens were embedded in paraffin, cut to a thickness of 8 μm, and then stained with hematoxylin and eosin and Masson Trichrome for morphologic analyses.
Statistical analysis
Data were shown as the mean ± one standard deviation. Statistical comparisons were made using a one-way ANOVA test. P < 0.05 was considered to be statistically significant.
Results and discussion
Microspheres
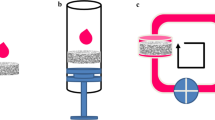
Figure 1a shows a schematic illustration representing the experimental procedures used in this study and a clinically feasible application. The PLDLA/PCL/camphene composite solution in chloroform was solidified to form spheroids with the aid of a PVA surfactant, during which the camphene was sublimated to result in highly interconnected pore networks throughout the microspheres (Hong et al. 2009a). The average size of the obtained microspheres was approximately 350 μm diameter, which is considered to be proper to populate tissue cells. The six-times-higher amount of camphene relative to polymer was optimal for the preparation of the microspheres with well-developed pore structure, according to our previous studies (Hong et al. 2009b; Park et al. 2013b). Figure 1b shows the typical morphology of microspheres with different biopolymer compositions (PCL, PLPC3, PLPC1, and PLDLA) generated after the camphene sublimation. Regular-sized large pores (~100 μm) and small pores (~50 μm) between the large pores were generated in PCL and PLCL3 microspheres, which is known to be a suitable pore structure for cell penetration and growth (Lee et al. 2008; Park et al. 2013a). In contrast, PLCL1 microspheres showed irregular and relatively small-sized pores that cannot be considered effective for cell loading in tissue engineering. The pure PLDLA microspheres showed a smooth surface with no pores.
a Schematic illustration showing the fabrication of biopolymer microcarriers with a highly porous structure for effective cell culture and expansion, as well as their injectable applications for bone tissue engineering. b SEM morphology showing porous structure of PCL, PLPC3, PLPC1, and PLDLA microcarriers. c Water contact angle measurements of these biopolymer microcarriers. Contact angle data are shown as the mean ± SD from ten measurements
In addition to the scaffold structure, the scaffold surface plays an important role in cell adhesion and compatibility. Hydrophilic substrates are more favorable for tissue compatibility, and largely depend on surface energy (Kilpadi and Lemons 1994), considerably promoting the rate and extent of bone formation (Zhao et al. 2005). Despite excellent pore structure, PCL scaffolds are relatively hydrophobic and much effort has been made to improve their hydrophilicity (Yu et al. 2009; Xu et al. 2010; Jin et al. 2011a, b). We thus examined the hydrophilicity of PLDLA, PLPC1, PLPC3, and PCL materials by measuring water contact angles on the sample surface, as shown in Fig. 1c. The water contact angle of PCL decreased substantially due to the blending with PLDLA (82° for PCL, 60° for the blends, and 56° for PLDLA). The enhanced water affinity in the blend will affect the initial cell adhesion. Furthermore, the biodegradation of PLDLA is known to be much faster than that of PCL, which also influences the cellular responses, particularly in later periods. Based on the morphological and physico-chemical traits, the PCL and PLPC3 were chosen as microcarriers for further tests of the dynamic cell culture.
This type of porous-structured microcarriers has previously been produced including our group (Jin et al. 2012; Park et al. 2013b). A number of preparation techniques for porous microcarriers have been developed on the basis of conventional methods, such as phase separation (Madihally and Matthew 1999; Fang et al. 2014) and emulsion evaporation (Crotts and Park 1995; Brown et al. 2008). Compared to these conventional methods, a camphene sublimation technique fabricates much larger pore channels into porous microcarriers. Furthermore, compared to PCL porous microcarrier, which was also generated for the culture of cells, the blended composition PLPC3 showed improved surface wettability and possibly higher degradation rate, which is considered to be beneficial for the cellular adhesion and proliferation.
Cell adhesion and proliferation on the microsphere surface
A uniform distribution of isolated cells within the spherical porous scaffolds is the first step in establishing 3D culture and further bone tissue formation. In general, when cells were populated throughout the scaffold at high density and uniformly, the tissue formation was enhanced (Hutmacher 2000). Hence, the initial cell distribution within the scaffold after seeding could establish the basis of uniform tissue formation. In this regard, we investigated the initial cell distribution and expansion on the porous microcarriers. Figure 2a shows phalloidin-fluorescent images of cell constructs with the PCL and PLPC porous microcarriers at 6 h after shaking incubation with a sway of 45° at 3 rpm. The fluorescence spots corresponding to cell constructs were more vividly shown in the PLPC porous microcarriers than those shown in PCL microcarriers, indicating that the cell plating efficiency was higher on PLPC than on PCL porous microcarriers despite their similar pore structure. Next, the optimal rotation speed was determined.
a Fluorescence images of cells initially loaded onto microcarriers (PCL and PLPC3) for 6 h with sway of 45° at 3 rpm. b Cell expansion rates on the microcarriers recorded for up to 14 days at different rotating speed (30 and 50 rpm), by CCK-8 assay. c Fluorescence images of cells expanded over 14 days onto microspheres (PCL and PLPC3) at 30 rpm. Actin cytoskeletons were stained with Alexa Fluor 546-conjugated phalloidin (orange). Scale bars 400 μm. d SEM micrographs of cells expanded on the microcarriers for 7 and 14 days (color figure online)
The minimum speed of our commercially-available spinner flasks used in this study is 30 rpm, at which the porous microcarriers are just fully suspended. The viability results of cells cultured on the porous microcarriers were compared and analyzed at 30 and 50 rpm for up to 14 days (Fig. 2b). Initially (at 1 day), cells adhered better on the PLPC than on the PCL at both rotation speeds. The adherent cells proliferated actively on the porous microcarriers with culture time up to 14 days, and the culture conditions at 30 rpm of the PLPC porous microcarriers showed the highest cell viability during the culture period. The higher water affinity of PLPC should favor cellular adhesion processes, including cell anchorage and spreading, which subsequently increase cell growth and mitosis (Ehrlich et al. 2002; Borghi et al. 2010; Kitt and Nelson 2011).
Subsequently, cell/microcarrier constructs were captured at 30 rpm to confirm the cell expansion rates under the dynamic culture. Figure 2c shows phalloidin-fluorescent images of the cell constructs with PCL and PLPC porous microcarriers at 14 days after dynamic culture at 30 rpm. As expected, cell/PLPC constructs showed more vivid color than cell/PCL constructs, suggesting that PLPC porous microcarriers provided a better potential for cell expansion in the spinner flask culture than PCL porous microcarriers. SEM micrographs further support the cell expansion results and cell morphologies grown on the PCL and PLPC porous microcarriers at 30-rpm cultures on days 7 and 14 (Fig. 2d). Both porous microcarriers showed elongated but less-populated cells at day 7, with some cells found to migrate well into the pore structure. At day 14, cells numbered significantly to cover the surface and pores almost completely (no pores were revealed due to a thick cell layer formation). The cells on the PLPC compared to those on the PCL appeared to be much denser, forming more compacted cellular constructs.
Collectively, the PLPC provided conditions for better initial cell adhesion than the PCL, at both 30 and 50 rpm. With subsequent spinner flask culturing, the rotation at a lower speed improved the cell proliferation for up to 7 and 14 days, and this was general for the PLPC and PCL. The lower mechanical shear force at the lower rotation speed was considered to provide better conditions for cellular proliferation. A rotation speed of 30 rpm is relatively low compared to those reported in other studies, where the speeds more than 50–100 rpm have been applied to gain optimal cellular anchorage and proliferation (Bilgen and Barabino 2007; Tamura et al. 2012; Wang et al. 2013). It is considered that compared to the dense polymer microcarriers used in other studies, the currently used porous microcarriers, even when retaining relatively large size, have much lower density, which should enable easier floating even at a minimal rotation speed.
Cell differentiation
The differentiation ability of the dynamically cultured cells on both porous microcarriers were evaluated by a colorimetric ALP activity (Fig. 3a) and mRNA expression levels (Fig. 3b) on days 7 and 14. The ALP staining intensity on cell constructs with the PLPC at 14 days was the highest. As supported, bone-specific mRNA levels of ALP and Col I expressed by the cell constructs on the both microcarriers significantly increased at 14 days in comparison with those at 7 days. Notably, the mRNA levels of two bone-specific genes on cell constructs with the PLPC in 14-day cultures were significantly higher than those expressed on cell constructs with the PCL. The results confirmed that the cells cultured on the PCL and PLPC were properly induced to an osteogenic differentiation under the dynamic culture conditions.
Osteogenic activity of cells cultured on the microcarriers in the dynamic conditions (at 30 rpm) for up to 14 days. a Digital camera images of ALP activity showing violet-stained cells cultured on the microcarriers on day 7 and 14. Size bars 5 mm. b mRNA levels of bone-specific genes, including ALP and type I collagen, expressed by the cells on the microspheres, assayed by quantitative real-time PCR assessment. * p < 0.05 and ** p < 0.01
Spinner flask cell culture using microcarriers have been employed for scalable expansion of stem cell (Eibes et al. 2010; Oh et al. 2009) or tissue regeneration including cartilage (Sommar et al. 2010; Lee et al. 2011), skin (Voigt et al. 1999), and adipose (Kang et al. 2008a). Specific for the bone repair, Kang et al. (2008b) reported apatite-coated poly(lactide-co-glycolic acid) microspheres as osteoblast carriers in spinner flasks. Similarly, Grellier et al. (2009) performed a stirring culture of osteoprogenitor and endothelial cells using alginate microbeads for bone defect healing. Compared to the previous work on dense microcarriers, the currently-developed porous microcarriers are considered to provide more space and surface area for the loading of cells and their subsequent delivery into in vivo bone defects. Based on the current in vitro results, including cell adhesion, proliferation, and differentiation, the porous polymeric microcarriers are considered as a promising platform for expanding cells under proper spinner flask cultures and generating cellular constructs for further bone tissue engineering. We next performed an in vivo test to find out the feasibility of the cell-microcarrier constructs.
In vivo findings
PCL and PLPC microcarriers constructed with cells for 7 days under the dynamic culture were tested. After the implantation in mice subcutaneously for 4 weeks, tissue samples were excised and histologically examined after H&E and MT staining (Fig. 4a and b, respectively). No significant inflammatory reaction was noticed on both sample groups, confirming good tissue compatibility. The periphery of the microcarriers was filled with tissues including connective tissues. The pore space of both microcarriers was occupied by existing or migrating tissue cells, and the presence of cells was more conspicuous on the PLPC than on the PCL porous microcarriers, resulting in the formation of a thicker tissue layer inside the PLPC than inside the PCL. These in vivo findings of excellent cellular penetration and connective tissue formation within the porous microcarriers support the feasibility of the cell-constructed microcarriers under the dynamic culture conditions for applications in bone tissue engineering, warranting further study.
Conclusion
We report for the first time the dynamic seeding and culture of cells within porous polymeric microcarriers as a potential platform for bone tissue engineering. The highly porous structure of the microcarriers in conjunction with the properly-adjusted spinner flask cultures enabled uniform cellular loading at large quantity. Subsequent cultures over periods of weeks resulted in substantial cell expansion and osteogenic differentiation. The cell-microcarrier constructs exhibited good in vivo compatibility and tissue formation within the pore space, suggesting that the current system may be potentially applicable for future bone tissue engineering.
References
Bang S-H, Kim T-H, Lee H-Y, Shin US, Kim H-W (2011) Nanofibrous-structured biopolymer scaffolds obtained by a phase separation with camphene and initial cellular events. J Mater Chem 21:4523–4530
Bilgen B, Barabino GA (2007) Location of scaffolds in bioreactors modulates the hydrodynamic environment experienced by engineered tissues. Biotechnol Bioeng 98:282–294
Borghi N, Lowndes M, Maruthamuthu V, Gardel ML, Nelson WJ (2010) Regulation of cell motile behavior by crosstalk between cadherin-and integrin-mediated adhesions. Proc Natl Acad Sci USA 107:13324–13329
Brown JL, Nair LS, Laurencin CT (2008) Solvent/non-solvent sintering: a novel route to create porous microsphere scaffolds for tissue regeneration. J Biomed Mater Res B 86:396–406
Chun KW, Yoo HS, Yoon JJ, Park TG (2004) Biodegradable PLGA microcarriers for injectable delivery of chondrocytes: effect of surface modification on cell attachment and function. Biotechnol Prog 20:1797–1801
Crotts G, Park TG (1995) Preparation of porous and nonporous biodegradable polymeric hollow microspheres. J Control Release 35:91–105
Cukierman E, Pankov R, Stevens DR, Yamada KM (2001) Taking cell-matrix adhesions to the third dimension. Science 294:1708–1712
Dorj B, Park J-H, Kim H-W (2012) Robocasting chitosan/nanobioactive glass dual-pore structured scaffolds for bone engineering. Mater Lett 73:119–122
Drury JL, Mooney DJ (2003) Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials 24:4337–4351
Ehrlich JS, Hansen MD, Nelson WJ (2002) Spatio-temporal regulation of Rac1 localization and lamellipodia dynamics during epithelial cell–cell adhesion. Dev Cell 3:259–270
Eibes G, dos Santos F, Andrade PZ, Boura JS, Abecasis M, da Silva CL, Cabral J (2010) Maximizing the ex vivo expansion of human mesenchymal stem cells using a microcarrier-based stirred culture system. J Biotechnol 146:194–197
Fang J, Zhang Y, Yan S, Liu Z, He S, Cui L, Yin J (2014) Poly(l-glutamic acid)/chitosan polyelectrolyte complex porous microspheres as cell microcarriers for cartilage regeneration. Acta Biomater 10:276–288
Frauenschuh S, Reichmann E, Ibold Y, Goetz PM, Sittinger M, Ringe J (2007) A microcarrier-based cultivation system for expansion of primary mesenchymal stem cells. Biotechnol Prog 23:187–193
Frith JE, Thomson B, Genever PG (2009) Dynamic three-dimensional culture methods enhance mesenchymal stem cell properties and increase therapeutic potential. Tissue Eng C 16:735–749
Grellier M, Granja PL, Fricain J-C, Bidarra SJ, Renard M, Bareille R, Bourget C, Amédée J, Barbosa MA (2009) The effect of the co-immobilization of human osteoprogenitors and endothelial cells within alginate microspheres on mineralization in a bone defect. Biomaterials 30:3271–3278
Hoffman AS (2012) Hydrogels for biomedical applications. Adv Drug Del Rev 64:18–23
Hong S-J, Yu H-S, Kim H-W (2009a) Preparation of porous bioactive ceramic microspheres and in vitro osteoblastic culturing for tissue engineering application. Acta Biomater 5:1725–1731
Hong SJ, Yu HS, Kim HW (2009b) Tissue engineering polymeric microcarriers with macroporous morphology and bone-bioactive surface. Macromol Biosci 9:639–645
Hutmacher DW (2000) Scaffolds in tissue engineering bone and cartilage. Biomaterials 21:2529–2543
Jin G-Z, Kim M, Shin US, Kim H-W (2011a) Neurite outgrowth of dorsal root ganglia neurons is enhanced on aligned nanofibrous biopolymer scaffold with carbon nanotube coating. Neurosci Lett 501:10–14
Jin GZ, Kim M, Shin US, Kim HW (2011b) Effect of carbon nanotube coating of aligned nanofibrous polymer scaffolds on the neurite outgrowth of PC12 cells. Cell Biol Int 35:741–745
Jin G-Z, Kim J-H, Park J-H, Choi S-J, Kim H-W, Wall I (2012) Performance of evacuated calcium phosphate microcarriers loaded with mesenchymal stem cells within a rat calvarium defect. J Mater Sci Mater Med 23:1739–1748
Kang S-W, Seo S-W, Choi CY, Kim B-S (2008a) Porous poly(lactic-co-glycolic acid) microsphere as cell culture substrate and cell transplantation vehicle for adipose tissue engineering. Tissue Eng C 14:25–34
Kang SW, Yang HS, Seo SW, Han DK, Kim BS (2008b) Apatite-coated poly(lactic-co-glycolic acid) microspheres as an injectable scaffold for bone tissue engineering. J Biomed Mater Res A 85:747–756
Kilpadi DV, Lemons JE (1994) Surface energy characterization of unalloyed titanium implants. J Biomed Mater Res 28:1419–1425
Kitt KN, Nelson WJ (2011) Rapid suppression of activated Rac1 by cadherins and nectins during de novo cell–cell adhesion. PLoS ONE 6:e17841
Lee KY, Mooney DJ (2001) Hydrogels for tissue engineering. Chem Rev 101:1869–1880
Lee H–H, Hong S-J, Kim C-H, Kim E-C, Jang J-H, Shin H-I, Kim H-W (2008) Preparation of hydroxyapatite spheres with an internal cavity as a scaffold for hard tissue regeneration. J Mater Sci Mater Med 19:3029–3034
Lee T-J, Bhang SH, La W-G, Yang HS, Seong JY, Lee H, Im G-I, Lee S-H, Kim B-S (2011) Spinner-flask culture induces redifferentiation of de-differentiated chondrocytes. Biotechnol Lett 33:829–836
Macchetta A, Turner IG, Bowen CR (2009) Fabrication of HA/TCP scaffolds with a graded and porous structure using a camphene-based freeze-casting method. Acta Biomater 5:1319–1327
Madihally SV, Matthew HW (1999) Porous chitosan scaffolds for tissue engineering. Biomaterials 20:1133–1142
Maeno S, Niki Y, Matsumoto H, Morioka H, Yatabe T, Funayama A, Toyama Y, Taguchi T, Tanaka J (2005) The effect of calcium ion concentration on osteoblast viability, proliferation and differentiation in monolayer and 3D culture. Biomaterials 26:4847–4855
Oh SK, Chen AK, Mok Y, Chen X, Lim U, Chin A, Choo AB, Reuveny S (2009) Long-term microcarrier suspension cultures of human embryonic stem cells. Stem Cell Res 2:219–230
Park J-H, Lee E-J, Knowles JC, Kim H-W (2013a) Preparation of in situ hardening composite microcarriers: calcium phosphate cement combined with alginate for bone regeneration. J Biomater Appl. doi:10.1177/0885328213496486
Park J-H, Pérez RA, Jin G-Z, Choi S-J, Kim H-W, Wall IB (2013b) Microcarriers designed for cell culture and tissue engineering of bone. Tissue Eng B 19:172–190
Seo S-J, Choi Y-J, Akaike T, Higuchi A, Cho C-S (2006) Alginate/galactosylated chitosan/heparin scaffold as a new synthetic extracellular matrix for hepatocytes. Tissue Eng 12:33–44
Sommar P, Pettersson S, Ness C, Johnson H, Kratz G, Junker JP (2010) Engineering three-dimensional cartilage-and bone-like tissues using human dermal fibroblasts and macroporous gelatine microcarriers. J Plastic Reconst Aesth Surg 63:1036–1046
Tamura A, Kobayashi J, Yamato M, Okano T (2012) Temperature-responsive poly(N-isopropylacrylamide)-grafted microcarriers for large-scale non-invasive harvest of anchorage-dependent cells. Biomaterials 33:3803–3812
Voigt M, Schauer M, Schaefer D, Andree C, Horch R, Stark G (1999) Cultured epidermal keratinocytes on a microspherical transport system are feasible to reconstitute the epidermis in full-thickness wounds. Tissue Eng 5:563–572
Wang Y, Chou B-K, Dowey S, He C, Gerecht S, Cheng L (2013) Scalable expansion of human induced pluripotent stem cells in the defined xeno-free E8 medium under adherent and suspension culture conditions. Stem Cell Res 11:1103–1116
Xu F, Wang Z, Yang W (2010) Surface functionalization of polycaprolactone films via surface-initiated atom transfer radical polymerization for covalently coupling cell-adhesive biomolecules. Biomaterials 31:3139–3147
Yu HS, Hong SJ, Park JH, Jeong I, Kim HW (2009) Bioactive and degradable composite microparticulates for the tissue cell population and osteogenic development. Adv Eng Mater 11:B162–B168
Zhao G, Schwartz Z, Wieland M, Rupp F, Geis-Gerstorfer J, Cochran D, Boyan B (2005) High surface energy enhances cell response to titanium substrate microstructure. J Biomed Mater Res A 74:49–58
Acknowledgment
This work was supported by a grant from Dankook University in 2013.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jin, GZ., Park, JH., Seo, SJ. et al. Dynamic cell culture on porous biopolymer microcarriers in a spinner flask for bone tissue engineering: a feasibility study. Biotechnol Lett 36, 1539–1548 (2014). https://doi.org/10.1007/s10529-014-1513-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-014-1513-6