Abstract
Culture of three-dimensional (3D) cell-laden bone tissue engineering constructs poses significant challenges to researchers. Traditional static culture techniques allow for the growth and maintenance of cells on scaffolds within the millimeter range. Above this size, lack of media and oxygen transport to cells on the inner portions of scaffolds results in a sharp nutrient gradient, non-homogenous tissue formation, and cell death. Because of these difficulties, production of regenerative bone tissue engineering constructs of clinically relevant size is limited. To ameliorate the issues associated with static culture, several bioreactor systems have been developed. These technologies improve nutrient, oxygen, and waste transport through convective mixing of media within the 3D culture vessel. Convective transport within the scaffolds results in more homogenous cell seeding and increased viability and proliferation. The dynamic mixing environment also introduces the application of fluid shear forces to cells, which has been demonstrated to improve the process of osteogenesis. Bioreactors allow for the culture of large volumes of engineered bone and increase the potential of clinical translation by automating culture processes. Current bioreactor designs employed in bone tissue engineering applications include spinner flasks, rotating wall vessels, perfusion cartridges, and mechanical forces systems. This chapter provides an overview of each of these systems, including their design rationale, applications, and limitations. It also covers the potential for bioreactor systems to produce clinically relevant volumes of bone tissue, as well as future considerations for clinical translation.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Engineered bone
- Bioreactors
- Dynamic culture
- Mass transport
- Cell seeding
- Perfusion
- Shear stress
- Clinical translation
1 Introduction
Tissue engineering strategies for bone defect repair typically consist of cells, scaffolds, and bioactive signals that, when combined, produce a regenerative bone template that degrades within the host over time and stimulates the body to produce new, functional bone tissue to take its place [1]. For strategies utilizing cell-based approaches, scaffolds are seeded with cells and pre-cultured in vitro to allow for cell proliferation and osteogenic differentiation prior to implantation [2]. However, traditional static culture technique has proven an inefficient method for culture of three-dimensional (3D) scaffolds. Static culture provides sufficient nutrients and oxygen to cells on outer surfaces of these scaffolds, but cells within the center of the scaffold rely on diffusive transport of nutrients. Consequently, a nutrient gradient forms within the scaffolds, resulting in cell death toward the center. These limitations restrict the production of regenerative bone tissue engineering (BTE) scaffolds of clinically relevant sizes, demonstrating these effects in constructs as small as 10 mm3 [3, 4]. The difficulties associated with static culture conditions are ameliorated through the use of bioreactor culture. These systems provide dynamic culture environments in which media is constantly mixed and convectively transported to cells throughout the scaffold [2].
Although bone is a regenerative tissue that often heals without surgical intervention, defects of critical size do not spontaneously heal over time. Critical size bone defects, which were previously discussed in chapters “3D Printing for Oral and Maxillofacial Regeneration” and “Bone Grafting in the Regenerative Reconstruction of Critical-Size Long Bone Segmental Defects”, are on the scale of 2.5 cm, and their geometry varies with anatomical location [5]. When generating tissue engineering constructs to repair these large defects, bioreactor culture is often required to increase the nutrient transport throughout the scaffold via convective flow. Transporting media and oxygen through convective mechanisms allows cells within the entire construct to readily receive necessary nutrients during the culture period, leading to greater cell survival and more homogenous tissue formation. This same effect is related to bone tissue development and maintenance in vivo. Lack of appropriate vasculature limits new tissue formation in the body to sizes of 100–200 μm, which is the diffusion limit of oxygen in highly cellular tissues [6]. However, the presence of vasculature, which is further discussed in chapter “Strategies for 3D Printing of Vascularized Bone”, within these tissues allows for the transport of the growth factors necessary to instruct bone regeneration, such as bone morphogenic protein (BMP) and vascular endothelial growth factor (VEGF) [6, 7]. Bioreactor culture not only improves the transport of nutrients and oxygen from surrounding media but also has a positive impact on the formation of vascular-like networks within these constructs. These systems also have the potential to culture BTE constructs in a manner that replicates the in vivo environment of bone. Due to the nature of bone acting as a supportive tissue within the body, forces of tension and compression are constantly applied. Because these forces heavily regulate bone metabolism, their application is included within several bioreactor systems to generate more functional bone-like structures.
Advantages of Bioreactor Culture:
-
1.
Homogenous cell seeding: Even cell seeding is essential in the formation of well-organized tissue within the scaffold [8]. Two dimensional cell seeding involves deposition of cell solution directly onto the scaffold and incubation for a period of time in static culture. Although this seeding method is relatively popular, it results in uneven matrix deposition in 3D bone tissue applications, typically on the periphery of the scaffolds, leading to nutrient gradients and cell death [8]. Perfusion bioreactor systems are used to combat this issue because they deliver cells and nutrients throughout BTE constructs [4, 9].
-
2.
Increased cell proliferation: Along with consistent cell distribution, bioreactor culture increases cell proliferation on scaffolds as well. Convection mechanisms of transport lead to improved delivery of nutrients and oxygen, as well as better waste removal processes that are linked to greater cell survival and proliferation.
-
3.
Application of mechanical loading and fluid shear stress: In order to more closely mimic the in vivo environment of bone, bioreactor systems are utilized to generate mechanical loading conditions and application of shear stress [2, 8]. The bone matrix is subjected to mechanical forces of cyclic loading while performing everyday functions. This loading cycle on the bone matrix then transmits forces to cells within the tissue via fluid shear through channels called canaliculi [8]. These forces trigger bone cells to activate the bone remodeling process in order to maintain the necessary mechanical strength that bone provides in the body [2]. Also, research suggests that application of fluid shear stress to mesenchymal stem cells (MSCs), the most heavily utilized stem cell source in BTE applications, increases their osteoblastic differentiation [10]. Both compression and magnetic force bioreactor systems recreate the cyclic loading conditions that bone experiences. Several bioreactor systems apply fluid shear directly to cells within cultured scaffolds, including spinner flasks and perfusion systems.
-
4.
Vascularization: Bioreactors are utilized to improve in vitro culture processes prior to scaffold implantation to produce more mature and complex tissue constructs [2]. One important aspect of generating these mature tissues in vitro includes scaffold vascularization, which will be discussed further in chapter “Strategies for 3D Printing of Vascularized Bone”. Bone vasculature provides the matrix with the necessary nutrients and oxygen it needs to maintain normal physiological functions, such as development, regeneration, and remodeling, and serves as a mechanism for waste removal [7]. Many vascularization strategies are available, but when combining a vascularization strategy with bioreactor culture, more highly organized and functional bone tissue substitutes can be generated. Both rotating wall vessels and perfusion systems have been utilized to produce preliminary vascular networks within tissue constructs, however there is still significant growth and optimization necessary to improve vascularization strategies [11].
-
5.
Biomanufacturing: Bioreactor systems allow for the generation and maintenance of clinically relevant volumes of engineered bone on the centimeter scale. This provides a clear advantage over traditional static culture techniques that can only produce constructs on the millimeter scale. These systems also enhance and expedite the production process through minimization of necessary culture times and monitoring. Because culture processes often produce variable results, bioreactors standardize these processes and allow for the generation of multiple patient-specific grafts in parallel, so as to ensure that patients receive the most effective graft.
-
6.
Clinical translation: To enhance the clinical potential for tissue engineering constructs, in vitro culture steps must be optimized to achieve maximum rate of production and automation, while minimizing risk of infection and necessary manual labor [2]. Bioreactor culture allows for cell seeding, proliferation, differentiation, and tissue maturation to occur within the same closed vessel, minimizing transfers of the cells and scaffolds during in vitro culture, and therefore minimizing contamination risks [2]. Its potential for automation, combined with increased nutrient delivery, and tissue maturation allows for the generation of BTE constructs of clinically relevant sizes by overcoming the size limitations that static culture conditions produce. These systems significantly augment the capacities of BTE scaffolds to serve as functional replacements for diseased or defective bone tissue in clinical scenarios.
2 Bioreactor Systems
Some commercially available bioreactor systems and their uses are presented in Table 1. Several bioreactor designs will be discussed in this chapter. These systems and a summary of their results are presented in Table 2
2.1 Spinner Flasks
Rationale and design: Spinner flask systems consist of a three-armed flask, magnetic stir bar, and scaffolds that are suspended in media via wires, needles, or thread [4]. A schematic of a spinner flask system is pictured in Fig. 1a. Typical stirring speeds are set between 30 and 50 rpm, so as to ensure dynamic mixing of media, but not to cause significant damage to cells seeded on the scaffolds [16, 18]. The rationale behind the use of spinner flasks for in vitro culture is that the dynamic mixing of media allows for convective transport of nutrients and oxygen to cells throughout the entire scaffold, which increases cell viability and proliferation [4, 16, 18]. Furthermore, the dynamic environment results in the application of fluid shear forces to cells seeded on the scaffolds, particularly on the peripheral regions, which also enhances osteogenic differentiation and bone extracellular matrix (ECM) deposition [17, 18, 44]. Also, spinner flasks are utilized as a means of cell seeding, which increases cellularity in comparison to static culture and other mechanisms of dynamic seeding [20].
Schematics of bioreactor systems. (a) Spinner flask. (b) Rotating wall vessel (arrow depicts rotation of outer cylinder) [2]. (c) Perfusion system. Perfusion chamber design varies with scaffold geometry. (Created with BioRender.com)
Applications: Several studies have investigated the effectiveness of spinner flasks for dynamic culture in BTE compared to other systems, such as rotating wall vessels (RWV), perfusion cartridges, and static culture. Sikavitsas et al. compared spinner flask culture to both RWV and static culture conditions of poly(lactic-co-glycolic acid) (PLGA) scaffolds seeded with rat bone marrow-derived MSCs [16]. In contrast with static conditions, scaffolds cultured in spinner flasks demonstrated increased cellularity, as well as an upregulation in markers of osteogenic differentiation, demonstrating the positive effect of the dynamic mixing environment on the culture of BTE constructs. However, both Sikavitsas et al. and Meinel et al. observed that mineralization occurs mostly on the outer periphery of these constructs, whereas other systems produce more homogenous mineral deposition [16, 17]. These studies demonstrate that spinner flask systems generate an uneven distribution of shear stress to cells within 3D scaffolds, resulting in uneven bone tissue development. Kim et al. sought to investigate how manipulation of scaffold design can eliminate this uneven tissue formation. By generating silk scaffolds with macroscale pores, more homogenous ECM deposition and mineralization was observed [18]. However, the use of larger scale pores resulted in mechanical properties that are less than desirable for BTE applications. This highlights the need for optimization strategies of both scaffold design and culture conditions to balance desired tissue strength and homogenous tissue formation.
One available strategy for understanding, predicting, and optimizing nutrient transport across 3D scaffolds is computational fluid dynamics (CFD) analysis. In the case of spinner flask culture systems, various stir bar rotation speeds have been examined to determine velocity fields and shear rates within the culture vessel, providing greater insight about processing parameters that yield homogenous bone tissue formation. Most CFD analyses in spinner flask systems have been conducted to investigate optimal environments for culture of articular cartilage tissue. However, Melke et al. predicted and examined the localization of mineral deposits within silk fibroin scaffolds seeded with human bone marrow-derived MSCs as a function of wall shear stress generated by different stir bar speeds [19]. This analysis demonstrated that with increasing stirring speeds, mineral deposits were more homogeneously distributed. Although this modeling approach is a useful tool for optimization of culture parameters, cell culture experiments conducted in parallel observing cell viability as a function of shear stress generated could provide greater insight on producing more homogenous bone tissue constructs.
These systems have also been examined as a means of dynamic cell seeding for tissue engineering constructs [20, 45]. Griffon et al. investigated a variety of dynamic seeding mechanisms, including spinner flasks, perfusion bioreactors, and orbital shakers for seeding of mouse-derived MSCs on a variety of scaffold materials. Spinner flasks demonstrated the greatest levels of DNA and cellularity in comparison to other culture methods [20]. However, other groups have seen greater success in cell seeding with perfusion bioreactors in comparison to spinner flask systems [45].
Limitations: A major restriction is the size of the constructs that can be successfully cultured using these systems. This is due to a difficult and delicate balance between convective transport via stirring and the resultant shear stress. In order to increase nutrient transport, stirring speeds are increased, which applies more shear stress to cells on the outer edges of scaffolds [4]. As these scaffolds are cultured over time, this results in greater matrix and mineral deposition on the periphery of scaffolds, creating a sharp nutrient gradient and inefficient waste removal [16, 17] on the inner portions of scaffolds, leading to necrosis [4]. The resulting non-homogeneous distribution of the deposited bone matrix severely impacts the mechanical integrity of these constructs. Because of these associated issues, culture of clinically relevant volumes of bone tissue is not currently possible using this method. The capacity of spinner flask systems to increase osteogenic differentiation and homogeneous bone matrix formation in bone tissue constructs seems promising, but significant optimization of both computational analyses and cell culture experiments needs to be conducted in order to enhance these systems’ capabilities.
2.2 Rotating Wall Vessels
Rationale and design: The rotating wall vessel (RWV) bioreactor system, which is represented in the schematic in Fig. 1b, consists of two concentric cylinders, with a rotating outer cylinder and stationary inner cylinder, which contains an oxygen-permeable membrane for gas exchange [2, 4]. Media and scaffolds are present in the space between the two cylinders. RWV bioreactors create a microgravity environment in which scaffolds are in a constant state of “free fall” by balancing drag forces, centrifugal forces, and net gravitational forces [4, 8]. This design results in lower shear stresses and turbulence with increased nutrient delivery [8]. Several variations of these systems exist, including slow turning lateral vessels (STLV) and high-aspect ratio vessels (HARV). STLV systems allow for greater control over oxygen supply, pH, and temperature of the culture vessel, and HARV systems have reduced speeds and improved gas exchange [4].
Applications: One application of RWV bioreactors is to simulate the effects of “weightlessness” on the culture of bone-forming cells. The interest in studying these effects stems from observations of bone loss due to space travel, leading researchers to believe that lack of mechanical stimulation of the tissue in microgravity environments alters bone metabolic processes. Rucci et al. utilized a RWV to culture rat osteoblast-like cells that formed organoids to observe the impact of weightlessness on osteoblastic phenotype [21]. In comparison to cells cultured in a traditional static environment, RWV-cultured cells displayed upregulations in markers indicative of osteoclastic phenotypes, demonstrating that this environment can result in greater bone resorption. Similar studies evaluated the impact of microgravity on osteogenic differentiation of rat and human bone marrow-derived MSCs. Decreased proliferation and osteoblastic differentiation were observed [22], as well as upregulations in markers associated with adipogenic differentiation [23, 24]. These studies provide great insight into the underlying mechanisms of bone loss that result from microgravity environments.
To determine the efficacy of RWV to culture constructs for BTE applications, Sikavitsas et al. compared the use of RWV systems to spinner flask and static conditions for the culture of rat MSCs on PLGA scaffolds [16]. Cell proliferation and osteogenic differentiation were significantly lower in RWV than other culture conditions, leading researchers to believe that scaffold collisions with the culture vessel were detrimental to these processes. To overcome the issues associated with scaffold-wall collisions, Botchwey et al. and Yu et al. investigated the use of lighter than water (LTW) and heavier than water (HTW) PLGA microcarriers to culture osteoblast-like cells [25, 26]. The purpose of these studies was to manipulate the densities of these scaffolds to obtain greater control over scaffold movement within the vessels, therefore optimizing culture parameters to lead to increased osteogenesis. By varying the ratios of HTW and LTW microspheres, they were able to achieve a more homogenous cell distribution and upregulation in osteogenic markers in RWV systems [26]. Another optimization study conducted by Varley et al. investigated ideal operating parameters of both single and dual axis rotational reaction vessels on fetal human osteoblast proliferation on collagen-glycosaminoglycan (GAG) scaffolds [27]. By determining optimal media fill volume and rotational speed of both RWV systems, cell proliferation increased significantly in comparison to static controls. Even though this optimization strategy improved cell growth on the scaffolds within RWV structures, the researchers suggested that developing a variation of the RWV structure including perfusion could significantly enhance in vitro culture [27].
Limitations: Although some RWV systems demonstrate upregulation in osteogenic markers, they are not significantly different from static cultures. The microgravity environment results in low shear applications to cells within the scaffolds, which was initially hypothesized to lead to greater cell survival [8]. These vessels provide a mechanism to understand the impact of weightlessness on bone tissue formation, but they remain relatively ineffective for culturing regenerative BTE constructs. Scaffold collisions with the outer vessel walls also lead to decreased cell survival and disruption of bone tissue formation [2, 16]. To combat this, a variation was designed in which scaffolds are fixed on the outer wall, which was demonstrated to increase cell proliferation, ECM deposition, and mineralization [2]. Another variation that was developed by Zhang et al. combined perfusion and biaxial rotation (BXR) within the same culture vessel, which led to increased proliferation and upregulation of osteogenic markers in comparison to spinner flasks, RWV, and perfusion systems [46]. Although these results are promising, researchers are turning to other dynamic culture systems due to the lack of efficacy observed in RWV systems.
2.3 Perfusion Systems
Rationale and design: Perfusion-based systems typically consist of a media reservoir, pump, and a perfusion cartridge which contains the scaffold, represented in Fig. 1c [2]. Scaffolds are press-fit to the bioreactor cartridges to allow media to perfuse directly through scaffold pores, rather than around the scaffold [2]. Two main design types for perfusion cartridges include packed and fluidized beds. Packed beds are typically used for microparticle-based scaffolds or scaffolds consisting of one piece [8]. Fluidized bed designs are mainly used for microparticle or particulate based biomaterials, which are mobilized due to the fluid flow of dynamic culture [8]. However, numerous perfusion bioreactor designs exist because their design is reliant on scaffold architecture. Media is perfused directly through scaffold pores in perfusion systems, resulting in more uniform cell seeding, osteogenic differentiation, and bone matrix production [8]. The efficiency of these systems is co-dependent on optimal scaffold properties, such as porosity [2, 47]. Because of the exciting potential that these systems have to improve the generation of bone-like tissue in vitro, several groups have also applied these systems to produce constructs of clinically relevant size, which will be discussed later in this chapter.
Applications: Because of the need to create scaffold-specific perfusion cartridges, several designs have been investigated in the context of BTE. One design that has been utilized is the tubular perfusion system (TPS) bioreactor [29,29,31]. Developed by Yeatts et al., this system was used to culture hMSCs in alginate beads to test the growth and differentiation of the cells within the system, as well as the effects of flow rate on these processes. Perfusion culture increased viability, proliferation, and osteogenic differentiation of hMSCs within the scaffolds [29]. The same group then investigated the effects of dynamic culture and application of shear on osteogenic differentiation and proliferation of hMSCs as a function of radial position within the same scaffolds. Over the course of the culture period, increases in DNA and osteogenic marker expression levels were observed in dynamically cultured scaffolds, whereas statically cultured scaffolds suffered from greater levels of cell death, specifically in larger scale constructs [30]. This demonstrates the capability of bioreactor culture to mitigate nutrient and oxygen diffusion limitations associated with static culture. The TPS system was used again by Yeatts et al. to culture hMSCs on electrospun nanofibrous PLGA/poly(ε-caprolactone) (PCL) scaffolds to understand the impact of dynamic culture on in vivo bone formation [31]. Bioreactor-cultured scaffolds exhibited the greatest increase in new bone area and better integration with host tissue, which demonstrates the positive effects of in vitro dynamic culture on bone tissue healing in vivo.
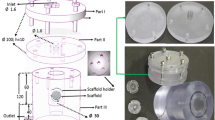
To demonstrate the capability of perfusion systems to produce complex anatomical structures of bone tissue, Grayson et al. developed a bioreactor system specifically to culture temporomandibular joints (TMJ) from decellularized bovine bone scaffolds and hMSCs [28]. As shown in Fig. 2, anatomical geometry was obtained via computed tomography (CT) imaging, which was then used to generate the polydimethylsiloxane (PDMS) fitting mechanism for the perfusion cartridge and the computer numerical control (CNC) milled scaffolds [28]. Static conditions resulted in the formation of bone matrix and mineralization primarily on the periphery of the scaffolds, while bioreactor culture groups exhibited a more even distribution of matrix formation and greater cell proliferation [28]. This not only validates the advantages of bioreactor culture over static conditions, but also the flexibility of these systems to culture anatomically accurate bone grafts.
Tissue engineering of anatomically shaped bone grafts. (a, b) Clinical CT imaging techniques were used to generate 3D models of TMJ. (c) Resulting machined decellularized bone scaffold. (d) Resulting scaffolds from different CT scans. (e) Culture schematic and (f) photograph of bioreactor utilized to culture TMJ constructs. (g–i) Assembly process of bioreactor. (Image adapted from [28])
Frölich et al. compared static and perfusion culture conditions in control and osteogenic media using human adipose-derived stem cells (hASCs)-seeded decellularized bone scaffolds [32]. Although increases in mineralization and ECM deposition were observed, they were not statistically different from static controls, potentially due to low fluid shear rates [32]. Results such as this highlight the need for flow rate optimization studies to maximize the effectiveness of in vitro culture.
Grayson et al. conducted a flow rate optimization study to determine the impact of this parameter on osteogenic differentiation of hMSCs on decellularized bovine bone plug scaffolds in a perfusion bioreactor [3]. Flow rates and their corresponding interstitial fluid flow were determined via mathematical modeling. By observing cell and ECM distribution and gene expression, they were able to determine an optimal range of flow rates that enhanced the process of bone formation in the system. Another variation of flow optimization studies was conducted by Kim et al. by investigating the effects of parallel and transverse media flow through scaffold pores. By culturing hMSCs on polyethylene terephthalate (PET) scaffolds, it was demonstrated that transverse flow conditions increased the rate of MSC differentiation toward an osteoblastic lineage, whereas parallel flow allowed them to maintain greater proliferative potential [33]. Direction of perfusion was also investigated for its effects on osteogenic differentiation of hMSCs in alginate bead scaffolds, as a function of scaffold position within a TPS bioreactor. Nguyen et al. hypothesized that cells within these scaffolds at the inlet and outlet regions of the perfusion bioreactor experience different biochemical cues that guide osteogenic differentiation due to their differences in position [34]. Greater levels of osteogenic marker expression were observed toward the outlet region under unidirectional flow. Alternating the flow direction after 24 h mitigated these differences. The culmination of these results demonstrates the significant effort required to optimize perfusion systems in order to foster homogenous bone tissue formation.
Limitations: Although these systems show great promise for the culture of regenerative BTE constructs, several limitations exist. First, these systems are prone to leakage and contamination due to ill-fitting connections within the system. Significant optimization of processing parameters is also necessary. Ideal perfusion flow rates are necessary to determine, so as to aid in the process of osteogenic differentiation, but not to cause significant cell death within the scaffold. Bioreactor and scaffold designs must also be tuned in order to maximize the effects of the in vitro culture process. However, the use of CFD analyses allows for significant optimization within these areas prior to experimentation. Combining these analyses with perfusion bioreactor systems provides more standardized methods for producing clinically relevant volumes of bone tissue. Because perfusion systems overcome the issues associated with other bioreactor systems and static culture, these bioreactors and their derivatives present the most potential for use in clinical scenarios.
2.4 Mechanical Force Systems
Rationale and design: The objective of mechanical force bioreactor systems is to more accurately mimic the mechanical loading forces, such as tension and compression, in the in vivo bone microenvironment. These forces are a result of regular physical movements such as walking, running, or jumping and are instrumental in guiding bone growth, remodeling, and other metabolic processes [4, 8]. Several designs have been investigated in the context of BTE, but the most common includes the application of compressive forces, which is often used in combination with perfusion. The synergistic relationship between fluid perfusion and compressive forces both mimics natural loading conditions of bone, while also recreating the in vivo microenvironment of interstitial canalicular flow in response to those loading conditions. These systems are typically composed of similar elements as traditional perfusion systems, but culture chambers include mechanisms for compressive force application, such as a piston driven by pneumatic pressure. Using compression systems to apply physiologically relevant loads in cyclic loading conditions increases cellularity, matrix production, and osteogenic gene expression [8]. Another variation is the magnetic force bioreactor (MFBs), in which magnetic nanoparticles are attached to the cell membrane and force is applied via a magnetic field [4]. This system allows for forces to be applied directly to the cell membrane, rather than to the scaffold and then transmitted to cells. Because other systems require an external mechanism for forces of compression, this damages scaffold integrity and increases risk of infection. These are the limitations that magnetic force bioreactors seek to address. Both compression and magnetic force systems are represented in Fig. 3.
Schematics of mechanical force bioreactor systems. (a) Compression bioreactor—arrows indicate direction of force applied to the system & the scaffold. (b) Magnetic force bioreactor—arrows indicate direction of movement of magnetic field and nanoparticle. (Created with Biorender.com)
Applications: Matziolis et al. developed a compression-based system to simulate the in vivo fracture healing environment and investigate cell response [35]. The upper membrane of the system, driven by pneumatic pressure, applied compressive forces that mimic the cyclic loading cycle experienced by bone. Mechanically loaded scaffolds demonstrated an increase in ECM deposition and calcification, validating that the application of compressive forces aids in the process of bone tissue formation [35].
The application of compressive forces has been demonstrated to upregulate osteogenesis, but several groups investigated the use of compression-perfusion culture systems to more accurately replicate the in vivo fluid microenvironment of bone [36, 37]. Both Bölgen et al. and Jagodinski et al. conducted studies comparing perfusion, compression-perfusion, and static conditions. Although both dynamic environments resulted in homogenous tissue formation, markers of osteogenesis were significantly upregulated in compression-perfusion cultures, demonstrating the potential for these combined systems to enhance the process of bone tissue development.
One mechanical loading bioreactor system that has been investigated extensively for its use in culturing bone explants is the Zetos system [38,38,40, 48, 49]. Developed by Davies et al., this system has been utilized to culture human, bovine, and ovine trabecular bone explants to determine the effect of mechanical loading on cell and tissue response. Mann et al. investigated how mechanical loading simulating a jumping exercise impacted cellular apoptosis and bone formation in human trabecular bone. Their study established that mechanical stimulation decreased levels of apoptosis, whereas unloaded conditions resulted in decreased osteocyte viability and increased apoptotic behavior [38]. This same system was employed to investigate its effects on the mechanical properties of bovine bone, demonstrating that cyclic compression increases the thickness of trabeculae and compressive modulus [39, 40]. Endres et al. investigated several loading conditions for their effects on bovine bone stiffness and osteoid deposition, finding that stiffness increases with applied strain, but also depends on the amount of matrix deposited [49]. Overall, these studies demonstrate that viable bone explants are better maintained under loading conditions, but the system has yet to be applied to engineered bone tissue.
Previous studies reported various levels of loading conditions, all of which seemed to have a positive impact on bone formation or mechanical properties of the engineered or explanted constructs. However, Ravichandran et al. sought to investigate how physiologically relevant strains impact early osteogenesis on constructs in a compression bioreactor [41]. This study demonstrated that physiological loading conditions caused an upregulation in osteogenic markers and ECM calcification earlier in the culture period than other strain levels and static controls. This presents a potential benefit for future clinical translation because culture times can be reduced due to the increased rate of bone tissue formation.
Another variation of mechanical loading bioreactors are MFBs. These systems apply different mechanical forces directly to cell membranes via membrane-attached magnetic nanoparticles [4]. The magnitude of the force applied changes based on the strength of the magnetic field. In comparison to other mechanical conditioning bioreactor systems, this system has several advantages, such as lesser risk of infection, precise control over applied force, no necessary optimization of scaffold parameters, and a scalable system [4]. Several groups have investigated the use of these bioreactors in BTE [42, 43]. Kanczler et al. targeted membrane potassium channels and integrin receptors to assess how loading on these targeted proteins affects osteogenesis and chondrogenesis in hMSCs. Markers of both processes were significantly upregulated when these proteins were mechanically stimulated. [42] Henstock et al. targeted the same membrane proteins in hMSCs and injected these mechanically conditioned cells into a chick fetal femur model for endochondral bone formation [43]. Mechanically stimulated cells mineralized the injection site better than unlabeled controls. The culmination of these results demonstrates the potential for MFBs to enhance bone regeneration by stimulating specific membrane proteins. However, it has yet to be investigated how they compare to other well-established systems.
Limitations: One major limitation associated with the design of mechanical force bioreactors is that the mechanisms that apply compressive forces enter the sterile bioreactor environment, which leads to potential contamination [4]. While mechanical conditioning has been demonstrated to increase the mechanical properties of BTE constructs, the scaffold material must withstand the forces applied over the culture period without rapid degradation in order to maintain relevance for clinical applications. It is therefore necessary to optimize scaffold material properties and fabrication strategy in order to ensure compatibility with these systems. Optimization of scaffold properties and operating conditions is challenging, but the use of finite element analysis and computational modeling aids in this process in silico prior to experimentation [50,50,52].
3 Biomanufacturing and Scale-Up
One of the greatest challenges is translating bioreactor technologies to a clinical setting. Several factors influence their translational capacity, but the most prevalent is their potential to generate clinically relevant volumes of bone. Although dynamic culture enhances osteogenesis and cell survival within scaffolds, the scale at which they are typically produced is that of a small in vivo animal model. Several groups have recently demonstrated that these systems can maintain clinically relevant volumes of bone for larger animal studies or human-sized defects, and they are summarized in Table 3. Most of these systems consist of perfusion bioreactors, but several groups have also investigated the use of in vivo bioreactors. In vivo bioreactors consist of a polymethyl methacrylate (PMMA) chamber filled with autograft or synthetic bone substitutes that are implanted into the rib periosteum to allow for ingrowth of vasculature and tissue development. The tissue developed in this chamber is then resected and used to repair a defect site within the same animal model.
Because perfusion systems demonstrated the most potential for BTE applications, these systems have also been applied in the scale-up movement for clinical relevance. Gardel et al. designed a perfusion-based system called the bidirectional continuous perfusion bioreactor (BCPB), in which the scaffold chamber consists of a center tube with perforations that the scaffold surrounds [53]. Using this system, researchers maintained the culture of a 42-mm thick scaffold containing goat bone marrow-derived MSCs. Similarly, Kleinhans et al. used another perfusion system to culture hMSCs on large-scale poly(l-lactide-co-caprolactone) (P[LLA-co-CL]) porous scaffolds [60]. Comparing static and dynamic seeding of cells, bioreactor-cultured constructs demonstrated more homogenous cell distributions, as well as an upregulation of markers of osteogenesis. These studies validate the feasibility in culturing large-scale constructs and inducing bone tissue formation through the use of bioreactor culture.
To confirm the possibility of culturing BTE constructs mimicking anatomical geometry and size of the greater half of a human femur, Nguyen et al. utilized a larger scale TPS bioreactor to culture hMSC-laden alginate beads, creating a volume of 200 cm3 of bone-like tissue, shown in Fig. 4 [54]. Cell viability was successfully maintained throughout the entire tissue. Although osteogenic marker expression was upregulated toward outer portions of the construct, the researchers hypothesized that this could lead to the development of cortical and cancellous bone tissue within the femur, more closely mimicking the structure of native bone. This study establishes both the scalability and the exciting potential of dynamic culture systems to maintain clinically relevant volumes and geometries of BTE constructs.
Design, fabrication, and culture of human femur graft. (a) 3D CAD model of superior half of human femur. (b) 3D printed mold containing cell-laden alginate beads. (c) Aggregated construct. (d) Image of large scale TPS bioreactor setup. (Image adapted from [54])
Several in vivo studies have been conducted utilizing large-scale engineered bone tissue constructs cultured in dynamic conditions. Janssen et al. adapted a direct perfusion bioreactor system to culture goat bone marrow-derived MSCs or hMSCs on β-TCP scaffolds with an online monitoring system of oxygen consumption [55, 56]. Although no concrete differences were observed between static and dynamically cultured constructs in vivo, the addition of online monitoring capabilities increases the clinical relevance by reducing the possibility of infection, minimizing transfers, and reducing the necessary monitoring procedures. To demonstrate the ability of these systems to be used in larger in vivo models, Wang et al. developed a perfusion-based system to culture goat bone marrow-derived MSCs on β-TCP scaffolds for goat tibial defects [57]. Constructs cultured dynamically exhibited greater mineral deposition and host tissue integration in vivo. While these results show promise for the use of dynamically cultured constructs in vivo, further research is needed to understand exactly how these culture environments influence bone tissue formation.
Another bioreactor design for the generation of large-scale BTE constructs is the in vivo bioreactor. Tatara et al. utilized this design to compare autograft and synthetic materials and their potential to regenerate mandibles in sheep models [58, 59]. Although these materials exhibited significantly lower bone densities than native mandibles when evaluated after resection, this study revealed the feasibility of this approach to generate vascularized bone tissue substitutes. The group also used 3D printed bioreactors of the same design to culture similar materials [61]. Using 3D printing to fabricate bioreactors allows for the culture and maintenance of patient-specific grafts, further increasing clinical translation potential. These strategies utilize common clinical imaging techniques, such as CT or MRI, to obtain scans of the patient defect, which can be reconstructed into 3D models that are compatible with printing software.
These studies validate the potential for bioreactor platforms to produce larger scale constructs of both clinically relevant size and shape. Although several in vivo studies have been conducted, further research is needed to understand the relationship between dynamic culture and bone regeneration. There are also several other factors that need to be optimized before these technologies are able to be utilized as new clinical standards of care.
4 Future Considerations for Clinical Translation
Bioreactor systems are utilized to overcome the issues associated with static culture of three-dimensional constructs. However, several improvements need to be made to increase their relevance to clinical applications.
4.1 Culture of Patient-Specific Grafts
Generation of patient-specific grafts from synthetic materials and readily available cells would mitigate the need for harvesting autologous tissue. Because these defects often require complex geometrical structures, it is necessary to find methods to successfully generate and culture these constructs. Traditional clinical imaging techniques can be utilized to generate 3D models of the defect site and additive manufacturing technologies can be used to fabricate both synthetic grafts and bioreactors for dynamic culture. Costa et al. utilized additive manufacturing technologies to fabricate scaffolds and a perfusion bioreactor chamber for ovine tibia defects [62]. After conducting computational studies to optimize fluid flow rate in these bioreactors, scaffolds were seeded and cultured with primary human osteoblasts, resulting in relatively high cell viability [62]. Although this shows promise for the use of 3D printing technologies as a means of fabrication of bioreactors, further research is needed to confirm the potential of enhancing osteogenesis within these systems in comparison to static culture. Not only would 3D-printed bioreactors allow for the generation of patient specific grafts, but they would also enhance the process of biomanufacturing such scaffolds due to the ease with which these systems could be fabricated. This would be beneficial for the production of multiple patient-specific grafts at one time because the culture of 3D constructs varies significantly based on processing parameters and cell behavior. Generating multiple grafts at one time would allow for the “best” graft to be implanted [63]. However, optimizing processing parameters and cell seeding could further improve bioreactor systems.
4.2 Automation and Monitoring
Another crucial step toward clinical translation for these bioreactor systems is the inclusion of monitoring systems and automation of processes such as cell seeding and culture. Current research strategies rely heavily on manual labor and the technique of researchers. However, in order for these systems to be implemented on a large biomanufacturing scale, the level of manual labor and manipulation of tissue engineered constructs must be reduced in order to decrease variability. First, these systems should be implemented for cell seeding, rather than relying on manual seeding methods. Also, online and in situ monitoring of culture environment would reduce manual labor. Several groups have investigated the use of oxygen monitoring systems within culture vessels to monitor tissue culture without having to manipulate the constructs [55, 56]. These systems can be further improved by introducing feedback monitoring loops of quantitative markers for osteogenesis [63]. The generation of imaging-compatible bioreactor systems to monitor the growth and development of tissue engineered bone has also been investigated [63]. In addition to quantifying growth, online monitoring systems should be designed for easy manipulation without significant technical training. Automating these processes and designing user-friendly interfaces would optimize the potential for clinical translation and significantly reduce the variability associated with traditional tissue culture techniques.
4.3 Optimization
The greatest challenge that researchers face in translating bioreactor systems to clinical scenarios involves the process of optimization. Although bioreactors have been investigated extensively in the context of BTE, each specific design requires optimization of processing parameters so as to generate the most functional tissue constructs. From the cell type to flow rates and culture times, there are several variables that need to be optimized and standardized in order to make these systems more clinically relevant. For this reason, computational fluid dynamic studies within specific culture vessels should be conducted prior to experimentation. This will allow for the most ideal generation of synthetic bone grafts. Although these systems show great promise, significant research is needed in establishing a standardized use of bioreactors before they can be translated to the clinics.
References
Amini AR, Laurencin CT, Nukavarapu SP. Bone tissue engineering: recent advances and challenges. Crit Rev Biomed Eng. 2012;40:363. https://doi.org/10.1615/CritRevBiomedEng.v40.i5.10.
Yeatts AB, Fisher JP. Bone tissue engineering bioreactors: dynamic culture and the influence of shear stress. Bone. 2011;48:171. https://doi.org/10.1016/j.bone.2010.09.138.
Grayson WL, et al. Optimizing the medium perfusion rate in bone tissue engineering bioreactors. Biotechnol Bioeng. 2011;108:1151. https://doi.org/10.1002/bit.23024.
El Haj AJ, Cartmell SH. Bioreactors for bone tissue engineering. Proc Inst Mech Eng H J Eng Med. 2010;224:1523. https://doi.org/10.1243/09544119JEIM802.
Kim YS, Majid M, Melchiorri AJ, Mikos AG. Applications of decellularized extracellular matrix in bone and cartilage tissue engineering. Bioeng Transl Med. 2019;4:83. https://doi.org/10.1002/btm2.10110.
Rouwkema J, Koopman BFJM, Blitterswijk CAV, Dhert WJA, Malda J. Supply of nutrients to cells in engineered tissues. Biotechnol Genet Eng Rev. 2010;26:163. https://doi.org/10.5661/bger-26-163.
Filipowska J, Tomaszewski KA, Niedźwiedzki Ł, Walocha JA, Niedźwiedzki T. The role of vasculature in bone development, regeneration and proper systemic functioning. Angiogenesis. 2017;20:291. https://doi.org/10.1007/s10456-017-9541-1.
Carpentier B, Layrolle P, Legallais C. Bioreactors for bone tissue engineering. Int J Artif Organs. 2011;34:259. https://doi.org/10.5301/IJAO.2011.6333.
Martin Y, Vermette P. Bioreactors for tissue mass culture: design, characterization, and recent advances. Biomaterials. 2005;26:7481. https://doi.org/10.1016/j.biomaterials.2005.05.057.
Yourek G, McCormick SM, Mao JJ, Reilly GC. Shear stress induces osteogenic differentiation of human mesenchymal stem cells. Regen Med. 2010;5:713. https://doi.org/10.2217/rme.10.60.
Lovett M, Lee K, Edwards A, Kaplan DL. Vascularization strategies for tissue engineering. Tissue Eng Part B Rev. 2009;15:353. https://doi.org/10.1089/ten.teb.2009.0085.
Hansmann J, Groeber F, Kahlig A, Kleinhans C, Walles H. Bioreactors in tissue engineering-principles, applications and commercial constraints. Biotechnol J. 2013;8:298. https://doi.org/10.1002/biot.201200162.
Hutmacher DW, et al. Bioreactor for growing cell or tissue cultures. U.S. Patent Application Publication; 2006.
Ahmed S, Chauhan VM, Ghaemmaghami AM, Aylott JW. New generation of bioreactors that advance extracellular matrix modelling and tissue engineering. Biotechnol Lett. 2019;41:1. https://doi.org/10.1007/s10529-018-2611-7.
Buesch S, Schroeder J, Bunger M, D’Souza T, Stosik M. A novel in vitro liver cell culture flow system allowing long-term metabolism and hepatotoxicity studies. Appl Vit Toxicol. 2018; 4:232. https://doi.org/10.1089/aivt.2018.0009
Kim HJ, et al. Bone regeneration on macroporous aqueous-derived silk 3-D scaffolds. Macromol Biosci. 2007;7:643. https://doi.org/10.1002/mabi.200700030.
Melke J, Zhao F, Rietbergen B, Ito K, Hofmann S. Localisation of mineralised tissue in a complex spinner flask environment correlates with predicted wall shear stress level localisation. Eur Cells Mater. 2018;36:57. https://doi.org/10.22203/eCM.v036a05.
Griffon DJ, Abulencia JP, Ragetly GR, Fredericks LP, Chaieb S. A comparative study of seeding techniques and three-dimensional matrices for mesenchymal cell attachment. J Tissue Eng Regen Med. 2011;5:169. https://doi.org/10.1002/term.302.
Rucci N, Migliaccio S, Zani BM, Taranta A, Teti A. Characterization of the osteoblast-like cell phenotype under microgravity conditions in the NASA-approved rotating wall vessel bioreactor (RWV). J Cell Biochem. 2002;85:167. https://doi.org/10.1002/jcb.10120.
Sikavitsas VI, Bancroft GN, Mikos AG. Formation of three-dimensional cell/polymer constructs for bone tissue engineering in a spinner flask and a rotating wall vessel bioreactor. J Biomed Mater Res. 2002;62:136. https://doi.org/10.1002/jbm.10150.
Meinel L, et al. Bone tissue engineering using human mesenchymal stem cells: effects of scaffold material and medium flow. Ann Biomed Eng. 2004;32:112. https://doi.org/10.1023/B:ABME.0000007796.48329.b4.
Gomes ME, Sikavitsas VI, Behravesh E, Reis RL, Mikos AG. Effect of flow perfusion on the osteogenic differentiation of bone marrow stromal cells cultured on starch-based three-dimensional scaffolds. J Biomed Mater Res A. 2003;67:87. https://doi.org/10.1002/jbm.a.10075. PMID: 14517865
Chen X, et al. Mechanical stretch-induced osteogenic differentiation of human jaw bone marrow mesenchymal stem cells (hJBMMSCs) via inhibition of the NF-κ B pathway. Cell Death Dis. 2018; 9:207. https://doi.org/10.1038/s41419-018-0279-5.
Nettelhoff L, et al. Influence of mechanical compression on human periodontal ligament fibroblasts and osteoblasts. Clin Oral Investig. 2016; 20:621. https://doi.org/10.1007/s00784-015-1542-0.
Chen J, et al. Chromium oxide nanoparticle impaired osteogenesis and cellular response to mechanical stimulus. Int J Nanomedicine. 2021; 16:6157. https://doi.org/10.2147/IJN.S317430.
Dai ZQ, Wang R, Ling SK, Wan YM, Li YH. Simulated microgravity inhibits the proliferation and osteogenesis of rat bone marrow mesenchymal stem cells. Cell Prolif. 2007;40:671. https://doi.org/10.1111/j.1365-2184.2007.00461.x.
Zayzafoon M, Gathings WE, McDonald JM. Modeled microgravity inhibits osteogenic differentiation of human mesenchymal stem cells and increases adipogenesis. Endocrinology. 2004;145:2421. https://doi.org/10.1210/en.2003-1156.
Sheyn D, Pelled G, Netanely D, Domany E, Gazit D. The effect of simulated microgravity on human mesenchymal stem cells cultured in an osteogenic differentiation system: a bioinformatics study. Tissue Eng Part A. 2010;16:3403. https://doi.org/10.1089/ten.tea.2009.0834.
Botchwey EA, Pollack SR, Levine EM, Laurencin CT. Bone tissue engineering in a rotating bioreactor using a microcarrier matrix system. J Biomed Mater Res. 2001;55:242. https://doi.org/10.1002/1097-4636(200105)55:2<242::AID-JBM1011>3.0.CO;2-D.
Yu X, Botchwey EA, Levine EM, Pollack SR, Laurencin CT. Bioreactor-based bone tissue engineering: the influence of dynamic flow on osteoblast phenotypic expression and matrix mineralization. Proc Natl Acad Sci U S A. 2004;101:11203. https://doi.org/10.1073/pnas.0402532101.
Varley MC, Markaki AE, Brooks RA. Effect of rotation on scaffold motion and cell growth in rotating bioreactors. Tissue Eng A. 2017;23:522. https://doi.org/10.1089/ten.tea.2016.0357.
Grayson WL, et al. Engineering anatomically shaped human bone grafts. Proc Natl Acad Sci U S A. 2010;107:3299. https://doi.org/10.1073/pnas.0905439106.
Yeatts AB, Fisher JP. Tubular perfusion system for the long-term dynamic culture of human mesenchymal stem cells. Tissue Eng Part C Methods. 2011;17:337. https://doi.org/10.1089/ten.tec.2010.0172.
Yeatts AB, Geibel EM, Fears FF, Fisher JP. Human mesenchymal stem cell position within scaffolds influences cell fate during dynamic culture. Biotechnol Bioeng. 2012;109:2381. https://doi.org/10.1002/bit.24497.
Yeatts AB, et al. In vivo bone regeneration using tubular perfusion system bioreactor cultured nanofibrous scaffolds. Tissue Eng Part A. 2014;20:139. https://doi.org/10.1089/ten.tea.2013.0168.
Fröhlich M, et al. Bone grafts engineered from human adipose-derived stem cells in perfusion bioreactor culture. Tissue Eng Part A. 2010;16:179. https://doi.org/10.1089/ten.tea.2009.0164.
Kim J, Ma T. Perfusion regulation of hMSC microenvironment and osteogenic differentiation in 3D scaffold. Biotechnol Bioeng. 2012;109:252. https://doi.org/10.1002/bit.23290.
Nguyen BNB, Ko H, Fisher JP. Tunable osteogenic differentiation of hMPCs in tubular perfusion system bioreactor. Biotechnol Bioeng. 2016;113:10805. https://doi.org/10.1002/bit.25929.
Matziolis G, et al. Simulation of cell differentiation in fracture healing: mechanically loaded composite scaffolds in a novel bioreactor system. Tissue Eng. 2006;12:201. https://doi.org/10.1089/ten.2006.12.201.
Bölgen N, et al. Three-dimensional ingrowth of bone cells within biodegradable cryogel scaffolds in bioreactors at different regimes. Tissue Eng Part A. 2008;14:1743. https://doi.org/10.1089/ten.tea.2007.0277.
Jagodzinski M, et al. Influence of perfusion and cyclic compression on proliferation and differentiation of bone marrow stromal cells in 3-dimensional culture. J Biomech. 2008;41:1885. https://doi.org/10.1016/j.jbiomech.2008.04.001.
Mann V, Huber C, Kogianni G, Jones D, Noble B. The influence of mechanical stimulation on osteocyte apoptosis and bone viability in human trabecular bone. J Musculoskelet Neuronal Interact. 2006;6:408.
David V, et al. Ex vivo bone formation in bovine trabecular bone cultured in a dynamic 3D bioreactor is enhanced by compressive mechanical strain. Tissue Eng Part A. 2008;14:117. https://doi.org/10.1089/ten.a.2007.0051.
Vivanco J, et al. Apparent elastic modulus of ex vivo trabecular bovine bone increases with dynamic loading. Proc Inst Mech Eng Part H J Eng Med. 2013;227:904. https://doi.org/10.1177/0954411913486855.
Ravichandran A, et al. In vitro cyclic compressive loads potentiate early osteogenic events in engineered bone tissue. J Biomed Mater Res B Appl Biomater. 2017;105:2366. https://doi.org/10.1002/jbm.b.33772.
Kanczler JM, et al. Controlled differentiation of human bone marrow stromal cells using magnetic nanoparticle technology. Tissue Eng Part A. 2010;16:3241. https://doi.org/10.1089/ten.tea.2009.0638.
Henstock JR, Rotherham M, Rashidi H, Shakesheff KM, El Haj AJ. Remotely activated mechanotransduction via magnetic nanoparticles promotes mineralization synergistically with bone morphogenetic protein 2: applications for injectable cell therapy. Stem Cells Transl Med. 2014;3:1363. https://doi.org/10.5966/sctm.2014-0017.
Wang TW, Wu HC, Wang HY, Lin FH, Sun JS. Regulation of adult human mesenchymal stem cells into osteogenic and chondrogenic lineages by different bioreactor systems. J Biomed Mater Res A. 2009;88:935. https://doi.org/10.1002/jbm.a.31914.
Wendt D, Marsano A, Jakob M, Heberer M, Martin I. Oscillating perfusion of cell suspensions through three-dimensional scaffolds enhances cell seeding efficiency and uniformity. Biotechnol Bioeng. 2003;84:205. https://doi.org/10.1002/bit.10759.
Zhang ZY, et al. A comparison of bioreactors for culture of fetal mesenchymal stem cells for bone tissue engineering. Biomaterials. 2010;31:8684. https://doi.org/10.1016/j.biomaterials.2010.07.097.
Gomes ME, Reis RL, Mikos AG. Bone tissue engineering constructs based on starch scaffolds and bone marrow cells cultured in a flow perfusion bioreactor. In: Materials Science Forum; 2006.
Davies CM, et al. Mechanically loaded ex vivo bone culture system ‘Zetos’: systems and culture preparation. Eur Cells Mater. 2006;11:57. https://doi.org/10.22203/eCM.v011a07.
Endres S, Kratz M, Wunsch S, Jones DB. Zetos: a culture loading system for trabecular bone. Investigation of different loading signal intensities on bovine bone cylinders. J Musculoskelet Neuronal Interact. 2009;9:173.
Wood MA, et al. Correlating cell morphology and osteoid mineralization relative to strain profile for bone tissue engineering applications. J R Soc Interface. 2008;5:899. https://doi.org/10.1098/rsif.2007.1265.
Baas E, Kuiper JH, Yang Y, Wood MA, El Haj AJ. In vitro bone growth responds to local mechanical strain in three-dimensional polymer scaffolds. J Biomech. 2010;43:733. https://doi.org/10.1016/j.jbiomech.2009.10.016.
Birmingham E, Niebur GL, McNamara LM, McHugh PE. An experimental and computational investigation of bone formation in mechanically loaded trabecular bone explants. Ann Biomed Eng. 2016;44:1191. https://doi.org/10.1007/s10439-015-1378-4.
Gardel LS, Correia-Gomes C, Serra LA, Gomes ME, Reis RL. A novel bidirectional continuous perfusion bioreactor for the culture of large-sized bone tissue-engineered constructs. J Biomed Mater Res B Appl Biomater. 2013;101:1377. https://doi.org/10.1002/jbm.b.32955.
Nguyen BNB, Ko H, Moriarty RA, Etheridge JM, Fisher JP. Dynamic bioreactor culture of high volume engineered bone tissue. Tissue Eng Part A. 2016;22:263. https://doi.org/10.1089/ten.tea.2015.0395.
Janssen FW, Oostra J, Van Oorschot A, Van Blitterswijk CA. A perfusion bioreactor system capable of producing clinically relevant volumes of tissue-engineered bone: in vivo bone formation showing proof of concept. Biomaterials. 2006;27:315. https://doi.org/10.1016/j.biomaterials.2005.07.044.
Janssen FW, et al. Human tissue-engineered bone produced in clinically relevant amounts using a semi-automated perfusion bioreactor system: a preliminary study. J Tissue Eng Regen Med. 2010;4:12. https://doi.org/10.1002/term.197.
Wang C, et al. Repair of segmental bone-defect of goat’s tibia using a dynamic perfusion culture tissue engineering bone. J Biomed Mater Res A. 2010;92:1145. https://doi.org/10.1002/jbm.a.32347.
Tatara AM, et al. Autologously generated tissue-engineered bone flaps for reconstruction of large mandibular defects in an ovine model. Tissue Eng Part A. 2015;21:1520. https://doi.org/10.1089/ten.tea.2014.0426.
Tatara AM, et al. Reconstruction of large mandibular defects using autologous tissues generated from in vivo bioreactors. Acta Biomater. 2016;45:72. https://doi.org/10.1016/j.actbio.2016.09.013.
Kleinhans C, et al. A perfusion bioreactor system efficiently generates cell-loaded bone substitute materials for addressing critical size bone defects. Biotechnol J. 2015;10:1727. https://doi.org/10.1002/biot.201400813.
Tatara AM, et al. Biomaterials-aided mandibular reconstruction using in vivo bioreactors. Proc Natl Acad Sci U S A. 2019;116:6954. https://doi.org/10.1073/pnas.1819246116.
Costa PF, et al. Biofabrication of customized bone grafts by combination of additive manufacturing and bioreactor knowhow. Biofabrication. 2014;6:035006. https://doi.org/10.1088/1758-5082/6/3/035006.
Salter E, et al. Bone tissue engineering bioreactors: a role in the clinic? Tissue Eng Part B Rev. 2012;18:62–75.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Nature Switzerland AG
About this chapter
Cite this chapter
McLoughlin, S.T., Mahadik, B., Fisher, J. (2022). Bioreactors and Scale-Up in Bone Tissue Engineering. In: Guastaldi, F.P., Mahadik, B. (eds) Bone Tissue Engineering. Springer, Cham. https://doi.org/10.1007/978-3-030-92014-2_10
Download citation
DOI: https://doi.org/10.1007/978-3-030-92014-2_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-92013-5
Online ISBN: 978-3-030-92014-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)