Abstract
IR-789, a novel near-infrared fluorescent probe, was designed, synthesized, and applied to living cells. The probe exhibited better response fluorescence characteristics than the only FDA-approved agent, indocyanine green. Cell experiments showed that the probe had high affinity and without apparent cytotoxicity. Fluorescent image experiments in living MCF-7 cells (human breast adenocarcinoma cell line) further demonstrated the potential applications of the probe in biological systems. The probe effectively prevented the influence of autofluorescence and native cellular species in biological systems. It also exhibited high sensitivity, good photostability, and excellent cell membrane permeability.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fluorescence imaging is an optical imaging technique that is useful for biological imaging without the complications that often accompany the use of nuclear contrast agents (Hilderbrand and Weissleder 2010). A near-infrared (NIR) fluorescent probe is administered in fluorescence imaging and NIR irradiation allows the collection of remitted fluorescence for imaging. The development of NIR dyes as fluorescence labels and sensors has attracted increasing interest over the last decade because of their potential to advance and facilitate optical imaging translation to humans. NIR fluorescence imaging has great potential for probing molecular markers associated with tumor proliferation, growth, and metastasis. Compared with other dyes, NIR fluorescent probes have the following advantages: (a) NIR light is poorly absorbed (Tromberg et al. 2000; Klohs et al. 2008); (b) background autofluorescence is negligible; and (c) light scattering in tissue is relatively low.
NIR fluorescent dyes provide numerous opportunities for non-invasive in vivo applications. Indocyanine dyes are useful NIR probes and important abiotic molecules. The clinically-approved indocyanine green (ICG), which has been extensively applied for NIR fluorescence imaging, is an important member of this family (Fox and Wood 1960; Padhani 2005). ICG is the only FDA-approved agent that can be used to test for hepatic function (Caesar et al. 1961) and fluorescence angiography in ophthalmology (Brancato and Trabucchi 1998). Since 1970, it has been investigated as a potential contrast agent for tumor detection in animal models (Reynolds et al. 1999) and humans (Ntziachristos et al. 2000; Haglund et al. 1996; Zhao et al. 1995). The high molecular weight, specific metabolic features, and infrared absorption and emission spectra of ICG create the specificity of fluorescent images obtained with this dye in ophthalmology (Landsman et al. 1976). However, ICG applications are mitigated by several limitations, including low quantum yield, optical instability in the body, and unrestrained leakage in blood vessels.
We considered the aforementioned limitations, which affect the NIR imaging of biological systems, when we designed our novel NIR fluorescent probe. Thus, we synthesized an NIR fluorescent probe, which contains trifluoromethanesulfonic and carboxylic acid groups that could provide better water solubility when applied in cellular analysis in an aqueous environment. We selected tricarbocyanine, an NIR fluorescent dye with high extinction coefficients (Tang et al. 2007) as fluorophore that could provide good photostability when applied in biological system.
Materials and methods
Apparatus and materials
Absorption spectra were obtained using a Pharmaspect UV-3600 spectrophotometer (Shimadzu, Japan). Fluorescence spectra were recorded on an F-4600 spectrofluorometer (Hitachi, Japan). 1H NMR data were recorded on 500 and 300 MHz spectrometers at ambient temperature in DMSO-d 6 and referenced to tetramethylsilane as an internal standard. A Q-TOF Micro Mass Spectrometer (Waters, USA) was used to identify the products. Fluorescent images were obtained using a confocal laser-scanning microscope (Nikon) with an objective lens (×10). Compounds 4 and 2 were synthesized in our laboratory. MTT was from Sigma-Aldrich Co. All reagents and solvents were commercially available and used without further purification, unless otherwise indicated. MCF-7 (human breast adenocarcinoma cell line), HepG2 (liver hepatocellular cell line), and L929 (mouse fibroblast cell line) cells were purchased from ATCC.
Synthesis and characterization of IR-789
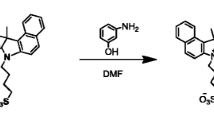
Scheme 1 shows the synthetic route of the designed heptamethine cyanine dye IR-789. Compound 5 (100 mg, 0.25 mmol) was dissolved in THF (50 ml), and compound 4 (100 mg, 0.3 mmol) was added. The solution was stirred under argon at 0 °C for 2 h. Vacuum filtration was performed to isolate the resulting precipitate to obtain a crude solid that was recrystallized to yield compound 3 a white solid (0.11 g, 61.1 %). Mp = 247–248 °C. IR (KBr, cm−1) 3,431 (w, –OH), 2,989 (w, –CH3), 1,721 (s, C=O), 1,628 (w, C=N), 1292 (–CF3), 1,227 (s, C–O), 1,157, 1,031 (s, S=O). 1H NMR (500 MHz, DMSO-d 6 ), δ (ppm) = 8.37 (s, 1H), 8.18–8.19 (d, J = 8.05 Hz, 1H), 8.00–8.01 (d, J = 7.90 Hz, 1H), 2.79 (s, 3H), 1.56 (s, 6H), 1.29 (s, 3H). TOF–MS m/z: 219.1 [M+H]+ : 149.0 (IR, 1H NMR, and MS spectra of compound 3; see Fig. 1).
Compound 3 (200 mg, 0.54 mmol) was dissolved in acetic anhydride (20 ml), and compound 2 (50 mg, 0.27 mmol) and anhydrous sodium acetate (50 mg, 0.54 mmol) were added. The solution was stirred under argon at 30 °C for 12 h. The resulting solution was added into the methyl tert-butyl ether to yield a crude solid. The solid was subjected to column chromatography on silica gel using methanol/dichloromethane (1:6 v/v) as an eluent, affording sensor IR-789 (55 mg, 13.97 %) as a dark green solid. IR (KBr, cm−1) 3,422 (w, –OH), 2,931 (w, –CH3), 1,699 (w, C=O), 1,612 (w, C=N), 1,513 (w, C=C), 1,385, 1,357 (S=O). 1H NMR (300 MHz, DMSO-d 6 ), δ (ppm) = 8.26–8.30 (d, J = 12 Hz, 2H), 8.14 (s, 2H), 8.01–8.04 (m, 2H), 7.50–7.53 (d, J = 9.00 Hz, 2H), 6.36–6.41 (d, J = 15.00 Hz, 2H), 3.71 (s, 6H), 2.70–2.74 (d, J = 12.00 Hz, 4H), 1.87 (m, 2H), 1.70 (s, 12H). TOF–MS m/z: 574.2 [M+H]+. (1H NMR and MS spectra of IR-789; see Fig. 2).
Absorption analysis
Probe IR-789 (0.1 ml, 0.1 mM) and ICG (0.1 ml, 0.1 mM) were separately added to the 10 ml color comparison tubes. After dilution to 1 μM with 50 mM PBS at pH 7.4, the mixture was equilibrated for 3 min prior to spectrophotometry measurements. All experiments were performed in the presence of 0.1 M NaCl to maintain a constant ionic strength.
Fluorescence analysis
Probe IR-789 (0.1 ml, 0.1 mM) and ICG (0.1 ml, 0.1 mM) were separately added into the 10 ml color comparison tubes. After dilution to 1 μM with 50 mM PBS at pH 7.4, the mixture was equilibrated for 3 min before spectrofluorometry measurements. Fluorescence intensity was measured at λ ex = 844 nm. All experiments were conducted in the presence of 0.1 M NaCl to maintain a constant ionic strength.
MTT assay
MCF-7 cells were seeded in 96-well plates at 5,000 cells/well. After overnight culture, the medium in each well was replaced by fresh medium containing different concentrations of IR-789. Each well was treated for 24 h, and 10 μl MTT (5 mg/ml in PBS) was added. The remaining MTT solution was removed after 4 h, and 100 μl DMSO was added into each well to dissolve the formazan crystals. After 6 h at 37 °C, the absorbance of each well at 490 nm was recorded by a plate reader (Huber and Koella 1993).
Confocal imaging
Fluorescent images were observed using a confocal laser-scanning microscope. The excitation wavelength was 789 nm. The medium was removed prior to cell imaging. Confocal imaging was performed after the cells were washed three times with PBS (0.1 M).
Results and discussion
Absorption and fluorescence spectra of IR-789
The absorption and fluorescence spectral property of IR-789 and ICG were examined in buffered aqueous solution (50 mM PBS, IR-789 1.0 μM, pH 7.4) with 5 % DMSO as a cosolvent. IR-789 showed λ max of excitation and emission at 789/844 nm (Fig. 1a). Figure 1b shows that the absorption of IR-789 was 789 nm, which exhibits a stronger blue excitation peak at a neutral pH than ICG (784 nm). The fluorescence of IR-789 was 844 nm, which emits a stronger emission peak at a neutral pH than ICG (825 nm; Fig. 1c).
Test for probe selectivity
Considering the complexity of the intracellular environment, we performed an additional test to determine whether other ions were potential interferents to the photostability. Amines can bind to various metal cations in solution (Silva et al. 1997; Czarnik 1993, 1994). A probe is also used to determine whether other ions were potential interferents. The relative fluorescence intensity of IR-789 (1 and 0.5 μM) at 789 nm in the absence or presence of 200 μM Cu2+, Ca2+, Mg2+, and Zn2+ ions in buffer solution (HEPES 40 mM) at pH 6.50 (λ ex = 789 nm) is shown in Fig. 2, which indicates that the effect of such metals on the measurement is negligible, and the probe has good photostability.
MTT assay
Biocompatibility is always the first property to consider when using a probe in live cells (Seidl and Zinkernagel 2013). MTT assay was conducted to evaluate IR-789 cytotoxicity on MCF-7 cells with probe concentrations from 0.1 μM to 10 mM. The results showed that cell viability was not significantly affected even when IR-789 was as high as 1,210 μM in the culture medium. The probe concentration with good cell viability was later used for in vivo imaging (1 μM).
Determination of quantum yield
To determine the quantum efficiency of fluorescence (Φ FX), ICG was used as a reference standard, which has a 0.078 value in methanol (Benson and Kues 1978). Values were calculated according to the following equation (Demas and Crosby 1971):
where Φ F(X) is the fluorescence quantum yield, A is the absorbance, F is the area under the emission curve, n is the refractive index of the solvents used in the measurement, and the subscripts S and X represent the standard and unknown, respectively. The fluorescence quantum yield was obtained by integrating the emission spectra over 750–900 nm for IR-789, and a value of 0.8 was obtained.
Fluorescent confocal microscopy image
Finally, we applied IR-789 to MCF-7 cells to investigate its applicability in biological systems. Confocal fluorescence images of 1 μM IR-789 were obtained in Dulbecco’s modified Eagle’s medium (DMEM) containing 0.1 % DMSO and 10 % fetal bovine serum as a co-solvent. The DMSO stock solution of IR-789 was first dissolved in DMEM, and confocal fluorescence images of MCF-7 cells were measured. The cells were incubated with IR-789 at pH 7.4 and washed three times with PBS buffer (0.1 M). The total time of fluorescence stability (Fig. 3) was 40 min. The distribution of the probe within the cells was observed under a fluorescence microscope following excitation at 789 nm. Figure 3 shows that IR-789 is a permeable membrane, and the fluorescence intensities vary within single cells. Cell viability was confirmed using bright field transmission measurements after IR-789 incubation. Studies have shown that the IR-789 had excellent membrane permeability and good photostability. We also examined the effects on HepG2 and L929 cells (see Fig. 3).
Conclusion
We have synthesized IR-789, a small organic fluorogen with excellent fluorescent characteristics. It is a novel NIR fluorescent probe for optical imaging in living cells. The probe exhibited stronger excitation and emission characteristics than ICG under similar conditions. The probe effectively prevented the influence of autofluorescence in biological systems and exhibited positive results when tested at a near neutral pH both in aqueous solution and MCF-7 cells. The real-time imaging of cells was successfully achieved. The results demonstrated that IR-789 was based on a PET mechanism, and the IR-789 value was obtained by monitoring the intracellular environment of MCF-7 cells. Therefore, IR-789 has great potential in the investigation of a pivotal function in a biological context through direct intracellular imaging. Efforts to utilize the probe for biological imaging application are in progress. This simple, sensitive NIR fluorescent probe will be of great benefit to biomedical researchers on biological systems.
References
Benson RC, Kues HA (1978) Fluorescence properties of indocyanine green as related to angiography. Phys Med Biol 23:159–163
Brancato R, Trabucchi G (1998) Fluorescein and indocyanine green angiography in vascular chorioretinal diseases. Semin Ophthalmol 13:189–198
Caesar J, Shaldon S, Chiandussi L, Guevara L, Sherlock S (1961) The use of indocyanine green in the measurement of hepatic blood flow and as a test of hepatic function. Clin Sci 21:43–57
Czarnik AW (1993) Fluorescent chemosensors for ion and molecule recognition. ACS, Washington DC 538
Czarnik AW (1994) Chemical communication in water using fluorescent chemosensors. Acc Chem Res 27:302–308
Demas JN, Crosby GA (1971) Measurement of photoluminescence quantum yields. J Phys Chem 75:991–1023
Formica M, Fusi V, Giorgi L, Micheloni M (2012) New fluorescent chemosensors for metal ions in solution. Coord Chem Rev 256:170–192
Haglund MM, Berger MS, Hochman DW (1996) Enhanced optical imaging of human gliomas and tumor margins. Neurosurgery 38:308–317
Hilderbrand SA, Weissleder R (2010) Near-infrared fluorescence: application to in vivo molecular imaging. Curr Opin Chem Biol 14:71e9
Huber W, Koella JC (1993) A comparison of three methods of estimating EC50 in studies of drug resistance of malaria parasites. Acta Trop 55:257–261
Klohs J, Wunder A, Licha K (2008) Near-infrared fluorescent probes for imaging vascular pathophysiology. Basic Res Cardiol 103:144–151
Landsman ML, Kwant G, Mook GA, Zijlstra WG (1976) Light-absorbing properties, stability, and spectral stabilization of indocyanine green. J Appl Physiol 40:575–583
Ntziachristos V, Yodh AG, Schnall M, Chance B (2000) Concurrent MRI and diffuse optical tomography of breast following indocyanine green enhancement. Proc Natl Acad Sci USA 97:2767–2772
Padhani AR (2005) Where are we with imaging oxygenation in human tumours? Cancer Imag 5:128–130
Reynolds JS, Troy TL, Mayer RH, Thompson AB, Waters DJ, Cornell KK, Snydera PW, Sevick-Muraca EM (1999) Imaging of spontaneous canine mammary tumors using fluorescent contrast agents. Photochem Photobiol 70:87–94
Seidl K, Zinkernagel AS (2013) The MTT assay is a rapid and reliable quantitative method to assess Staphylococcus aureus induced endothelial cell damage. J Microbiol Meth 92:307–309
Silva AP, Gunaratne HQN, Gunnlaugsson T, Huxley AJM, McCoy CP, Rademacher J, Rice TE (1997) Signaling recognition events with fluorescent sensors and switches. Chem Rev 97:1515–1566
Tang B, Liu X, Xu K, Huang H, Yang G, An L (2007) A dual near-infrared pH fluorescent probe and its application in imaging of HepG2 cells. Chem Commun 36:3726–3728
Tromberg BJ, Shah N, Lanning R, Cerussi A, Espinoza J, Pham T, Svaasand L, Butler J (2000) Non-invasive in vivo characterization of breast tumors using photon migration spectroscopy. Neoplasia 2:26–40
Zhao S, O’Leary MA, Nioka S, Chance B (1995) Breast tumor detection using continuous wave light source. Proc SPIE 2389:789–798
Acknowledgments
This work is supported by the National Basic Research Program of P. R. China (No. 2011CB933503) and Technology Supporting Program of Jiangsu province (BE2012657).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sun, C., Wu, Y., Cai, J. et al. Synthesis of a near-infrared fluorescent probe and its application in imaging of MCF-7 cells. Biotechnol Lett 36, 1203–1207 (2014). https://doi.org/10.1007/s10529-014-1478-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-014-1478-5