Abstract
Endophytic actinomycetes from five Asteraceae plants were isolated and evaluated for their bioactivities. From Parthenium hysterophorus, Ageratum conyzoides, Sonchus oleraceus, Sonchus asper and Hieracium canadense, 42, 45, 90, 3, and 2 isolates, respectively, were obtained. Of the isolates, 86 (47.2 %) showed antimicrobial activity. Majority of the isolates were recovered from the roots (n = 127, 69.7 %). The dominant genus was Streptomyces (n = 96, 52.7 %), while Amycolatopsis, Pseudonocardia, Nocardia and Micromonospora were also recovered. Overall, 36 of the 86 isolates were significantly bioactivity while 18 (20.9 %) showed strong bioactivity. In total, 52.1 and 66.6 % showed potent cytotoxicity and antioxidant activities. The LC50 for 15 strains was <20 μg/ml. Compared to the ascorbate standard (EC50 0.34 μg/ml), all isolates gave impressive results with notable EC50 values of 0.65, 0.67, 0.74 and 0.79 μg/ml.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With the continuing search for novel natural products, it has become evident that the rate of re-isolation of known compounds has increased; therefore new microbial habitats need to be examined. One relatively overlooked but biologically important niche is the inner tissues of higher plants (Qin et al. 2009). All plants retain a microbiota (Bascom-Slack et al. 2009) and organisms that establish an endosymbiotic relationship with plants are defined as endophytes. Actinomycetes are saprophytes well known for producing a wide range of secondary metabolites. However, very little information is available to us regarding the biodiversity, tissue distribution, and biosynthetic potential of endophytic actinomycetes residing in wild, native plants (Janso and Carter 2010).
The Asteraceae family includes some of the most valued and oldest medicinal plants (Jeschke et al. 2009). Ageratum conyzoides L. is widely distributed all over Pakistan (Singh et al. 2012) and locally known as ‘Neeli Booti’ (Zafar et al. 2006). It has been used in traditional medicine for the treatment of innumerous disorders. However, no report of the in vitro anticancer activity of the plant has been evaluated or published (Adebayo et al. 2010).
Sonchus asper is another important herbal plant used as an alternative in the treatment of various diseases. It is a rich source of phenolic compounds, flavonoids, carotenoids, sesquiterpene lactones glycosides, ascorbic acid, and ω-3 fatty acids. (Khan et al. 2010).
The aim of the study was to explore the diversity and analyze the endophytic actinomycetes isolated from parts of medicinal plants belonging to Asteraceae family for specific biological activities. Actinomycetes were isolated from the medicinal plants from several of its natural habitats in Pakistan. Keeping in mind there is a lack of data on the antioxidant and cytotoxicity studies of endophytic actinomycetes from these plants therefore this study provides new and useful information.
Materials and methods
Plant material and collection site
Healthy plants of Parthenium hysterophorus were collected from the agricultural lands at the department of microbiology and molecular genetics (MMG) located at the University of the Punjab, Lahore. Ageratum conyzoides, Sonchus oleraceus, Sonchus asper and Hieracium canadense were collected from shady, moist areas around the department (31.49°N, 74.29°E, elevation ~210 m).
Sample treatment and isolation
Each plant sample was thoroughly washed in running tap water to remove soil particles and adhered epiphytes. After drying in sterile conditions, tissues surfaces were divided into 0.5 cm segments and surface sterilized by the five step sequential process as described by Tanvir et al. (2013) and plated.
Test organisms and antibiosis assay
For detection of antimicrobial activity, Staphylococcus aureus (ATCC 25923), Escherichia coli (ATCC 25922), Klebsiella pneumoniae (ATCC 706003), Methicillin-resistant Staphylococcus aureus (MRSA), Pseudomonas, Enterobacter, Bacillus, Candida tropicalis, and Chlorella vulgaris were used. The minimum inhibitory concentration (MIC) of the extracts obtained from endophytic actinomycetes were tested by broth dilution method as described by Andrews (2001). Extraction of fermentation broth of endophytic strains was done with ethyl acetate which was dried on a rotary evaporator to yield crude extracts.
Brine shrimp lethality test
For brine shrimp cytotoxicity assay, the extracts were dried and dissolved in DMSO to obtain the desired concentrations. The final concentration of DMSO in the assay volume was kept at 1 % (v/v) to prevent possible false effects originating from DMSO toxicity. Actinomycin D was used a positive control and artificial sea water was used as a negative control for the assay. Toxicity of the crude extracts was tested at 10, 50, 100, 150, 500, 1,000 μg/ml in seawater containing 1 % (v/v) DMSO. The mortality rate M was calculated using the formula:
where M is the percent of the dead nauplii after 24 h; A is the number of the dead nauplii after 24 h; B is the average number of the dead nauplii in the blind samples after 24 h; N is the number of dead nauplii before starting the test; G is the total number of nauplii.
Cytotoxicity was considered significant if the LC50 value was less than 20–30 μg/ml (Geran et al. 1972).
Free radical scavenging activity assay using diphenylpicrylhydrazyl (DPPH)
Antioxidant activity was tested according to the procedure by Pajero et al. (2000) with slight modifications. Antioxidant activity of the crude extracts was tested at 100, 500, 1,000, 5,000 and 10,000 μg DPPH/ml dissolved in methanol. An ascorbate standard was used as a positive control and DPPH stock was used as a negative control. The DPPH radical scavenging activity of the sample was calculated as:
The antioxidant activity was expressed in terms of EC50, which was defined as the dilution of the crude extract needed to decrease initial DPPH chemilumininescence intensity by 50 %.
Statistical analysis
All experiments were performed in triplicates and results are presented as mean values. The calculations for LC50 were done through probit regression analysis and the determination of EC50 was done through dose response curve. Analysis was done using statistical package SPSS version 20.0 (SPSS, USA).
Results and discussion
A variety of plant species have been explored for the isolation of endophytic actinobacteria ranging from different woody tree species, ferns, club mosses even crop plants, like rice, wheat, carrots, potato, tomato and citrus (Qin et al. 2011a, b). However, there are few reports on bioactive compounds from actinomycetes of such origin (Radhakrishnan et al. 2010). This situation encouraged us to explore and investigate the endophytic actinomycetes community of medicinal plants in family Asteraceae. Therefore this study is one of its kind to describe the biodiversity and bioactivities of endophytic actinomycetes associated with the plants described.
Our study revealed that the tissues of these plants harbor a variety of endophytic actinomycetes. A total of 182 isolates were obtained from the stem, root and leaf of the plants. Most isolates were identified as Streptomyces spp. after cultural characterization. Of these 182 isolates, a majority were recorded from the roots (n = 127, 69.7 %) then shoots (n = 32, 17.5 %) and leaves (n = 23, 12.6 %). The Streptomyces genus was dominant (n = 96, 52.7 % of all isolates), followed by Micromonospora (n = 3, 1.5 %), Amycolatopsis (n = 2, 1 %) and Pseudonocardia, Nocardia (n = 1, 0.5 %) (Table S1).
Among the isolates, various subgroups were identified and among Streptomyces spp., the subgroup Streptomyces rochei (n = 3, 7.1 %) was also isolated. Apart from our previous study (Tanvir et al. 2013), no other study has reported its isolation from Asteraceae plants. The roots of Asteraceae plants harbored besides Streptomyces spp., other genera like Amycolatopsis, Nocardia, Pseudonocardia, and Micromonospora. There are reports of isolation of Micromonospora tulbaghia sp. nov., from the leaves of Tulbaghia violacea (Kirby and Meyers 2010), and Pseudonocardia sichuanensis sp. nov., from the root of Jatropha curcas L. (Qin et al. 2011a, b).
Considering these findings, it is apparent that a selected group of actinomycetes namely Streptomyces, Amycolatopsis, Nocardia, Pseudonocardia, and Micromonospora are able to form endophytic associations with a wide range of plant hosts.
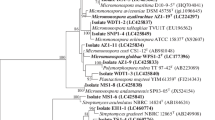
Eighty-six of the isolates (47.2 %) showed growth inhibitory activity against pathogenic strains of microorganisms and among them 36 (41.8 %) isolates had significant broad spectrum antimicrobial activity while 25 (29.0 %) showed strong bioactivity. The isolates RT 6, 13, 18, 36, 49, 56, 67 and AGRS8, SORS80 (all isolated from the roots) were further characterized by 16S rRNA sequencing (Table S2).
Streptomyces strains, such as RT 46, 49, 54 and 63, exhibited major MIC values ranging from 4–32 μg/ml against all nosocomial pathogens (Table 1). One of the isolate AGRS8 with 99 % homology to Streptomyces albovinaceus showed the most significant MIC value of 2 μg/ml against all multidrug resistant isolates whereas another strain AGLS2 identified as Pseudonocardia carboxydivorans and SORS80 identified as Amycolatopsis japonicum also showed 4–8 μg/ml MIC values against all test organisms as shown in Table 2.
Exposure of brine shrimp to extracts also revealed interesting results: in case of strains RT 13, 50, 59 and 67 the percentage mortality after 24 h ranged from 96.6 % at 100 μg/ml to 100 % at 500, 1 and 5 mg/ml. A high mortality rate was also observed in the isolates RT 18, 36 and 53 which gave 100 % mortality at 100 μg/ml which was the lowest concentration for these extracts tested (Fig. S1). The LC50 value for the extracts was very potent at 20 μg/ml, which is considered extremely toxic when compared to values provided by Geran et al. (1972). Overall, 15 of the 33 isolates selected for cytotoxicity showed an extremely potent cytotoxicity value of LC50 10 μg/ml whereas two isolates showed LC50 values between 23 and 50 μg/ml which is also very toxic (Table 3).
It is evident from our study that the endophytic actinomycetes residing in the plants of Asteraceae family have significant capability of producing cytotoxic compounds. Previous reports also support our results with novel antibiotics such as coronamycin from endophytic Streptomyces sp. with cytotoxicity of IC50 5–10 μg ml (Ezra et al. 2004). It establishes the fact that endophytes are excellent source for the isolation of novel cytotoxic compounds.
In light of the previous reports regarding the antioxidant activity for the Streptomyces, the activity for endophytic actinomycetes was quantified using the DPPH free-radical scavenging activity assay. As compared to the ascorbate standard for which the EC50 was 0.34 μg/ml, the EC50 of all of the actinomycetes strains gave impressive results. The EC50 of the isolates SORS64b, SORS124, AGRS16, AGLS2, AGRS19, gave EC50 values of 0.55, 0.57, 0.62, 0.65, and 0.67 μg/ml, respectively, with 0.552 and 0.567 μg/ml being the highest EC50 value (Table 4). The remaining isolates also gave good results with EC50 value of 0.7 ± 2 μg/ml when compared to the ascorbate standard. According to our study, the antioxidant activity of the crude extracts increased with the increase in concentration which is similar to the results of Kekuda et al. (2010) who reported that with higher concentration the scavenging activity of the Streptomyces was also increased.
Summary
This study suggests that the Asteraceae plant family harbors endophytic actinomycetes that produce a wide range of novel natural products with pharmaceutical potential. As such to the best of our knowledge, there is a lack of data on exploring the diversity of endophytic actinomycetes residing in the plant tissue of Asteraceae plant family, therefore this study not only describes an untapped environment but also highlights the potential usage of these isolates in biotechnological, medical and pharmaceutical applications.
References
Adebayo AH, Tan NH, Akindahunsi AA, Zeng GZ, Zhang YM (2010) Anticancer and antiradical scavenging activity of Ageratum conyzoides L. (Asteraceae). Pharmacogn Mag 6:62–66
Andrews JM (2001) Determination of minimum inhibitory concentrations. J Antimicrob Chemother 48:5–16
Bascom-Slack CA, Ma C, Moore E et al (2009) Multiple, novel biologically active endophytic actinomycetes isolated from upper Amazonian rainforests. Microbiol Ecol 58:374–383
Ezra D, Castillo UF, Strobel GA et al (2004) Coronamycins, peptide antibiotics produced by a verticillate Streptomyces sp. (MSU-2110) endophytic on Monstera sp. Microbiology 150:785–793
Geran RI, Greenberg HM, McDonald M, Abbott BJ (1972) Protocols for screening chemical agents and natural products against animal tumors and other biological systems. Cancer Chemother Rep 33:1–17
Janso JE, Carter GT (2010) Biosynthetic potential of phylogenetically unique endophytic actinomycetes from tropical plants. Appl Environ Microbiol 76:4377–4386
Jeschke E, Ostermann T, Luke C, Tabali M, Kroz M, Bockelbrink A, Witt CM, Willich SN, Matthes H (2009) Remedies containing Asteraceae extracts a prospective observational study of prescribing patterns and adverse drug reactions in German primary care. Drug Saf 32:691–706
Kekuda TRP, Shobha KS, Onkarappa R (2010) Studies on antioxidant and anthelmintic activity of two Streptomyces species isolated from Western Ghat soils of Agumbe, Karnataka. J Pharm Res 3:26–29
Kester DR, Duedall IW, Connors DN, Pytkowicz RM (1967) Preparation of artificial seawater. Limnol Oceanogr 12:176–179
Khan RA, Khan MR, Sahreen S, Bokhari J (2010) Prevention of CCl4-induced nephrotoxicity with Sonchus asper in rat. Food Chem Toxicol 48:2469–2476
Kirby BM, Meyers PR (2010) Micromonospora tulbaghia sp. nov., isolated from the leaves of wild garlic, Tulbaghia violacea. Int J Syst Evol Microbiol 60:1328–1333
Kuster E, Williams ST (1964) Selection of media for the isolation of streptomycetes. Nature 202:928–929
Pajero I, Codina C, Petrakis C, Kefalas P (2000) Evaluation of scavenging activity assessed by Co(II)/EDTA-induced luminol chemiluminescence and DPPH (2,2,-diphenyl-1-piccrylhydrazyl) free radical assay. J Pharm Toxicol Meth 44:507–512
Qin S, Li J, Chen HH, Zhao GZ, Zhu WY, Jiang CL, Xu LH, Li WJ (2009) Rare actinobacteria from medicinal plants of tropical rainforests, Xishuangbanna: isolation, diversity and antimicrobial activity. Appl Environ Microbiol 75:6176–6186
Qin S, Xing K, Fei SM et al (2011a) Pseudonocardia sichuanensis sp. nov., a novel endophytic actinomycete isolated from the root of Jatropha curcas L. Antonie Van Leeuwenhoek 99:395–401
Qin S, Xing K, Jiang JH, Xu LH, Li WJ (2011b) Biodiversity, bioactive natural products and biotechnological potential of plant-associated endophytic actinobacteria. Appl Microbiol Biotechnol 89:457–473
Radhakrishnan M, Suganya S, Balagurunathan R, Kumar V (2010) Preliminary screening for antibacterial and antimycobacterial activity of actinomycetes from less explored ecosystems. World J Microbiol Biotechnol 26:561–566
Shirling EB, Gottlieb D (1966) Methods for characterization of Streptomyces species. Int J Syst Bacteriol 16:313–340
Singh J, Singh P, Prakash B, Dubey NK (2012) Insecticidal activity of Ageratum conyzoides L, Coleus aromaticus Benth and Hyptis suaveolens (L.) Poit essential oils as fumigant against storage grain insect Tribolium castaneum Herbst. J Food Sci Technol. doi:10.1007/s13197-012-0698-8
Tanvir R, Sajid I, Hasnain S (2013) Screening of endophytic streptomycetes isolated from Parthenium hysterophorus L against nosocomial pathogens. Pak J Pharm Sci 26:277–283
Zafar M, Khan MA, Ahmad M, Sultana S (2006) Palynological and taxonomic studies of some weeds from flora of Rawalpindi. Pak J Weed Sci Res 12:99–109
Acknowledgments
The authors thank the late Prof. Dr. Ghazala Nasim of the Institute of Plant Pathology and Dr. Sikandar Sultan at the Department of Microbiology and Molecular Genetics, University of the Punjab for the help with the identification of the plants.
Conflict of interest
The authors declare they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10529_2013_1430_MOESM3_ESM.jpg
Cytotoxicity test using extracts of Asteraceae plant endophytic actinomycetes against Artemia salina nauplii (JPEG 88 kb)
Rights and permissions
About this article
Cite this article
Tanvir, R., Sajid, I. & Hasnain, S. Biotechnological potential of endophytic actinomycetes associated with Asteraceae plants: isolation, biodiversity and bioactivities. Biotechnol Lett 36, 767–773 (2014). https://doi.org/10.1007/s10529-013-1430-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-013-1430-0