Abstract
A cDNA encoding for a laccase was isolated from the white-rot fungus Lenzites gibbosa by RT-PCR and expressed in the Pichia pastoris. The laccase native signal peptide efficiently directed the secretion of the recombinant laccase in an active form. Factors influencing laccase expression, such as pH, cultivation temperature, copper concentration and methanol concentration, were optimized. The recombinant enzyme was purified to electrophoretic homogeneity, and was estimated to have a MW of ~61.5 kDa. The purified enzyme behaved similarly to the native laccase produced by L. gibbosa and efficiently decolorized Alizarin Red, Neutral Red, Congo Red and Crystal Violet, without the addition of redox mediators. The decolorization capacity of this recombinant enzyme suggests that it could be a useful biocatalyst for the treatment of dye-containing effluents. This study is the first report on the synthetic dye decolorization by a recombinant L. gibbosa laccase.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Laccases (benzenediol:oxygen oxidoreductases, EC 1.10.3.2) belong to the family of blue multicopper oxidases that catalyze the oxidation of an array of aromatic substrates by the reduction of O2 to water (Paola 2010). Laccases are widely distributed in many bacteria, fungi and plants (Baldrian 2006). The substrate spectrum of laccase is very broad and can be expanded considerably in the presence of appropriate oxidation–reduction mediators (Camarero et al. 2005). Therefore, laccases have a promising potential in many industrial and biotechnology applications (Couto and Herrera 2006). The successful applications of laccases in these areas require the production of large quantities of laccases at low-cost.
Lenzites gibbosa is a white rot fungi. Whilst white rot fungi produce high activities of laccases (Yan and Chi 2013), they often express multiple laccase genes encoding isozymes with a highly similar primary structure but different physicochemical characteristics (Baldrian 2006). This complexity makes it difficult to purify individual isozymes for analysis. Instead, heterologous expression in an alternative host can provide an effective mechanism of recombinant laccase production. Many laccase genes or cDNAs have been cloned and some have been expressed in yeasts (Saccharomyces cerevisiae and Pichia pastoris) and in filamentous fungi (Trichoderma reesei and Aspergillus oryzae) (Otterbein et al. 2000; Faraco et al. 2008; Téllez-Jurado et al. 2006). Although filamentous fungi are generally good hosts for protein secretion, they are more time consuming to work with than yeasts. The heterologous expression of laccase would not only generate large quantities of enzyme but also allow site-directed mutagenesis or in vitro evolution for design of improved laccases for biotechnical applications (Zumárraga et al. 2007; Madzak et al. 2006). The methylotrophic yeast, P. pastoris, is particularly suited to foreign protein expression. In addition, methods for molecular manipulation of P. pastoris are rapid and well developed and purification of secreted recombinant proteins is easily achieved (Macauley-Patrick et al. 2005).
Laccases was employed in larger-scale applications, such as removal of polyphenols from wine and beverages, conversion of toxic compounds and textile dyes in waste waters and in bleaching and removal of lignin from wood and non-wood fibers (Riva 2006). Synthetic dyes are increasingly used in the textile, leather, paper, cosmetics and pharmaceutical industries. These compounds are anthraquinones, azo compounds, heterocyclics, triphenylmethane or phthalocyanine dyes and cause serious environmental pollution (Claus et al. 2002). Most synthetic dyes are toxic and highly resistant to degradation. Laccases and laccase mediator systems can efficiently decolorize a variety of synthetic dyes (Wesenberg et al. 2003). So far, a number of studies have focused on the ability of white rot fungi to decolorize and degrade these dyes (Wesenberg et al. 2003). Laccase dye decolorization is an efficient method and is attracting increasing interest (Couto and Herrera 2006); however only limited research into purified recombinant laccase dye decolorization has been carried out (Soares et al. 2001; Guo et al. 2008; Dubé et al. 2008). In this study, we describe the heterologous production of the laccase from Lenzites gibbosa via cDNA gene expression in P. pastoris. The recombinant enzyme was purified and we investigated its ability to decolorize several chemically different synthetic dyes.
Materials and methods
Microbial strains, media and vectors
Lenzites gibbosa was maintained by Forest Pathology Laboratory of Northeast Forestry University, China. Escherichia coli JM109 was grown in low salt LB medium. E. coli JM109 Tiangen, Beijing, China was used for subcloning. P. pastoris SMD1168H (Invitrogen) was used for expression of laccase. Minimal methanol (MM), buffered minimal methanol (BMM), buffered glycerol-complex (BMGY) and yeast extract/peptone/dextrose (YPD) media were prepared according to the EasySelect Pichia Expression Kit manual (Invitrogen). The pMD18-T simple vector (TaKaRa,). In addition, the Pichia expression vector pPICZ B (Invitrogen) was used to clone the entire L. gibbosa laccase cDNA.
Vector construction
All enzymes used for cloning and restriction analysis were obtained from TaKaRa. Laccase cDNA was isolated and amplified by RT-PCR as described previously (Ong et al. 1997). The forward primer (5′-CGGAATTC ATGATGAATCTGGGCTTTCTCAACT-3′) and reverse primer (5′-GCTCTAGACTACTGGTCCCCCACATCCAG-3′) were used to generate an EcoRI and an XbaI restriction site, respectively (in italics). The PCR amplification program was initiated at 94 °C for 3 min, followed by 30 cycles of 94 °C for 30 s, 55 °C for 30 s and 72 °C for 2 min, and a final extension at 72 °C for 10 min. The PCR products were purified using the DNA Gel Extraction Kit (TaKaRa), and cloned into the pMD18-T simple vector. The expression vector pPICZB and the recombinant pMD18-T simple vector containing the entire laccase cDNA were digested with EcoRI and XbaI, and the resulting fragments were purified as described above. The purified laccase cDNA was ligated with the digested pPICZB fragment using T4 DNA ligase overnight at 4 °C. The ligation mixture was transformed into E. coli JM109 and selected on low salt LB medium supplemented with 25 μg zeocin ml−1. Transformants were isolated and the recombinant pPICZB/lac2 vector was verified by restriction enzyme digestion, agarose gel electrophoresis and sequence analysis.
Transformation of P. pastoris
The pPICZB-Lg-lac2 and the control vector pPICZB were linearized by BamHI and SacI digestion, respectively, and transformed into P. pastoris by electroporation. The electroporated cells were plated out on YPD agar with 100 μg zeocin+ ml−1 for selecting the transformants. The selected transformants were transferred to MM agar plates containing 0.2 mM ABTS and 0.1 mM CuSO4. The plates were incubated inverted at 28 °C and 100 μl methanol was added to the lid of the plate each day to compensate for loss by evaporation. Laccase-producing transformants were identified by the presence of a dark green halo around the Pichia colonies.
Assay of laccase activity
Laccase activity was determined at 25 °C using 0.5 mg ABTS ml−1 as substrate. The samples were centrifuged at 5,000×g for 5 min at 4 °C to obtain supernatants. Laccase activity was measured by the ABTS method as described by Eggert et al. (1996). There were three replicates of each treatment and standard deviation did not exceed 5 % of the average values.
Optimization of laccase production in P. pastoris
All chemicals were analytical grade, unless stated otherwise. The recombinant laccase was obtained from BMMY with 0.1 mM CuSO4. Cultures were grown at 28 °C with shaking at 240 rpm, and filter-sterilized methanol was added daily to 0.5 % (v/v). The cell extract was obtained by centrifugation at 10,000×g for 15 min at 4 °C and was used for determination of intracellular laccase activity. The transformant with highest laccase-secretion was selected for investigating the effects of temperature, pH, copper concentration and methanol concentration on the recombinant laccase production. There were three replicates of each treatment.
Purification and characterization of recombinant laccase
The purification of heterologous laccase was carried out as follows: the supernatant (550 ml) was filtered and concentrated to 50 ml in a Labscale TFF system (Millipore) using a 30 kDa filter cassette. The sample was applied to a DEAE-Sepharose Fast Flow column (1.6 × 50 cm2) equilibrated with 20 mM sodium phosphate buffer (pH 6.5). The column was washed with the same buffer and the proteins were eluted by a linear concentration gradient of NaCl (150 ml, 0–1 M) at 1 ml min−1. SDS-PAGE was performed with a 5 % stacking gel and a 15 % resolving gel. The molecular mass of the recombinant laccase was estimated by calculating the relative mobility of standard protein markers (TaKaRa). The optimal pH and temperature, Km, Vmax, and the extent of glycosylation of the purified enzyme were also determined. There were three replicates of each treatment.
Results
Heterologous expression of laccase in P. pastoris
Lensites gibbosa laccase cDNA, including the native signal sequence, was cloned into the vector pPICZB and transformed into electro-competent P. pastoris. Transformants were tested for laccase expression by two days growth on MM plates supplemented with ABTS and CuSO4 (see supplementary materials). An intense green zone halo formed around the P. pastois containing the recombinant plasmid, pPICZB-Lg-lac2, while no halo was observed around the colony containing empty vector, pPICZB. Subsequent experiments using liquid cultures also included control transformants. Laccase activity was found in the supernatant of the culture medium indicating that it was secreted in an active form. Laccase activity was not detected in the cell extract nor in the supernatant of control culture.
Optimization of the recombinant laccase production
The transformant with high laccase-secreting ability was selected to optimize the heterologous expression of laccase under various cultivation conditions. The culture supernatants were assayed daily for seven consecutive days. The culture medium (BMM) was supplemented with 0.1 mM CuSO4 to support copper incorporation into the laccase.
High laccase activity was observed in cultures with initial pH 6.5, as measured on day five. After 5 days cultivation, the laccase activity of the culture at 28 °C was 1.9 times higher than that at 20 °C. Subsequent growth tests were performed at 28 °C. Increasing copper concentrations enhanced laccase activity, peaking at 0.6 mM CuSO4. Methanol at 0.4 % produced the highest laccase activity. Under the optimal conditions, laccase activity reached 3108 U l−1 on day seven (Fig. 1), and the highest laccase output was 5406 U l−1 after 14 days of cultivation. Other detailed results see supplementary materials.
The activity of recombinant laccase in BMM medium under different cultivation conditions. Before optimization: pH 6.0, 30 °C, supplemented with 0.1 mM CuSO4 and 0.5 % methanol (filled triangle). After optimization: pH 6.5, 28 °C, supplemented with 0.8 % alanine, 0.6 mM CuSO4 and 0.4 % methanol (filled square). The values are mean of triplicates (standard deviation <5 %)
Purification and characterization of recombinant laccase
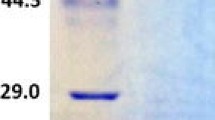
The purification process is summarized in Table 1. The purified enzyme appeared on SDS-PAGE as a single band with a molecular mass of 61.5 kDa (Fig. 2). The heterologous laccase activity was optimal at pH 2.3 and at 55 °C. The heterologous laccase exhibited Km and Vmax values of 170 mM and 24 mmol min−1, respectively, for ABTS at pH 2.3 and 25 °C.
The ability of the purified laccase to decolorize synthetic dyes differed (see Supplementary materials). The decolorization of Alizarin Red occurred rapidly within 30 min, and remained unchanged (98.8 %) after 1 h. In contrast, decolorization of the other three dyes proceeded more slowly and required more than 1 h to decolorize 50 % of the dye (Fig. 3). After 18 h, decolorization of Congo Red (70 %), Neutral Red (60 %), and Crystal Violet (51 %) was achieved.
Decolorization of synthetic dyes by the purified recombinant laccase (3 U ml−1) at pH 3.0, 40 °C. Alizarin Red (filled rectangle); Congo Red (filled triangle); Neutral Red (open triangle); Crystal Violet (open square). The values are mean of triplicates (standard deviation <5 %). The reaction mixture contained a final concentration of 0.1 M citrate/phosphate buffer (pH 3.4), different dyes (each 50 mg l−1), and 0.5 ml culture supernatant as enzyme solution (containing 0.005 U laccase) in a total volume of 3 ml. The reactions were initiated with enzyme and incubated at 40 °C under mild shaking conditions. Decolorization was measured from the absorbance at 467 nm for Alizarin Red, 465 nm for Neutral Red, 466 nm for Congo Red, and 502 nm for Crystal violet. The decolorization of dye, expressed as dye decolorization (%), was calculated with the formula: decolorization (%) = [(Ci − Ct)/Ci] × 100, where, Ci initial concentration of the dye, Ct dye concentration along the time (Lorenzo et al. 2006). Samples were assayed in parallel, without enzyme under identical conditions
Discussion
The laccase cDNA from L. gibbosa was successfully expressed in P. pastoris and the secreted protein was correctly processed and its productivity was optimized by the tailored expression conditions. However, laccase activity of the transformant was relatively low but may be further enhanced by optimization of the heterologous expression condition. The molecular weight of the recombinant laccase (61.5 kDa) was similar to the native enzyme (61.4 kDa). The molecular weight of the recombinant laccase may have been increased by P. pastoris glycosylation (Otterbein et al. 2000).
Four synthetic dyes that could be classified as azo, anthraquinone, triphenylmethane and heterocyclic dyes were decolorized by the purified laccase to different extents (see supplementary materials). The variation in the efficiency of decolorization could be attributed to the difference in the molecular structures of the dyes (Claus et al. 2002). Despite considerable research into azo dyes, limited information exists on the biotransformation of anthraquinone dyes (Epolitoa et al. 2005). There is no data on the decolorization efficiency of the anthraquinone dye, Alizarin Red, but the decolorization efficiency of anthraquinone dye in RBBR has been reported. The purified recombinant laccase from P. methanolica requires 16 h to decolorize about 90 % of RBBR under a higher laccase activity (25 U ml−1) (Guo et al. 2008). Among the four dyes tested, heterocyclic carmine was the most recalcitrant to decolorization, indicating it was not the suitable substrate for the purified enzyme but, in the presence of mediators, the decolorization of carmine by laccase might be enhanced (Dubé et al. 2008). Industrially, azo dyes are usually decolorized to aromatic amines by bacterial azoreductases. These colorless products though, may be toxic, mutagenic and carcinogenic to human and animals (Chen 2006). The oxidation mechanism of laccase may avoid the formation of these hazardous aromatic amines. Laccases alone, however, cannot efficiently decolorize azo dyes. In this case, laccase activity depends on the presence of some small redox mediators (Camarero et al. 2005). However, we have found in this present work that the purified recombinant laccase could efficiently decolorize the azo dye, Congo Red, in the absence of any mediators. These results suggest a potential use of this recombinant laccase in decolorizing and detoxifying of industrial synthetic dyes.
pH control is usually needed during laccase production as some acidic proteases may be activated at a lower pH and cause a detrimental effect on laccase production (Soden et al. 2002). Metabolism of alanine by P. pastoris could, however, stabilize the culture pH (O’Callaghan et al. 2002). We used 0.8 % alanine in the BMM growth medium to stabilize the pH around 6.5 during the cultivation. Although the activities of several heterologous laccases expressed by yeasts were improved by lowering the cultivation temperature to 20 °C (Hong et al. 2002), the expression of L. gibbosa laccase in P. pastoris was not improved in this way. Laccase activity in P. pastoris shake-flask cultures was much higher at 30 °C than at 20 °C and might be ascribed to the lower growth rate of the yeast at 20 °C. Laccases are copper-containing enzymes and the copper atoms are important for the activity of laccases. The heterologous expression of Pycnoporus coccineus laccase in A. oryzae and S. cerevisiae has demonstrated that copper is necessary for the correct folding and assembly of laccase at the post-transcriptional step (Hoshida et al. 2005). The activity of the recombinant laccase was directly proportional to the concentration of copper in the growth medium indicating a requirement of the enzyme for copper. The increasing of methanol concentration effected laccase production: the lower laccase activity obtained at higher methanol concentrations may be attributed to the accumulation of the toxic products of methanol metabolism which would retard the growth and decrease the observed biomass yield of P. pastoris (Khatri and Hoffmann 2006).
In conclusion, we have demonstrated the use of the P. pastoris expression system to produce heterologous laccase which could be used in the removal of dyes from industrial effluents. P. pastoris laccase efficiently decolorized alizarin red without additional redox mediators. This enzyme also catalyzed decolorization of other synthetic dyes in the absence of redox mediators.
References
Baldrian P (2006) Fungal laccases-occurrence and properties. FEMS Microbiol Rev 30:215–242
Camarero S, Ibarra D, Martínez MJ, Martínez AT (2005) Lignin-derived compounds as efficient laccase mediators for decolorization of different types of recalcitrant dyes. Appl Environ Microbiol 71:1775–1784
Chen H (2006) Recent advances in azo dye degrading enzyme research. Curr Protein Pept Sci 7:101–111
Claus H, Faber G, König H (2002) Redox-mediated decolorization of synthetic dyes by fungal laccases. Appl Microbiol Biotechnol 59:672–678
Couto SR, Herrera JLT (2006) Industrial and biotechnological applications of laccases: a review. Biotechnol Adv 24:500–513
Dubé E, Shareck F, Hurtubise Y, Daneault C, Beauregard M (2008) Homologous cloning, expression, and characterization of a laccase from Streptomyces coelicolor and enzymatic decolourisation of an indigo dye. Appl Microbiol Biotechnol 79:597–603
Eggert C, Temp U, Eriksson KE (1996) The ligninolytic system of the white-rot fungus Pycnoporus cinnabarinus: purification and characterization of the laccase. Appl Environ Microbiol 62:1151–1158
Faraco V, Ercole C, Festa G, Giardina P, Piscitelli A, Sannia G (2008) Heterologous expression of heterodimeric laccase from Pleurotus ostreatus in Kluyveromyces lactis. Appl Microbiol Biotechnol 77:1329–1335
Garavaglia S, Cambria MT, Miglio M, Ragusa S, Iacobazzi V, Palmieri F, D’Ambrosio C, Scaloni A, Rizzi M (2004) The structure of Rigidoporus lignosus Laccase containing a full complement of copper ions, reveals an asymmetrical arrangement for the T3 copper pair. J Mol Biol 342:1519–1531
Guo M, Lu F, Liu M, Li T, Pu J, Wang N, Liang P, Zhang C (2008) Purification of recombinant laccase from Trametes versicolor in Pichia methanolica and its use for the decolorization of anthraquinone dye. Biotechnol Lett 30:2091–2096
Hong F, Meinander NQ, Jönsson LJ (2002) Fermentation strategies for improved heterologous expression of laccase in Pichia pastoris. Biotechnol Bioeng 79:438–449
Hoshida H, Fujita T, Murata K, Kubo K, Akada R (2005) Copper-dependent production of a Pycnoporus coccineus extracellular laccase in Aspergillus oryzae and Saccharomyces cerevisiae. Biosci Biotechnol Biochem 69:1090–1097
Khatri NK, Hoffmann F (2006) Impact of methanol concentration on secreted protein production in oxygenlimited cultures of recombinant Pichia pastoris. Biotechnol Bioeng 93:871–879
Lorenzo M, Diego MM, Sanroman A (2006) Effect of heavy metals on the production of several laccase isoenzymes by Trametes versicolor and on their ability to decolourise dyes. Chemosphere 63:912–917
Macauley-Patrick S, Fazenda ML, McNeil B, Harvey LM (2005) Heterologous protein production using the Pichia pastoris expression system. Yeast 22:249–270
Madzak C, Mimmi MC, Caminade E, Brault A, Baumberger S, Briozzo P, Mougin C, Jolivalt C (2006) Shifting the optimal pH of activity for a laccase from the fungus Trametes versicolor by structure-based mutagenesis. Protein Eng Des Sel 19:77–84
O’Callaghan J, O’Brien MM, McClean K, Dobson ADW (2002) Optimization of the expression of a Trametes versicolor laccase gene in Pichia pastoris. J Ind Microbiol Biotechnol 29:55–59
Ong E, Pollock WBR, Smith M (1997) Cloning and sequence analysis of two laccase complementary DNAs from the ligninolytic basidiomycete Trametes versicolor. Gene 196:113–119
Otterbein L, Record E, Longhi S, Asther M, Moukha S (2000) Molecular cloning of the cDNA encoding laccase from Pycnoporus cinnabarinus I-937 and expression in Pichia pastoris. Eur J Biochem 267:1619–1625
Paola G (2010) Laccases: a never-ending story. Cell Mol Life Sci 67:369–385
Riva S (2006) Laccases: blue enzymes for green chemistry. Trends Biotechnol 24:219–226
Soares GMB, Costa-Ferreira M, de Amorim MTP (2001) Decolorization of an anthraquinone-type dye using a laccase formulation. Bioresour Technol. 79:171–177
Soden DM, O’Callaghan J, Dobson ADW (2002) Molecular cloning of a laccase isozyme gene from Pleurotus sajor-caju and expression in the heterologous Pichia pastoris host. Microbiology 148:4003–4014
Téllez-Jurado A, Arana-Cuenca A, González Becerra AE, Viniegra-González G, Loera O (2006) Expression of a heterologous laccase by Aspergillus niger cultured by solid-state and submerged fermentations. Enzyme Microb Technol 38:665–669
Wesenberg D, Kyriakides I, Agathos SN (2003) Whiterot fungi and their enzymes for the treatment of industrial dye effluents. Biotechnol Adv 22:161–187
Yan HB, Chi YJ (2013) Cloning and characterization of two novel manganese peroxidase genes from the white-rot basidiomycete Lenzites gibbosa. Curr Microbiol 23:224–247
Zumárraga M, Bulter T, Shleev S, Polaina J, Martínez-Arias A, Plou FJ, Ballesteros A, Alcalde M (2007) In vitro evolution of a fungal laccase in high concentrations of organic cosolvents. Chem Biol 14:1052–1064
Acknowledgments
This study was financially supported by National Natural Science Foundation of China (No. 30671700, “Study on cloning, characterization and expression of manganese peroxidase genes from white-rot fungi”), which is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zheng, M., Chi, Y., Yi, H. et al. Decolorization of Alizarin Red and other synthetic dyes by a recombinant laccase from Pichia pastoris . Biotechnol Lett 36, 39–45 (2014). https://doi.org/10.1007/s10529-013-1323-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-013-1323-2