Abstract
A new Lysinibacillus fusiformis strain with abundant laccase activity was isolated from soil under forest rotted leaf and identified as L. fusiformis W11 based on its 16S rRNA gene sequence and physiological characteristics. The laccase LfuLac was purified and characterized. The optimum temperature and pH of LfuLac on guaiacol were 45 °C and pH 9, respectively. LfuLac kept 78%, 88%, 92%, 74%, and 47% of activity at pH 7-11, respectively, suggesting the alkali resistance of the enzyme. The effects of various metal ions on LfuLac showed that Cu2+, Mg2+, and Na+ were beneficial to laccase activity and 10 mM Cu2+ increased the activity of LfuLac to 216%. LfuLac showed about 90% activity at 5% organic solvents and more than 60% activity at 20%, indicating its resistance to organic solvents. In addition, LfuLac decolorized different kinds of dyes. This study enriched our knowledge about laccase from L. fusiformis W11 and its potential industrial applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Laccase (benzenediol: oxygen oxidoreductase; EC 1.10.3.2) is a copper-containing polyphenol oxidase that is widely distributed in plants, insects, fungi, and bacteria [1]. Laccases are applied in food, pharmaceutical, and environmental industries because they can oxidize a wide variety of compounds and reduce oxygen into water without producing harmful by-products [2,3,4]. During the biotechnological applications of laccases, reactions are often carried out under extreme conditions, such as extremely acidic and alkaline pH and high concentration of organic solvents [5, 6]. Thus, searching for robust laccases that can tolerate harsh conditions is an ongoing effort to date.
Compared with plant and animal laccases, microbial laccases are abundant and classified into fungal and bacterial laccases [7]. Fungal laccases have been found in many species, such as Trametes trogii, Coriolopsis caperata, and Trametes versicolor [8,9,10]. Fungal laccases have been studied in a variety of biotechnological applications, but their applications are usually hampered due to long fermentation periods, narrow pH range, and intolerance to extreme conditions [11]. Many bacterial laccases are available under extreme conditions and expected to replace fungal laccases for application to bioremediation, industrial wastewater treatment, and dye decolorization [1, 7, 12]. Laccases are discovered in a number of bacteria, including Bacillus subtilis, Escherichia coli, Streptomyces coelicolor, Stenotrophomonas maltophilia, and Thermus thermophilus [13, 14]. More bacterial laccases need to be found to meet the biotechnological applications.
Lysinibacillus fusiformis is a Gram-positive and rod-shaped bacterium, which has been isolated from multiple environments [15]. L. fusiformis has been reported for its capability for azo dye decolorization, petroleum degradation, and deproteinization [16, 17]. Meanwhile, some extracellular enzymes of L. fusiformis, such as L-asparaginase and protease, have been reported, suggesting L. fusiformis was a multifunctional bacterium. The laccase of L. fusiformis has also been reported with ability of removal sulfonamides and tetracyclines residues, but other characteristics of the laccase need to be further studied [18]. In this study, a new strain of L. fusiformis W11 was isolated from soil under forest rotted leaf in Wuling Mountain, Chongqing, China, and showed significant laccase activity. This study purified the laccase LfuLac and investigated its characteristics.
Materials and methods
Isolation and identification
Sample was collected from soil under forest rotted leaf in Wuling Mountain, Chongqing, China. Ten grams of soil sample were properly mixed with 90 mL of distilled water in a 250-mL flask and serially diluted up to 10−6. A 0.1-mL sample diluent was spread over solid lysogeny broth (LB) medium (10-g/L tryptone, 5-g/L yeast extract, 10 g/L NaCl, 10 g/L agar) containing 0.04% (v/w) guaiacol, which is a typical substrate of laccase [19]. The plates were incubated at 30 °C for 1–5 d. The colonies that showed red–brown circle on the plates were repeatedly transferred on LB solid plates until pure cultures were obtained.
Molecular characterization of the pure bacterial colony was conducted using 16S rRNA gene sequencing [20]. Genomic DNA was isolated with the TIANamp bacteria DNA Kit (Tiangen, Beijing, China). The 16S bacterial rRNA gene sequence was amplified by 27F with sequence 5′-AGAGTTTGATCATGGCTCAG-3′ and 1492R with sequence 5′-CTACGGTTACCTTGTTAC GAC-3′. The gene sequence of 16S rRNA was verified by sequencing. A phylogenetic tree was constructed using neighbor-joining and maximum likelihood methods with MEGA 7 software, and bootstrap values were calculated from 1000 replications [21].
Purification of enzyme
LB medium was used for the production of laccase. Laccase was purified using the method described by Liu et al. [22]. The culture was centrifuged at 5000 rpm for 10 min. The supernatant was precipitated by ammonium sulfate, dialyzed in 0.1 M citrate-phosphate buffer (pH 7.0) for 48 h at 4 °C to remove ammonium sulfate and lyophilized. Then, the sample was loaded onto a DEAE-cellulose anion exchange column which was previously equilibrated with 0.1 M citrate-phosphate buffer (pH 7.0). After the unadsorbed proteins had been eluted, the adsorbed proteins were eluted with linear gradient concentration of NaCl from 0 to 1.0 M. All obtained fractions were monitored for laccase activity and the fractions containing laccase activity were pooled, concentrated, and dialyzed overnight. The sample was loaded onto a Sephadex G-100 column which was previously equilibrated with 0.1 M citrate-phosphate buffer (pH 7.0). Elution was collected with citrate-phosphate buffer and the eluted fractions with laccase activity were pooled and dialyzed. Protein content was then determined by SDS-PAGE, and protein concentration was determined by Nanovue Plus (GE).
Biochemical characterization
The activity of LfuLac was determined using guaiacol as substrate [23, 24]. The reaction mixture containing 1 μM LfuLac, 3 mM guaiacol, and 10 mM Cu2+ was incubated at set temperatures for 30 min. Absorbance of the reaction mixture at 465 nm was recorded to represent the production of tetraguaiacol. The optimum temperature was the temperature with the highest activity on guaiacol at the optimum pH during a 30-min reaction period. Optimum pH was determined by measuring the activity on guaiacol at 45 °C at different pH levels. Relative laccase activity was measured by setting the activity at the optimal temperature and pH as 100%. LfuLac was incubated at different temperatures at pH 9.0 for 0.5, 1.0, 2.0, 4.0, 8.0, and 16.0 h to determine its thermostability. LfuLac was incubated at different pH levels at room temperature for 0.5, 1.0, 2.0, 4.0, 8.0, and 16.0 h to determine pH stability. The amount of activity retained was detected. The activity of LfuLac without incubation was set as 100%. Cu2+ (CuCl2), Mg2+ (MgCl2), Zn2+ (ZnCl2), Ni2+ (NiCl2), Mn2+ (MnCl2), Co2+ (CoCl2), Na+ (NaCl), and K+ (KCl) were used to study the effects of metal ions on LfuLac. Five concentrations of metal ions (1, 2, 5, 10, and 20 mM) were tested. Five organic solvents (ethanol, acetone, methanol, chloroform, and DMSO) were tested at concentrations of 5%, 10%, and 20% (v/v) to study their effects on laccase activity.
Dye decolorization assays
Dye decolorization assays were performed as previously described [25, 26]. The reaction mixture of 1 μM LfuLac, 15 mg/L dyes, and 10 mM Cu2+ was incubated at 30 °C for 1 h. Dye decolorization was determined by recording changes in absorbance at the following wavelengths for different dyes: crystal violet (590 nm), malachite green (624 nm), Coomassie brilliant blue R-250 (556 nm), Congo red (497 nm), Acid red 27 (520 nm), methyl orange (465 nm), Acid blue 25 (595 nm), and Disperse blue 60 (670 nm). Samples without the enzyme were used as control. Dye decolorization was calculated using the following equation:
Results
Screening and identification of laccase-positive strain
The soil sample was cultured in LB–guaiacol medium to screen for laccase-positive strains. Eight strains showed a red–brown oxidized zone on the plates, indicating that they were laccase active. Strain W11 was screened as the best laccase-secreting strain because of the biggest red–brown oxidized zone on the LB–guaiacol plate (Fig. S1). The colony of W11 was irregular, moist, flat, and slightly convex (Fig. 1a). The strain was Gram-positive and had rod-shaped cells (Fig. 1b).
The 16S rRNA gene sequence (1403 nt) of the strain was amplified, purified, and sequenced to identify W11. The 16S rRNA sequence of W11 (accession no: OQ418029) showed the 99.67% similarity to the sequences of L. fusiformis DSM2898. A phylogenetic tree was constructed by neighbor-joining and maximum likelihood methods, and the results revealed that W11 clustered with the members of L. fusiformis (Fig. 1c). According to the analysis of 16S rRNA sequence and morphological characteristics, strain L-11 was identified as L. fusiformis and designated as L. fusiformis W11. The laccase secreted by L. fusiformis W11 was labeled as LfuLac.
Purification of LfuLac from L. fusiformis W11
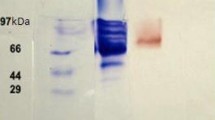
The laccase activities in the liquid medium were 0.11, 0.35, 0.87, and 1.01 U/mL in 1 to 4 days, respectively, and it reached the maximum level of 1.28 U/mL on the 5th day. The extracellular laccase from L. fusiformis W11 was purified, and a summary of purification data is shown in Table 1. LfuLac was purified by ammonium sulfate precipitation, DEAE-cellulose anion exchange, and Sephadex G-100 gel filtration chromatography, generating a 19.11-fold increase in purity and 44.31% yield. The purified LfuLac was examined by SDS-PAGE (Fig. 2).
Effect of pH and temperature on the activity and stability of LfuLac
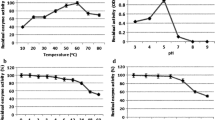
Assays were performed using guaiacol as substrate to determine the laccase activity of LfuLac. As shown in Fig. 3a, the laccase activity of LfuLac increased gradually from 25 °C to 45 °C. LfuLac reached its maximum activity of 76 U/mL at 45 °C and decreased at higher temperatures. The effect of pH on the laccase activity of LfuLac was examined at 45 °C. LfuLac kept more than 70% activity over a wide range of pH 7.0–11.0 and reached its highest activity at pH 9.0 (optimum pH) (Fig. 3b).
Biochemical characterization of LfuLac. a and b Optimum temperature and pH of LfuLac determined with guaiacol as the substrate. Relative laccase activity was measured by setting the activity at the optimal temperature and pH as 100%. c and d Thermostability and pH stability of LfuLac. The activity of PthLac without incubation was set as 100%. The error bars represent the standard error of the mean of triplicate measurements
Temperature and pH stability were examined. The activity of LfuLac remained almost 100% after incubation at 10 °C and 0 °C for 1 h and then decreased gradually with increasing incubation temperatures (Fig. 3c). LfuLac retained about 74% and 40% of its activity after incubation at 30 °C and 40 °C for 1 h, respectively. The activity of LfuLac almost reduced to 0 after incubation at 60 °C for 1 h. As shown in Fig. 3d, LfuLac kept 78%, 88%, 92%, 74%, and 47% of its activity at pH 7–11, suggesting the alkali resistance of the enzyme under these conditions. However, the activity of LfuLac decreased to 50% at pH 5 and was 9% at pH 4, indicating that it was unstable against strong acid conditions.
Effect of metal ions on the activity of LfuLac
The reaction mixtures were added with Cu2+, Mg2+, Zn2+, Ni2+, Mn2+, Co2+, Na+, and K+ to study the effects of metal ions on LfuLac. Five concentrations of metal ions (1, 2, 5, 10, and 20 mM) were tested. As shown in Fig. 4, Cu2+, Mg2+, and Na+ were beneficial to laccase activity. To be specific, 10 mM Cu2+ increased the activity of LfuLac to 216%, suggesting that LfuLac was a Cu2+-activated laccase. Moreover, 2 mM Mg2+ and Na+ 10 mM increased the laccase activity to 152% and 125%, respectively. The addition of K+, Mn2+, and Co2+ did not show obvious effects on LfuLac. Zn2+ and Ni2+ suppressed the activity of LfuLac and exhibited dose-dependent inhibition effects at high concentrations.
Effects of organic solvents on the activity of LfuLac
Five organic solvents (ethanol, acetone, methanol, chloroform, and DMSO) were tested at concentrations of 5%, 10%, and 20% (v/v). The laccase activity without any chemicals of 76 U/mL was set as 100%. As shown in Fig. 5, LfuLac showed more than 90% activity at 5% organic solvents and more than 60% activity at 20%, indicating that the enzyme displayed decent resistance to the tested chemicals. In 10% ethanol, acetone, methanol, chloroform, and DMSO, LfuLac exhibited 87.13%, 80.32%, 82.19%, 80.19%, 76.87%, 92.52%, and 89.89% activity, respectively. Compared with other organic solvents, LfuLac displayed stronger resistance to ethanol with more than 65% activity at 20% concentrations. Hence, LfuLac remained active at high concentrations of the chemicals and might have potential for industrial applications.
Dye decolorization capacity of LfuLac
As shown in Table 2, nine dyes from three categories were used to test the dye decolorization capacity of LfuLac. The results showed that LfuLac partly decolorized all the tested dyes. LfuLac showed the strongest decolorization capacity to azo dye than the two other dyes and the least decolorization rate to anthraquinone. The decolorization capacities of LfuLac to Congo red, Acid Red 27, and methyl orange were 45.18%, 45.11%, and 36.53%, respectively.
Discussion
L. fusiformis is one of the most recognized species in the genus Lysinibacillus. It was first isolated in 1901 from the surface of Beta vulgaris [15]. Since then, many strains of L. fusiformis have been isolated from various environmental samples, such as soil, wastewater, and potato phyllosphere [27, 28]. In this study, a new strain was isolated from soil under forest rotted leaf, which is habitable and rich in lignocellulose, and was identified as L. fusiformis W11 by its 16S rRNA sequence and morphological characteristics. Laccase was able to oxidize non-phenolic lignin, which might make L. fusiformis W11 secrete laccase to adapt to its habitat. The laccase activity of LfuLac was 44 U/mL when incubation with guaiacol as the substrate under the optimal reaction conditions. The value is higher than that of laccase from Agaricus blazei U2-4 (25.4 U/mL) and Colletotrichum gloeosporioides (38 U/mL) [29, 30]. Hence, L. fusiformis W11 could be a laccase-secreting microorganism. However, the activity is lower than that of laccase from Bacillus subtilis MTCC 2414 (100 U/mL), suggesting that the conditions need to be further optimized to increase the production of LfuLac.
Several extracellular laccases were purified with the three consecutive procedures, such as laccase from Marasmius scorodonius, laccase from Marasmius sp. BBKAV79, and ThLacc-S from Trametes hirsute [31, 32]. Compared with laccase activities of 432.8 U/mg from Marasmius scorodonius and 0.226 U/mg laccase from Marasmius sp. BBKAV79, the laccase activity of LfuLac was moderate 20 U/mg. The molecular weight of LfuLac was about 31 kDa, which was consistent with SLAC (PDB ID: 3CG8) from Streptomyces coelicolor, suggesting LfuLac might be a small bacterial laccase [13].
Alkali-stable laccases have been studied in many reports. ThLacc-S from Trametes hirsuta retained about 70% of its activity after incubation at pH 10 for 72 h, but the activity decreased to about 10% at pH 11 [24]. Laccase from Bacillus tequilensis SN4 kept more than 75% activity at pH 9.0 for 24 h, but higher pH was not tested [33]. The purified LfuLac protein kept more than 70% of its activity at pH 7–10, which was not prominent compared with other reported alkali-stable laccases. However, LfuLac retained 47% of its activity at pH11, suggesting its strong alkali resistance. Most of the known laccases are unstable at concentrations above 10% of organic solvents [34, 35] . However, LfuLac was stable and retained more than 80% of its activity in the presence of 10% ethanol, acetone, methanol, and chloroform. LfuLac kept about 60% activity in 20% organic solvents, indicating that it was an organic solvent-stable enzyme. Moreover, LfuLac decolorized triarylmethane, azo, and anthraquinone dyes. Therefore, LfuLac might have potential to industrial applications, such as pulp biobleaching and textile dye decolorization, due to its remarkable tolerance to alkali-organic solvents and decolorizing ability.
In conclusion, this study reported the laccase LfuLac obtained from L. fusiformis W11. Alkali tolerance, stability in organic solvents, and decolorizing ability were the important features of LfuLac. Overall, LfuLac could be a promising enzyme for biotechnological applications.
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
Khatami SH, Vakili O, Movahedpour A, Ghesmati Z, Ghasemi H, Taheri-Anganeh M (2022) Laccase: various types and applications. Biotechnol Appl Biochem 69(6):2658–2672. https://doi.org/10.1002/bab.2313
Arregui L, Ayala M, Gomez-Gil X, Gutierrez-Soto G, Hernandez-Luna CE, Herrera de Los Santos M, Levin L, Rojo-Dominguez A, Romero-Martinez D, MCN S, Trujillo-Roldan MA, Valdez-Cruz NA (2019) Laccases: structure, function, and potential application in water bioremediation. Microb Cell Fact 18(1):200. https://doi.org/10.1186/s12934-019-1248-0
Schmitz E, Leontakianakou S, Norlander S, Nordberg Karlsson E, Adlercreutz P (2022) Lignocellulose degradation for the bioeconomy: the potential of enzyme synergies between xylanases, ferulic acid esterase and laccase for the production of arabinoxylo-oligosaccharides. Bioresour Technol 343:126114. https://doi.org/10.1016/j.biortech.2021.126114
Mayolo-Deloisa K, Gonzalez-Gonzalez M, Rito-Palomares M (2020) Laccases in food industry: bioprocessing, potential industrial and biotechnological applications. Front Bioeng Biotechnol 8:222. https://doi.org/10.3389/fbioe.2020.00222
Feng H, Zhang D, Sun Y, Zhi Y, Mao L, Luo Y, Xu L, Wang L, Zhou P (2015) Expression and characterization of a recombinant laccase with alkalistable and thermostable properties from Streptomyces griseorubens JSD-1. Appl Biochem Biotechnol 176(2):547–562. https://doi.org/10.1007/s12010-015-1594-2
Neifar M, Chouchane H, Mahjoubi M, Jaouani A, Cherif A (2016) Pseudomonas extremorientalis BU118: a new salt-tolerant laccase-secreting bacterium with biotechnological potential in textile azo dye decolourization. 3 Biotech 6(1):107. https://doi.org/10.1007/s13205-016-0425-7
Chandra R, Chowdhary P (2015) Properties of bacterial laccases and their application in bioremediation of industrial wastes. Environ Sci Process Impacts 17(2):326–342. https://doi.org/10.1039/c4em00627e
Chaoua S, Chaouche NK, Songulashvili G, Gares M, Hiligsmann S, Flahaut S (2023) Yellow laccase produced by Trametes versicolor K1 on tomato waste: a comparative study with the blue one produced on semi-synthetic medium. J Biotechnol 361:99–109. https://doi.org/10.1016/j.jbiotec.2022.12.001
Nandal P, Ravella SR, Kuhad RC (2013) Laccase production by Coriolopsis caperata RCK2011: optimization under solid state fermentation by Taguchi DOE methodology. Sci Rep 3:1386. https://doi.org/10.1038/srep01386
Yan J, Chen Y, Niu J, Chen D, Chagan I (2015) Laccase produced by a thermotolerant strain of Trametes trogii LK13. Braz J Microbiol 46(1):59–65. https://doi.org/10.1590/S1517-838246120130895
Margot J, Bennati-Granier C, Maillard J, Blánquez P, Barry DA, Holliger C (2013) Bacterial versus fungal laccase: potential for micropollutant degradation. AMB Express 3(1):63. https://doi.org/10.1186/2191-0855-3-63
Siroosi M, Amoozegar MA, Khajeh K (2016) Purification and characterization of an alkaline chloride-tolerant laccase from a halotolerant bacterium Bacillus sp. strain WT. J Mol Catal B: Enzym 134:89–97. https://doi.org/10.1016/j.molcatb.2016.10.001
Dasgupta R, Gupta K, Nami F, de Groot HJM, Canters GW, Groenen EJJ, Ubbink M (2020) Chemical exchange at the trinuclear copper center of small laccase from Streptomyces coelicolor. Biophys J 119(1):9–14. https://doi.org/10.1016/j.bpj.2020.05.022
Unuofin JO (2020) Sustainability potentials of novel laccase tinctures from Stenotrophomonas maltophilia BIJ16 and Bordetella bronchiseptica HSO16: from dye decolourization to denim bioscouring. Biotechnol Rep (Amst) 25:e00409. https://doi.org/10.1016/j.btre.2019.e00409
Morioka H, Oka K, Yamada Y, Nakane Y, Komiya H, Murase C, Iguchi M, Yagi T (2022) Lysinibacillus fusiformis bacteremia: case report and literature review. J Infect Chemother 28(2):315–318. https://doi.org/10.1016/j.jiac.2021.10.030
Zhu L, Guo J, Sun Y, Wang S, Zhou C (2021) Acetic acid-producing endophyte Lysinibacillus fusiformis orchestrates jasmonic acid signaling and contributes to repression of cadmium uptake in tomato plants. Front Plant Sci 12:670216. https://doi.org/10.3389/fpls.2021.670216
Reyes-Cervantes A, Robles-Morales DL, Tellez-Jurado A, Huerta-Ochoa S, Jimenez-Gonzalez A, Medina-Moreno SA (2021) Evaluation in the performance of the biodegradation of herbicide diuron to high concentrations by Lysinibacillus fusiformis acclimatized by sequential batch culture. J Environ Manag 291:112688. https://doi.org/10.1016/j.jenvman.2021.112688
Ouyang B, Xu W, Zhang W, Guang C, Mu W (2022) Efficient removal of sulfonamides and tetracyclines residues by the laccase-mediator system employing a novel laccase from Lysinibacillus fusiformis. J Environ Chem Eng 10(6). https://doi.org/10.1016/j.jece.2022.108809
Karittapattawan P, Benchawattananon R (2021) Evaluation of laccase production by monokaryotic strains of edible mushrooms. Pak J Biol Sci 24(4):454–460. https://doi.org/10.3923/pjbs.2021.454.460
Qu JH, Ma WW, Li HF, Wang XF, Lu BB, Luo Y (2019) Altererythrobacter amylolyticus sp. nov., isolated from lake sediment. Int J Syst Evol Microbiol 69(4):1231–1236. https://doi.org/10.1099/ijsem.0.003299
Liao H, Li Y, Zhang M, Lin X, Lai Q, Tian Y (2017) Altererythrobacter mangrovi sp. nov., isolated from mangrove sediment. Int J Syst Evol Microbiol 67(11):4851–4856. https://doi.org/10.1099/ijsem.0.002393
Sun Y, Liu ZL, Hu BY, Chen QJ, Yang AZ, Wang QY, Li XF, Zhang JY, Zhang GQ, Zhao YC (2021) Purification and characterization of a thermo- and pH-stable laccase from the litter-decomposing fungus Gymnopus luxurians and laccase mediator systems for dye decolorization. Front Microbiol 12:672620. https://doi.org/10.3389/fmicb.2021.672620
Jiang YP, Cai JL, Pei JJ, Li Q, Zhao LG (2021) Cloning, overexpression, and characterization of a thermostable, organic solvent-tolerant laccase from Bacillus pumilus ARA and its application to dye decolorization. ACS Omega 6(14):9741–9749. https://doi.org/10.1021/acsomega.1c00370
Si J, Ma H, Cao Y, Cui B, Dai Y (2021) Introducing a thermo-alkali-stable, metallic ion-tolerant laccase purified from white rot fungus Trametes hirsuta. Front Microbiol 12:670163. https://doi.org/10.3389/fmicb.2021.670163
Sharma N, Leung IKH (2021) Characterization and optimisation of a novel laccase from Sulfitobacter indolifex for the decolourisation of organic dyes. Int J Biol Macromol 190:574–584
Park JH, Jeon SJ (2021) Production and characterization of crude laccase from Irpex sp. JS7 that decolorizes synthetic and natural melanin. Folia Microbiol (Praha) 66(6):1039–1046. https://doi.org/10.1007/s12223-021-00904-x
Passera A, Rossato M, Oliver JS, Battelli G, Shahzad GI, Cosentino E, Sage JM, Toffolatti SL, Lopatriello G, Davis JR, Kaiser MD, Delledonne M, Casati P (2021) Characterization of Lysinibacillus fusiformis strain S4C11: in vitro, in planta, and in silico analyses reveal a plant-beneficial microbe. Microbiol Res 244:126665. https://doi.org/10.1016/j.micres.2020.126665
Khadka S, Adhikari S, Thapa A, Panday R, Adhikari M, Sapkota S, Regmi RS, Adhikari NP, Proshad R, Koirala N (2020) Screening and optimization of newly isolated thermotolerant Lysinibacillus fusiformis strain SK for protease and antifungal activity. Curr Microbiol 77(8):1558–1568. https://doi.org/10.1007/s00284-020-01976-7
Valle JS, Vandenberghe LP, Oliveira AC, Tavares MF, Linde GA, Colauto NB, Soccol CR (2015) Effect of different compounds on the induction of laccase production by Agaricus blazei. Genet Mol Res 14(4):15882–15891. https://doi.org/10.4238/2015.December.1.40
Guetsky R, Kobiler I, Wang X, Perlman N, Gollop N, Avila-Quezada G, Hadar I, Prusky D (2005) metabolism of the flavonoid epicatechin by laccase of Colletotrichum gloeosporioides and its effect on pathogenicity on avocado fruits. Phytopathology 95(11):1341–1348. https://doi.org/10.1094/PHYTO-95-1341
Jeon SJ, Lim SJ (2017) Purification and characterization of the laccase involved in dye decolorization by the white-rot fungus Marasmius scorodonius. J Microbiol Biotechnol 27:1120–1127. https://doi.org/10.4014/jmb.1701.01004
Vantamuri AB, Kaliwal BB (2016) Purification and characterization of laccase from Marasmius species BBKAV79 and effective decolorization of selected textile dyes. 3 Biotech 6:189. https://doi.org/10.1007/s13205-016-0504-9
Sondhi S, Sharma P, Saini S, Puri N, Gupta N (2014) Purification and characterization of an extracellular, thermo-alkali-stable, metal tolerant laccase from Bacillus tequilensis SN4. PLoS One 9(5):e96951. https://doi.org/10.1371/journal.pone.0096951
Liu X, Deng W, Yang Y (2021) Characterization of a novel laccase LAC-Yang1 from white-rot fungus Pleurotus ostreatus strain Yang1 with a strong ability to degrade and detoxify chlorophenols. Molecules 26(2). https://doi.org/10.3390/molecules26020473
Motamedi E, Kavousi K, Sadeghian Motahar SF, Reza Ghaffari M, Sheykh Abdollahzadeh Mamaghani A, Hosseini Salekdeh G, Ariaeenejad S (2021) Efficient removal of various textile dyes from wastewater by novel thermo-halotolerant laccase. Bioresour Technol 337:125468. https://doi.org/10.1016/j.biortech.2021.125468
Funding
This study was funded by the Key Scientific Projects for colleges of Henan Province (Grant number 21A180004), the Henan Provincial Science and Technology Research Project (Grant number 222102320250), the Doctoral Scientific Research Start-up Foundation from Henan University of Technology (Grant number 2018BS080), and Natural Science Foundation of Henan Province (Grant number 232300420206).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
This study does not contain any studies with human participants or animals performed by any of the authors.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
The participant has consented to the submission of the case report to the journal.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Responsible Editor: Lucy Seldin
Supplementary information
ESM 1
(DOCX 19 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, X., Chen, P., Liu, Z. et al. Purification and characterization of an alkali-organic solvent-stable laccase with dye decolorization capacity from newly isolated Lysinibacillus fusiformis W11. Braz J Microbiol 54, 1935–1942 (2023). https://doi.org/10.1007/s42770-023-01091-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42770-023-01091-2