Abstract
Fungal treatment followed by FeCl3 treatment was used to improve saccharification of wood from Populus tomentosa. Combined treatments accumulated lignin and slightly degraded cellulose, whereas almost all hemicelluloses were removed. The white rot fungus, Trametes orientalis, and the brown rot fungus, Fomitopsis palustris, both accompanied by FeCl3 post-treatment resulted in 98.8 and 99.7 % of hemicelluloses loss at 180 °C, respectively, which were over twice than that of hot water pretreatment at the same level. In addition, the solid residue from the T. orientalis-assisted and F. palustris-assisted FeCl3 treatment at 180 °C released 84.5 and 95.4 % of reducing sugars, respectively: 1.4- and 1.6-fold higher than that of FeCl3 treatment alone at the same temperature. Combined treatments disrupted the intact cell structure and increased accessible surface area of cellulose therefore enhancing the enzymatic digestibility, as evidenced by XRD and SEM analysis data.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Microbial pretreatment of lignocellulosic materials with white rot fungi can effectively improve enzymatic hydrolysis. The process has a low energy input, uses little or no chemicals and does not generate a waste stream (Wan and Li 2010; Wang et al. 2012). However, microbial pretreatment has inherent disadvantages such as long pretreatment time and simultaneous degradation of carbohydrate and lignin. Combination of fungal pretreatment with a physical or chemical treatment can potentially overcome these drawbacks of fungal treatment alone since the combined pretreatment process could initiate a synergistic effect thereby improving the yields of end products. A combination of fungal pretreatment with one of the other types of treatment methods for biomass has been investigated, such as combined fungal pretreatment with mild alkaline (Yu et al. 2010), dilute acid (Ma et al. 2010), or hot water (Wang et al. 2012). The results suggest that combined treatments improve saccharification and biofuel production when compared to a single treatment process.
Metal salts can also increase the hydrolysis rate of cellulose and hemicelluloses during acid or liquid hot water (LHW) pretreatments. NaCl, KCl, CaCl2, MgCl2 and especially FeCl3 significantly increased xylose monomer and xylotriose degradation in water at 180 °C. When treated with 0.8 % FeCl3, the rate constants for the degradation of xylose and xylotriose increased 6- and 49-fold, respectively, compared to treatment with just pressurized hot water at the same temperature (Liu and Wyman 2006). In addition, NaCl, KCl, CaCl2, MgCl2, FeSO4, FeCl3, and Fe2(SO4)3, accelerated the degradation of hemicelluloses in corn stover and among these, FeCl3 gave the optimum performance (Liu et al. 2009a).
Wood-rotting fungi are the natural decomposers of wood in forest ecosystems. China is rich in wood-rotting fungi and around 1,200 species have been reported (Dai 2012). Some of these fungi have the potential to enhance the enzymatic hydrolysis of poplar wood (Wang et al. 2012). Based on a previous screening study, two promising wood-rotting fungi, Trametes orientalis (white rot fungus) and Fomitopsis palustris (brown rot fungus), were selected from 48 wood-rotting fungi strains for this investigation (data not shown). In this work, a facile two-step design, fungal treatment followed by FeCl3 treatment, was employed to treat Populus tomentosa. The synergy of this combined treatment on the modification of the chemical compositions of plant cell walls and improvement on saccharification, as well as crystallinity and morphology of poplar wood were all investigated.
Materials and methods
Microorganisms and inocula preparation
White rot fungus, T. orientalis (isolate BJFC004176), and brown rot fungus, F. palustris (isolate BJFC006103), were isolated, respectively, from Hainan and Guangdong Provinces in China. The fungi were identified by phylogeny-assisted morphological methods. The final organisms were isolated from fresh basidiocarps of the two fungi. They were activated in 100 ml basic medium (g/l: glucose 20, yeast extract 5, KH2PO4 1, MgSO4 0.5, vitamin B1 0.01), and cultured at 28 °C at 150 rpm. Mycelia were harvested after 5 days, mixed with 100 ml distilled water and then blended for 30 s at 5,000 rpm. This suspension acted as inoculum for further work.
Raw materials
Fresh poplar wood (P. tomentosa) was chopped into pieces and air-dried. The samples were ground and particles between 20 and 80 mesh were prepared for the subsequent pretreatment with wood-rotting fungi and FeCl3, respectively.
Biological treatment of poplar wood
Treatment was carried out in a 500 ml Erlenmeyer flask with 15 g air-dried poplar wood and 37.5 ml of distilled water. Samples were sterilized for 20 min at 121 °C and mixed with 15 ml inoculum. Cultures were incubated statically at 28 °C for 4 weeks. Non-inoculated samples served as controls.
FeCl3 treatment of poplar wood
Experiments were conducted in batch tube reactors fabricated from 316 stainless steel tubes (length 11 cm, outside diam. 2.5 cm, total volume 50 ml). The reactor was filled with 2.5 g raw or bio-treated poplar wood and 25 ml 0.1 M FeCl3. A set of LHW pretreatments of 2.5 g raw and 25 ml distilled water was also conducted. After the slurry was loaded, stainless steel caps were fitted onto each end of the tubes. The tubes were then placed in an oil bath preheated to either 140, 160 or 180 °C with vigorous magnetic stirring (300 rpm). After 30 min, the tubes were held in water at room temperature, which caused the temperature of the internal tube to drop below 100 °C in less than 5 min.
Wet material was vacuum filtered to obtain water-insoluble residues. The residues after filtration were washed to neutralize with distilled water, and then dried at 35 °C for 24 h for further analysis (Lü and Zhou 2011).
Enzymatic hydrolysis
Commercial cellulase (Celluclast 1.5 L produced by Tricoderma reesei) and β-glucosidase (from almonds) were from Sigma-Aldrich. Enzymatic hydrolysis was conducted in 10 ml 50 mM sodium acetate buffer (pH 4.8) supplemented with 40 μl tetracycline (1 % w/v in 70 % v/v ethanol) and 20 μl cycloheximide (1 % w/v in distilled water). The substrate and enzyme were loaded at 2 % (w/v), 30 FPU/g substrate of cellulase and 37.5 IU/g substrate of β-glucosidase, respectively. The mixture was incubated at 50 °C and shaken at 150 rpm for 96 h. Samples were taken from the reaction mixture, centrifuged for 10 min at ~8,000×g, and stored at −20 °C for reducing sugar assay. Experiments were all performed in duplicate.
Analytical methods
The chemical composition of the raw material and treated residues was determined according to Sluiter et al. (2008) using HPAEC. The HPAEC system (Dionex ISC 3000, USA) was equipped with an amperometric detector, AS50 autosampler, a CarbopacTM PA-20 column (4 × 250 mm2, Dionex), and a guard PA-20 column (3 × 30 mm2, Dionex). Cellulose contents were calculated based on glucose using anhydro corrections of 0.9, hemicellulose contents were calculated based on the sum of xylose, galactose and arabinose, using 0.88 as anhydro corrections for xylose and arabinose, and 0.9 for galactose.
The reducing sugars in the supernatant after enzymatic hydrolysis were measured by dinitrosalicylic acid method. The reducing sugar yield was calculated as follows:
XRD and crystallinity analysis
X-ray powder diffraction pattern of the raw and pretreated poplar wood was obtained using an XRD-6000 instrument (Shimadzu, Japan). The X-ray diffractograms were recorded from 2θ = 5 to 40° using reflection method at a scanning speed of 5°/min. The determination was performed with Ni-filtered Cu-Kα radiation (λ = 1.54 Å) at 40 kV and 40 mA. Crystallinity of sample was quantified from X-ray powder diffraction data using a crystallinity index (CrI) for cellulose.
Scanning electron microscopy of poplar wood
Changes in morphology of poplar wood before and after treatment were observed. Prior to imaging, samples were coated with gold/palladium in a sputter coater.
Results
Decay of poplar wood
After 4 weeks’ biological treatment with the white rot fungus, T. orientalis, or the brown rot fungus, F. palustris, the bio-treated samples and raw materials were further subjected to FeCl3 treatment at 140, 160 and 180 °C for 30 min, respectively. The changes of chemical components in residues after various treatments are shown in Fig. 1.
Changes in chemical components of Populus tomentosa after various treatments at different temperatures. % was defined as each component percent based on treated mass. Values were measured in duplicate (n = 2) and are reported as the mean. a LHW sole liquid hot water treatment, b sole FeCl3 treatment, c Trametes orientalis assisted-FeCl3 treatment, d Fomitopsis palustris assisted-FeCl3 treatment. Components AIL acid-insoluble lignin, ASL acid-soluble lignin
In Fig. 1, not only T. orientalis decayed 22.8 % of acid-insoluble lignin (AIL), but also F. palustris decayed 15.7 % AIL. When the bio-treated samples and raw materials were subjected to FeCl3 treatment, however, a significant increase of AIL was observed with the increasing temperature (p < 0.05) and as high as 56 % of AIL was achieved by the combination of F. palustris with FeCl3 treatment at 180 °C.
In addition, large quantities of hemicelluloses were degraded by T. orientalis-assisted FeCl3 treatment and F. palustris-assisted FeCl3 treatment, amounting to 98.8, 99.7 % of hemicelluloses loss at 180 °C, respectively, which were over twice larger than that of sole hot water pretreatment at the same level.
In the samples co-treated by fungi and FeCl3, the cellulose contents increased at low temperature (<140 °C) (p < 0.05). However, when the reaction was above 140 °C, cellulose gradually decayed with raising temperature and only 32 % of cellulose remained in the residues co-treated by F. palustris and FeCl3.
Effects of treatments on enzymatic hydrolysis
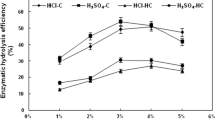
In Fig. 2, concerning the fungal treatment, the reducing sugar yield of T. orientalis and F. palustris increased by 61 and 30 %, respectively, compared to the untreated sample. When the raw and bio-treated poplar wood underwent FeCl3 treatment, the reducing sugars of sole treatment and combined treatments increased with temperature. Obviously, the solid residue of combined treatments released much more reducing sugar than sole FeCl3 treatment. 84.5 and 95.4 % of reducing sugar was obtained by T. orientalis-assisted and F. palustris-assisted FeCl3 treatment at 180 °C, respectively, being 1.4- and 1.6-fold higher than that of sole FeCl3 treatment at the same level.treatment. Components AIL acidinsoluble
Reducing sugar yield from 96-h saccharification of Populus tomentosa after various treatments. % was defined as the percent of reducing sugar released from cellulose and hemicellulose in enzymatic hydrolysis. Values were measured in duplicate (n = 2) and are reported as the mean. Treatments LHW liquid hot water, sole LHW treatment, FeCl3, sole FeCl3 treatment, Trametes orientalis + FeCl3, Trametes orientalis assisted-FeCl3 treatment, Fomitopsis palustris + FeCl3, Fomitopsis palustris assisted-FeCl3 treatment
CrI of poplar wood
The CrI of untreated poplar wood was 45.4 % but increased to >50 % after FeCl3 treatment alone and combined treatments from 140 to 160 °C (see Fig. 3). The CrI of FeCl3 treated and combination-treated poplar wood at 180 °C was lower than that of samples treated at 140 and 160 °C.
Degree of crystallinity of Populus tomentosa after various treatments. % was defined as the percent of crystalline cellulose in total cellulose. Treatments LHW liquid hot water, sole LHW treatment, FeCl3, sole FeCl3 treatment, Trametes orientalis + FeCl3, Trametes orientalis assisted-FeCl3 treatment, Fomitopsis palustris + FeCl3, Fomitopsis palustris assisted-FeCl3 treatment
Morphological characterizations of poplar wood
SEM images show that the untreated poplar wood had a flat, smooth, and highly fibrillar morphology whereas FeCl3 treatment and fungi-assisted FeCl3 treatment at 180 °C resulted in severe damage to the intact cell structure (Fig. 4). The particle size of the samples was reduced and delamination and increased porosity were also observed on the surface of the pretreated poplar wood.
SEM images of Populus tomentosa after various treatments. The scale bars of all 6 imagines are 50 μm, the same as that in 1. 1 untreated sample, 2 4-week fungal treated sample by Trametes orientalis, 3 4-week fungal treated sample by Fomitopsis palustris, 4 FeCl3 treated sample at 180 °C, 5 Trametes orientalis-assisted FeCl3 treated sample at 180 °C, 6 Fomitopsis palustris-assisted FeCl3 treated sample at 180 °C
Discussion
Decay of poplar wood
Brown rot fungi selectively degrade carbohydrates in wood without removing the surrounding lignin that normally prevent from the microbial attack (Kerem et al. 1999). The discrepancy in this study may relate to lignin modifications which can cause lignin to be solubilized (Tewalt and Schilling 2010). However, when the bio-treated samples and raw materials were subjected to FeCl3 treatment, the increase of ASL was consistent with previous studies concerning FeCl3 pretreatment (Liu et al. 2009b), in which thermochemical pretreatments, at temperatures above the range for the lignin phase transition caused lignin to coalesce into large molten bodies that migrated within and out of the cell wall, and could also redeposit on the surface of plant cell walls (Donohoe et al. 2008).
Transition metal cations, especially Fe3+, have a good electron acceptor capability and may coordinate to the oxygen donor atoms of carbohydrates and their derivates without the loss of the protons from the hydroxyl groups of the ligand, which might contribute to FeCl3’s excellent performance in hemicelluloses hydrolysis (Yu et al. 2011). Furthermore, the effect of sole FeCl3 pretreatment on hemicelluloses removal was so remarkable (p < 0.01) that the synergy of combined treatments was masked. Even so, a slight enhancement of combined treatment on hemicelluloses hydrolysis can still be observed at 140 and 160 °C, compared to FeCl3 treatment alone.
In this study, high temperature accelerated not only cellulose solubilization but also glucose degradation, which was the main reason for the cellulose diminishing during the pretreatment (Liu et al. 2009a). This investigation focuses on removing hemicellulose as much as possible while preventing an attack on cellulose, so as to diminish its losses. Thus, future work should be maintained below 180 °C during the treatment.
Effects of treatments on enzymatic hydrolysis
In the fungal treatment, the lignin degradation or modification during 4-week fungal colonization, which made the accessibility of cellulase to cellulose easier, might be responsible for the increase of reducing sugar yield (Ma et al. 2010). In the second-step FeCl3 treatment, higher temperatures lead to more hemicelluloses solubilization and removal, which exposed more cellulose to cellulase and facilitated the subsequent enzymatic digestibility.
FeCl3 treatment alone and in combined treatments displayed similar trends of hemicellulose degradation and both had no effects on delignification (p < 0.05). Thus some other factors must account for the higher reducing sugar yield of the combined treatments. One factor was that the synergy of combined treatments caused an increase in internal surface area and porosity thus reducing the unproductive binding of the enzyme onto the lignin and increasing enzyme accessibility to cellulose (Meunier-Goddik and Penner 1999). Another factor may be that the combination of fungal treatment with FeCl3 resulted in an the alteration to the structure of lignin rather than causing lignin degradation, such as the change in the content of hydrophilic phenolic hydroxyl groups also leading to a drop of enzyme’s irreversible adsorption (Widsten and Kandelbauer 2008).
In summary, fungal treatment followed by FeCl3 treatment can enhance enzymatic hydrolysis of woody biomass. The reducing sugar yield in this study was compared to those reports regarding metal salt pretreatment. When FeCl3 (0.1 M) was employed to pretreat corn stover at 160 °C for 20 min, the sugar yield after enzymatic hydrolysis was 91.6 % (Liu et al. 2009b). Yu et al. (2011) also reported that CuCl2 (0.1 %)—treated sweet sorghum bagasse at 184 °C resulted in 90.4 % recovery of sugars. For the more recalcitrant woody biomass, a combination of F. palustris with FeCl3 treatment in the present work still demonstrated a good performance, with a higher sugar yield (95.4 %) than any of the above results. Therefore, a combination of wood rotting fungi with FeCl3 treatment performed well at treating woody biomass with facile process.
CrI of poplar wood
The increase in CrI resulted from the removal of amorphous components in the biomass, mostly hemicellulose, potentially exposing the crystalline cellulose core and increasing the cellulose content in the treated poplar wood (Lü and Zhou 2011). However, when the temperature increased to 180 °C, this caused greater cellulose degradation and reduced the crystallinity of the cellulose, especially in co-treated residues, so that the enzymatic digestibility was high.
References
Dai YC (2012) Polypore diversity in China with an annotated checklist of Chinese polypores. Mycoscience 53:49–80
Donohoe BS, Decker SR, Tucker MP, Himmel ME, Vinzant TB (2008) Visualizing lignin coalescence and migration through maize cell walls following thermochemical pretreatment. Biotechnol Bioeng 101:913–925
Kerem Z, Jensen KA, Hammel KE (1999) Biodegradative mechanism of the brown rot basidiomycete Gleophyllum trabeum: evidence for an extracellular hydroquinone-driven fenton reaction. FEBS Lett 446:49–54
Liu CG, Wyman CE (2006) The enhancement of xylose monomer and xylotriose degradation by inorganic salts in aqueous solutions at 180 °C. Carbohydr Res 341:2550–2556
Liu L, Sun J, Cai C, Wang S, Pei H, Zhang J (2009a) Corn stover pretreatment by inorganic salts and its effects on hemicellulose and cellulose degradation. Bioresour Technol 100:5865–5871
Liu L, Sun J, Li M, Wang S, Pei H, Zhang J (2009b) Enhanced enzymatic hydrolysis and structural features of corn stover by FeCl3 pretreatment. Bioresour Technol 100:5853–5858
Lü JL, Zhou PJ (2011) Optimization of microwave-assisted FeCl3 pretreatment conditions of rice straw and utilization of Trichoderma viride and Bacillus pumilus for production of reducing sugars. Bioresour Technol 102:6966–6971
Ma FY, Yang N, Xu CY, Yu HB, Wu JG, Zhang XY (2010) Combination of biological pretreatment with mild acid pretreatment for enzymatic hydrolysis and ethanol production from water hyacinth. Bioresour Technol 101:9600–9604
Meunier-Goddik L, Penner MH (1999) Enzyme-catalyzed saccharification of model cellulose in the presence of lignacious residues. J Agr Food Chem 47:346–351
Sluiter A, Hames B, Ruiz R, Scarlata C, Sluiter J, Templeton D, Crocker D (2008) Laboratory analytical procedure (LAP): determination of structural carbohydrates and lignin in biomass. Technical Report: NREL/TP-510-42618. National Renewable Energy Laboratory, Golden, Co, USA
Tewalt J, Schilling J (2010) Assessment of saccharification efficacy in the cellulase system of the brown rot fungus Gloeophyllum trabeum. Appl Microbiol Biotech 86:1785–1793
Wan CX, Li YB (2010) Microbial pretreatment of corn stover with Ceriporiopsis subvermispora for enzymatic hydrolysis and ethanol production. Bioresour Technol 101:6398–6403
Wang W, Yuan TQ, Wang K, Cui BK, Dai YC (2012) Combination of biological pretreatment with liquid hot water pretreatment to enhance enzymatic hydrolysis of Populus tomentosa. Bioresour Technol 107:282–286
Widsten P, Kandelbauer A (2008) Adhesion improvement of lignocellulosic products by enzymatic pre-treatment. Biotechnol Adv 26:379–386
Yu HB, Du WQ, Zhang J, Ma FY, Zhang XY, Zhong WX (2010) Fungal treatment of cornstalks enhances the delignification and xylan loss during mild alkaline pretreatment and enzymatic digestibility of glucan. Bioresour Technol 101:6728–6734
Yu QA, Zhuang XS, Yuan ZH, Qi W, Qiong W, Tan XS (2011) The effect of metal salts on the decomposition of sweet sorghum bagasse in flow-through liquid hot water. Bioresour Technol 102:3445–3450
Acknowledgments
This research was supported by the Fundamental Research Funds for the Central Universities (Project No. JC2013-1), the Program for New Century Excellent Talents in University (NCET-11-0585) and Major State Basic Research Projects of China (973-2010CB732204).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, W., Yuan, T.Q. & Cui, B.K. Fungal treatment followed by FeCl3 treatment to enhance enzymatic hydrolysis of poplar wood for high sugar yields. Biotechnol Lett 35, 2061–2067 (2013). https://doi.org/10.1007/s10529-013-1306-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-013-1306-3