Abstract
Antifungalmycin 702, a novel polyene macrolide antibiotic produced by Streptomyces padanus JAU4234, strongly inhibited mycelial growth of the rice blast fungus, Magnaporthe grisea, with EC50 of 37 μg/ml and EC90 of 136 μg/ml. Significant reduction in the number of conidia was observed at above 20 μg/ml. Conidia germination and appressorium formation were also suppressed and were not viable with >40 μg/ml. When treated with antifungalmycin 702, hyphae morphology became irregular. Based on microscopic examination, antifungalmycin 702 may exert its antifungal activity by changing the structure of cell membranes and the cytoskeleton and interacting with the organelles. Antifungalmycin 702 thus has potential as a new fungicide in the treatment of rice blast disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Each year almost 20 % yield reduction in the major food and cash crops worldwide are caused by plant diseases (Vicentini et al. 2002). Rice (Oryza sativa L.) is the most important staple crop for more than half of the world’s population, particularly in East and Southeast Asia. Outbreaks of rice blast disease (RBD) are a serious and recurrent problem in China and other rice-growing regions of the world, and this disease is extremely difficult to control (Dean et al. 2005). The filamentous ascomycete fungus, Magnaporthe grisea (anamorph. Pyricularia grisea), is an economically important pathogen with a broad host range and worldwide distribution which causes RBD (one of the most devastating of all cereal diseases) and severely lowers both rice yield and quality. It is estimated that every year the amount of rice enough to feed 60 million people is lost by this disease (Tani et al. 2005). Thus, rice blast disease caused by M. grisea is a significant and persistent problem to rice cultivation (Caracuel-Rios and Talbot 2007).
Strategies for control of RBD are limited. Biocontrol is a better choice against fungal plant pathogens, while its effectiveness is often strongly affected by environmental conditions (Almeida et al. 2007). Using chemical fungicides is still the most common method to effectively minimize the severity of RBD but is not considered to be a long term solution because of the potential health and environmental risks (Liu et al. 2012). Meanwhile, pathogenic microbes are rapidly adapting to fungicides, making them ineffective and leading to the emergence of resistance. The appearance of resistant strains has raised concerns about the use of chemical fungicides (Chen et al. 2011). Hence, one direct course of action to control fungicide-resistant pathogenic infections and to reduce the negative environmental impact because of the use of chemicals is to discover new natural product-based fungicides.
Polyene macrolide antibiotics are one of the most important subgroup of polyketides which represent a large and structurally diverse group of natural products with over 10,000 members so far identified (Stodulkova et al. 2011). All members of polyene macrolide family possess a typical polyene structure ranging from three to seven double bonds in length. Over 200 polyene macrolide antibiotics, such as rapamycin, nystatins, filipins and amphotericin B, have been isolated and characterized since 1950, most of which are produced by the genus Streptomyces belonging to Actinomycetes. In a search of novel microbial natural products, we have isolated a new polyene macrolide antibiotic, antifungalmycin 702, from Streptomyces padanus JAU4234 (Xiong et al. 2008). Antifungalmycin 702, containing a macrocyclic lactone ring with four double bonds (Fig. 1), has good antifungal activity and may have potential future agricultural/clinical application, e.g. as a new biopesticide/fungicide, owing to low toxicity with >1,500 mg/kg by the intraperitoneal and oral routes of administration in mice (Xiong et al. 2012). In this work, we examined in vitro antifungal activity of antifungalmycin 702 against M. grisea.
Materials and methods
Strain, antimicrobial agent and reagents
Magnaporthe grisea JAU130 was maintained by our laboratory. Antifungalmycin 702 was purified according to the method described elsewhere (Xiong et al. 2012). Antifungalmycin 702 solutions used for biological assay were prepared by diluting the stock solution in water to the required concentrations. Other chemicals were purchased from Sigma-Aldrich.
Suppression of mycelial growth of M. grisea by antifungalmycin 702
Concentrations of antifungalmycin 702 that resulted in 50 and 90 % inhibition (EC50 and EC90) on M. grisea were bioassayed on potato/dextrose/agar (PDA) in Petri dishes. Antifungalmycin 702 was mixed with PDA to produce a series of concentrations in the final test solution. The 6.5 mm-diameter inoculum plugs of M. grisea removed from the margin of a 4-day-old colony on PDA were placed at the center of the dishes. Linear growth of M. grisea at 28 °C was recorded 2 days after treatment. Each treatment consisted of three replicates. Inhibition percentage was obtained from the equation: Inhibition (%) = [(growth diameter in untreated control-growth diameter in treatment) × 100]/growth diameter in untreated control (Shih et al. 2003). The experiment was repeated twice.
Effect of antifungalmycin 702 on the formation of conidia
To test antifungalmycin 702 effect on the formation of conidia of M. grisea, antifungalmycin 702 were mixed with PDA to produce a series of different final concentrations. A 6.5-mm inoculum plug was inoculated with the mycelium at the center of the dishes. The conidia number of M. grisea was counted by cell counting plate (Eppendorf co., Germany) and the average number of the nine PDA plates (replicates) for each treatment was calculated after 14 days at 28 °C. The experiments were repeated for three times.
Effect of antifungalmycin 702 on the conidial germination and appressorium formation
Mycelial plugs of M. grisea were inoculated on PDA in petri dishes and incubated at 28 °C. After 14 days, the conidia of M. grisea formed in each dish were harvested and air-dried at room temperature. M. grisea conidia were submerged in 20 ml antifungalmycin 702 solutions (0–160 μg/ml) at 28 °C for 24 h (submerged in distilled water as a control). Then the conidia were rinsed in sterile distilled water for three times (1 min each time). The germination of spores and change in morphology were monitored microscopically. The percentage of germinated conidia forming appressoria was determined by performing a direct microscopic examination of at least 200 spores per replicate in at least three experiments with three replicates per treatment (Lee et al. 2007).
Effect of antifungalmycin 702 on the morphology of M. grisea
When treated with distilled water (the negative control) or antifungalmycin 702, samples of M. grisea were visualized by optical microscopy, scanning electron microscopy (SEM) and transmission electron microscopy (TEM). SEM: M. grisea cells were incubated with distilled water or 10 μg antifungalmycin 702/ml at 28 °C for 2 h, fixed with an equal volume of 5 % (w/v) glutaraldehyde for 2 h at 4 °C, and then washed with 0.1 M cacodylate buffer, pH 7.4. The samples were then treated with 1 % (w/v) osmium tetroxide, washed with 5 % (w/v) sucrose in cacodylate buffer, and subsequently dehydrated in a graded ethanol series (30, 70, 80, 90, 100 and 100 %). After lyophilization using a critical point dryer and gold coating using a sputtering system, the samples were visualized by SEM (Xiong et al. 2012). For TEM: samples were fixed with 2.5 % glutaraldehyde, post-fixed with 1 % osmium tetroxide followed by 1 % uranyl acetate, dehydrated through a graded series of ethanol, and embedded in EPON-812 resin. Ultra-thin sections were stained with uranyl acetate followed by lead citrate, and viewed on a TEM (Liu et al. 2012).
Results and discussion
The disease cycle of rice blast fungus M. grisea is important with regard to management and control of the pathogen. Magnaporthe grisea exists primarily as vegetative mycelium and/or conidia. Antifungalmycin 702 has a broad antifungal activity on yeast and plant pathogens such as Rhizoctonia solani, Penicillium notatum and Gibberella zeae (Xiong et al. 2012). In this work, antifungalmycin 702 significantly inhibited the mycelial growth of M. grisea and showed dose-dependent inhibitory effect with EC50 and EC90 values of 37 and 136.2 μg/ml, respectively (Fig. 2a). Moreover, antifungalmycin 702 displayed the significant inhibition to conidial formation (Fig. 2b). Significant reduction of conidial formation was observed >20 μg antifungalmycin 702/ml. Germination rate of conidia was also suppressed (Fig. 2c). When treated with >40 μg antifungalmycin 702/ml, conidial germination was not observed during the 14 days of incubation.
Magnaporthe grisea infects rice via appressoria which are specialized structures consisting of dome-shaped cells with enormous turgor that invade and rupture the cuticle of leaves (Lee et al. 2007). When M. grisea infects young rice seedlings, whole plants often die owing to the spread of this disease to the stems, nodes and panicle of older plants, which results in nearly total loss of the rice grain (Dean et al. 2005). Appressorium formation was strongly suppressed by antifungalmycin 702 (Fig. 1c). Appressoria were not viable when M. grisea treated with >40 μg antifungalmycin 702/ml, and was similar in trend to inhibition of conidial germination by antifungalmycin 702.
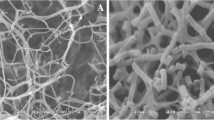
OM observations of the swollen germinating spores and hyphal tips of M. grisea revealed shrunken and vesicular cytoplasm as compared with the healthy cytoplasm of the control untreated hyphae. Although conidia germinated at lower concentrations of antifungalmycin 702, formation of the germ tube was abnormal (data not shown). Based on SEM assessment of the hyphal morphology, the control mycelia had regular hyphae with a smooth surface, but antifungalmycin 702 caused the increased hyphae branching and a swollen appearance with some hyphae bursting and others with a wrinkled surface (Fig. 3a, b). In TEM photographs, the cell wall, cell membrane nuclear envelope and cellular organelles were clearly seen in untreated hyphae, while cell wall and cell membrane of hyphae treated with antifungalmycin 702 were clearly wrinkled (Fig. 3c, d). The space between the cell wall and cell membrane widened. The cell organelles became visually much less distinct, indicating total degradation or significant destruction (Fig. 3d). Overall, the treated cells became abnormal and showed cellular disorganization, suggesting that antifungalmycin 702 penetrates the cell membrane and interacts with cellular organelles. The polyene antibiotics’ mechanism of action is commonly a specific interaction with membrane sterols that results in a changed permeability (Palacios and Serrano 1978). Our results suggested that, like other polyene macrolide antibiotics (Mulks et al. 1990), antifungalmycin 702 may exert its antifungal activity by changing the structure of cell membranes and the cytoskeleton and interacting with the organelles.
Effect of antifungalmycin 702 on the morphology of M. grisea. Top scanning electron microscopy of M. grisea, a treated with distilled water b 5 μg antifungalmycin 702/ml, bar 5 μm; Bottom transmission electron micrographs of M. grisea cellular organelles treated with, c distilled water and, d 5 μg/ml antifungalmycin 702/ml (d), bar 0.5 μm
Magnaporthe grisea Infection occurs when fungal spores release a specific adhesive from their tips to land and attach to leaves. The germinating spore then develops an appressorium to rupture the leaf cuticle and invade the underlying leaf tissue (Dean et al. 2005). Our results demonstrated that antifungalmycin 702 significantly inhibits mycelial growth, conidial formation/germination and appressorium formation of M. grisea. In addition, antifungalmycin 702 does not induce acute lethal toxicity at 1,500 mg/kg by the intraperitoneal and oral routes of administration in mice, suggesting low toxicity to mammal cells (Xiong et al. 2012), so it may be safe to use as a fungicide. Hence, owing to antifungal and toxicological characteristics of antifungalmycin 702, it has great potential for agricultural application in the treatment of fungal pathogen infections e.g., RBD caused by M. grisea. Further studies on antifungalmycin 702 such as in vivo antifungal activity, phytotoxicity tests and field experiments are required to determine its potential as a commercial fungicide.
References
Almeida FB, Cerqueira FM, Silva Rdo N, Ulhoa CJ, Lima AL (2007) Mycoparasitism studies of Trichoderma harzianum strains against Rhizoctonia solani: evaluation of coiling and hydrolytic enzyme production. Biotechnol Lett 29:1189–1193
Caracuel-Rios Z, Talbot NJ (2007) Cellular differentiation and host invasion by the rice blast fungus Magnaporthe grisea. Curr Opin Microbiol 10:339–345
Chen X, Zhu X, Ding Y, Shen Y (2011) Antifungal activity of tautomycin and related compounds against Sclerotinia sclerotiorum. J Antibiot 64:563–569
Dean RA, Talbot NJ, Ebbole DJ, Farman ML, Mitchell TK, Orbach MJ, Thon M, Kulkarni R, Xu JR, Pan H, Read ND, Lee YH, Carbone I, Brown D, Oh YY, Donofrio N, Jeong JS, Soanes DM, Djonovic S, Kolomiets E, Rehmeyer C, Li W, Harding M, Kim S, Lebrun MH, Bohnert H, Coughlan S, Butler J, Calvo S, Ma LJ, Nicol R, Purcell S, Nusbaum C, Galagan JE, Birren BW (2005) The genome sequence of the rice blast fungus Magnaporthe grisea. Nature 434:980–986
Lee HS, Lee TH, Lee JH, Chae CS, Chung SC, Shin DS, Shin J, Oh KB (2007) Inhibition of the pathogenicity of Magnaporthe grisea by bromophenols, isocitrate lyase inhibitors, from the red alga Odonthalia corymbifera. J Agric Food Chem 55:6923–6928
Liu H, Tian W, Li B, Wu G, Ibrahim M, Tao Z, Wang Y, Xie G, Li H, Sun G (2012) Antifungal effect and mechanism of chitosan against the rice sheath blight pathogen, Rhizoctonia solani. Biotechnol Lett 34:2291–2298
Mulks MH, Nair MG, Putnam AR (1990) In vitro antibacterial activity of faeriefungin, a new broad-spectrum polyene macrolide antibiotic. Antimicrob Agents Chemother 34:1762–1765
Palacios J, Serrano R (1978) Proton permeability induced by polyene antibiotics. A plausible mechanism for their inhibition of maltose fermentation in yeast. FEBS Lett 91:198–201
Shih HD, Liu YC, Hsu FL, Mulabagal V, Dodda R, Huang JW (2003) Fungichromin: a substance from Streptomyces padanus with inhibitory effects on Rhizoctonia solani. J Agric Food Chem 51:95–99
Stodulkova E, Kuzma M, Hench IB, Cerny J, Kralova J, Novak P, Chudickova M, Savic M, Djokic L, Vasiljevic B, Flieger M (2011) New polyene macrolide family produced by submerged culture of Streptomyces durmitorensis. J Antibiot 64:717–722
Tani H, Koshino H, Sakuno E, Nakajima H (2005) Botcinins A, B, C, and D, metabolites produced by Botrytis cinerea, and their antifungal activity against Magnaporthe grisea, a pathogen of rice blast disease. J Nat Prod 68:1768–1772
Vicentini CB, Forlani G, Manfrini M, Romagnoli C, Mares D (2002) Development of new fungicides against Magnaporthe grisea: synthesis and biological activity of pyrazolo[3,4-d][1,3]thiazine, pyrazolo[1,5-c][1,3,5]thiadiazine, and pyrazolo[3,4-d]pyrimidine derivatives. J Agric Food Chem 50:4839–4845
Xiong ZQ, Tu XR, Tu GQ (2008) Optimization of medium composition for actinomycin X2 production by Streptomyces spp JAU4234 using response surface methodology. J Ind Microbiol Biotechnol 35:729–734
Xiong ZQ, Zhang ZP, Li JH, Wei SJ, Tu GQ (2012) Characterization of Streptomyces padanus JAU4234, a producer of actinomycin X2, fungichromin, and a new polyene macrolide antibiotic. Appl Environ Microbiol 78:589–592
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grants No. 30960011 and 31071724), Natural Science Foundation of Jiangxi Province, China (Grants No. 2009GZN0030 and 2010GZN0037) and Key Technology R&D Program of Jiangxi Province, China (Grant No. 2007BN14002).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xiong, ZQ., Tu, XR., Wei, SJ. et al. In vitro antifungal activity of antifungalmycin 702, a new polyene macrolide antibiotic, against the rice blast fungus Magnaporthe grisea . Biotechnol Lett 35, 1475–1479 (2013). https://doi.org/10.1007/s10529-013-1229-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-013-1229-z