Abstract
Biological control of pathogens by endophytes is a promising approach. The present study reports the significant impact of an antifungal compound that is isolated from Streptoverticillium morookaense as a potential biocontrol agent. This antifungal compound demonstrated a significant inhibitory activity against the three phytopathogenic fungi, Ustilaginoidea virens, Rhizoctonia solani and Bipolaris maydis and resulted in severe morphological distortions in their structure. Minimal inhibitory concentrations of the compound ranged from 50 to 150 μg/ml. In vitro evaluation of the compound showed strong control efficacy against U. virens, a causative agent of rice false smut fungus, on susceptible rice seedlings. In addition, it promoted plant growth with increased rate of seed germination and displayed no phytotoxicity. This compound also showed stability after its exposure to a temperature of 100 °C. The antifungal metabolite produced by this actinomycete may be developed as a safe and ideal bio-fungicide for the control of different fungal plant diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ustilaginoidea virens (Cooke) Tak. is one of the economically important pathogen which causes false smut disease of rice worldwide and particularly in rice growing areas. It causes a severe yield loss which can reach up to 81% in different rice-producing areas depending on the rice variety planted and the specific disease intensity (Singh et al. 1992; Yang et al. 2012). Rice false smut is one of the fungal diseases that attacks rice and is subject to seeds certification. If there is a high disease level, seeds are rejected by Seed Certification Agency (SCA) on the basis of minimum seed certification standard (i.e. 0.5%) (Sharma and Gill 1997). Symptoms on rice infected by U. virens are entirely absent until after panicles begin flowering, at which time rice grains are replaced by spore balls that erupt through the glumes. Because of this reason, it became difficult to use fungicides to efficiently control the disease occurrence although there are few reports on the efficacy of fungicides against false smut (Muniraju et al. 2017; Mohiddin et al. 2012; Pannu et al. 2010; Bagga and Kaur 2006; Tsuda et al. 2006). The pathogen occasionally infects the anther, stigma, ovary and lodicules of rice (Mebeaselassie et al. 2015). In addition to rice yield losses as well as grain quality reduction, the disease caused by U. virens also results in the cautions of poisoning the live stock and humans who consume the rice grains contaminated by mycotoxins of this pathogen (Koiso et al. 1998; Wang et al. 2017).
Generally, the most feasible and economical disease management strategy is to develop resistant rice cultivars. There is a high degree of variation in resistance among rice cultivars, and unfortunately complete resistance to false smut disease has not been found in rice yet though some few quantitative resistance loci (QRL) have been identified which need to be confirmed before being used to improve rice resistance to false smut by MAS in breeding programmes (Zhou et al. 2014). So, protection of rice crops from U. virens infection is achieved by using different families of fungicides including prochloraz, carbendazim, propiconazole and tebuconazole as foliar treatments. However, the use of different types of fungicides brought up several problems by posing greater threat to ecology and biodiversity, toxicity to humans as well as emergence of resistant strains and detrimental effects on non-target populations (Fox et al. 2007). Therefore the need for new and useful compounds like microbes and their compounds are emerging as alternative strategies for pest control to provide protection and relief to crop plants from pathogens and pests and thereby sustenance of food production for human consumption (Montesinos 2003).
Biological control has become an attractive alternative strategy for the control of plant diseases to reduce the excessive use of agrochemicals and its health hazards (Moënne-Loccoz et al. 2001). In addition, biological control using antagonistic bacteria would be environmentally sound and can be implemented as an integrated disease management tool (Chung et al. 2015). Generally, microorganisms which simultaneously grow together with the pathogen, have been proved to be a rich source of bioactive secondary metabolites, and numerous compounds that have a potent biological activities to suppress the pathogen growth have been isolated (Vining 1990). Mebeaselassie et al. (2017) reported that a biocontrol agent, Antennariella placitae showed a significant in vitro inhibition of mycelial growth of U. virens and also gave protection for rice plants by improving yield in the in vivo study.
Actinomycetes, which are isolated from soil, the rhizosphere, and the phyllosphere, have a very great significance as they are potent producers of bioactive compounds with different biological properties (Strobel 2003; El-Tarabily and Sivasithamparam 2006) and have been established as potential biocontrol agents for protecting pathogenic microbes (Meij et al. 2017; Olaf et al. 2017). As reviewed by Berdy (2005) nearly 70–75% of secondary bioactive metabolites are isolated from these filamentous bacteria. Several studies have successfully used antagonistic microorganisms, especially actinomycetes and yeasts, to control different plant bacterial and fungal diseases (Alivizatos and Pantazius 1992; Ozaktan et al. 1999; Bressan 2003; Loliam et al. 2012; Law et al. 2017). In addition, the biocontrol potential of compounds that are obtained from different actinomycetes in controlling different fungal pathogens has also been previously reported (Hwang et al. 2001; Bordoloi et al. 2002; Kavitha et al. 2010).
With all the problems associated with synthetic chemicals, many scientists are investigating biological pesticide solutions (Martinez 2012; Nega 2014). Biological pesticides include chemicals derived from microorganisms, plants and animal sources. The potential use of microorganisms in the treatment of plant fungal diseases is based on the antagonistic nature of microbes towards the fungal pathogens. The results of experimental and field trials studies of microbial antagonistics against plant fungal pathogens are promising (Sharma et al. 2009). Several fungal and bacterial antagonistic commercial products including products like GiloGard (Gliocladium virens – seedling diseases of ornamentals and bedding plants), F-Stop (Trichoderma harzianum – several soilborne diseases), BINAB T (T. harzianum/T. polysporum – to control wood decay), Gallex or Galltrol (Agrobacterium radiobacter K-84 – crown rot), Dagger G (Pseudomonas fluorescens – Rhizoctonia and Pythium damping-off of cotton) and Kodiac (Bacillus subtillis – seed diseases) are effectively and successfully used worldwide to remedy problems associated with plant fungal diseases (Agrios 2005). Plant growth promoting microbials indirectly enhance plant growth via suppression of phytopathogens by producing chemicals that inhibit the growth of plant pathogens. Siderophores, antibiotics, biocidal volatiles, lytic enzymes and detoxification enzymes are some of the mechanisms that are employed by this antimicrobials (Jayaprakashvel and Mathivanan 2011; Tank et al. 2012; Saraf et al. 2014). The mechanism of antibiosisis is considered to be advantageous in biological control of plant diseases since antimicrobials can be able to diffuse rapidly in nature, and thus, direct contact between the pathogen and antagonist is not indispensable (Coleman et al. 2011). The present study reports the biological evaluation of a compound isolated from Streptoverticillium morookaense, a strong antagonist against various fungal phytopathogens (Feng et al. 2007). In addition, to controlling the disease causing agents, the ability to tolerate various factors like light, temperature, and pH in a natural environment is very important for any bioactive compound. Therefore, for commercial application purposes, the compound should be thermostable, photostable, and pH stable. This work will bring a great benefit in the development of biopesticides that are used in the future for plant disease control.
Materials and methods
Microorganisms and maintenance
S. morookaense was isolated from a soil sample collected in the pine (Pinus massoniana) forest at Dinghu Mountain Biosphere Reserve, Guangdong, China and maintained on agar slants and submerged cultures at refrigeration temperature (4 °C). The three test phytopathogenic fungi, Ustilaginoidea virens, Rhizoctonia solani and Bipolaris maydis were obtained from the culture collection of South China Botanical Garden, Chinese Academy of Sciences, Guangzhou, China. R. solani and B. maydis were maintained on potato dextrose agar (PDA) slants while U. virens was maintained on potato sucrose agar (PSA) slants at 4 °C.
Production of antifungal metabolites
Production of antifungal metabolites from S. morookaense was carried according to Feng et al. (2007). The actinomycete was grown on YMG medium (glucose 0.4%, malt extract 1.0%, yeast extract 0.4%, pH 5.5). The fermentation was carried out on a rotary shaker for 5 days in the dark at 25 °C with shaking at 150 rpm. Then the cultures were transferred into twenty 500-ml flasks containing 150 ml of YMG at the same incubation condition. At last the cultures were transferred into twenty 5.0-l flasks containing 1000 ml of YMG medium and 550 g of wheat grains, and the cultivation was continued in the stationary phase in the dark at 25 °C for 40 days.
Extraction of metabolites
For the recovery of active metabolites, the solid cultures of S. morookaense were extracted with 95% EtOH three times at room temperature. The resulting EtOH solution was suspended in water and this aqueous suspension was sequentially extracted three times each with petroleum ether, CHCl3, EtOAc, and n-BuOH.
Antifungal activity test of S. morookaense
The antifungal activity was tested against U. virens, R. solani and B. maydis causing different diseases on various host plants and the activity was determined in terms of zone of inhibition by using Kirby–Bauer well diffusion assay (Bauer et al. 1996). The diameters of the resultant zones of inhibition were measured in mm after 48–96 h of incubation. Each experiment was performed in duplicates and repeated three times and carbendazim was used as a positive control.
Effect of the antifungal metabolites on fungal morphology
The effect of the antifungal compound on the morphology of the fungal pathogens (i.e. U. virens and B. maydis) was studied using a microscope. Mycelia of U. virens and B. maydis were taken from the periphery of the inhibition zones which contained the antifungal compound as well as from the control plates. The glass slide was placed on the microscope and visualized under bright field at 40x (LeicaDM6000 B) then microphotographs were taken using a digital camera.
Effect of the antifungal metabolites on spore germination
To evaluate the effect of the culture filtrate on germination, 100 ml of U. virens and B. maydis PSA-spore suspension (1 × 105 spores ml−1) were mixed with 100 ml of the antifungal compound at different concentration rates (0, 1, 2, 3, 4%, v/v) and incubated for 24 and 48 h at 28 °C. In control similar concentration of U. virens and B. maydis spores were mixed with 100 ml PSA only. The percentage of germinated spores was determined by microscopic examination using a hemocytometer. A conidium was considered germinated if the germ tube was longer than one-half of the diameter of the conidium.
Antifungal effects of S. morookaense on the wet mycelia weight
The effect of the antifungal compound from S. morookaense on the wet mycelia weight was determined by the method of Tian et al. (2011). An aliquot of 1 ml of U. virens and B. maydis suspension (1 × 105 spores ml−1) was inoculated into 100 ml potato sucrose broth (PSA) medium and incubated at 28 °C, 160 rpm for 2 days, and then different concentrations (0, 0.5, 1, 2, 3, 4, 5 μl) of the antifungal compound were added in the fungal suspension respectively, no addition was made into the control group. The suspensions were incubated at 28 °C, 160 rpm for 96 h. Samples from the suspensions were collected at 12 h intervals during the incubation. The suspensions collected at different time were centrifuged at 7000 rpm for 7 min to obtain mycelium and supernatant, and then the mycelium was washed with distilled water twice. Finally, the wet weight of the mycelium was determined by using an electronic balance. Each test was run in triplicate.
Minimal inhibitory concentration (MIC)
Minimal inhibitory concentration of ethanol extract of the antifungal compound was evaluated based on the method of Díaz-Dellavalle et al. (2011) using broth microdilution assays. Different concentrations (12.5, 25, 50, 100, 250, 500, and 1000 mg/ml) of the ethanol extract were used to identify the MIC value. One-hundred ml of fungal spore suspension (1 × 105 spores ml−1) was mixed together with 100 ml of the antifungal extract at different concentrations while 100 ml of fungal spore suspension was mixed with 100 ml of ethanol as a control to confirm conidia viability and sensitivity to ethanol. The plates were incubated at 28 °C for 48 h. MIC values were calculated by comparing the growth in the plates containing the antifungal extract to the growth in the control plates. The lowest concentration that resulted in 90% inhibition growth compared to the growth in the control plates was recorded as MIC value. The experiments were conducted in triplicates.
Effects of the antifungal metabolites on cell membrane
The effect of the antifungal compound on cellular leakage was studied by determining the extracellular conductivity of supernatants obtained from mycelia suspensions of the fungal pathogen that was treated with the compound. The suspensions were collected at different time points, the first immediately after the addition of mycelium, the second after 12 h and the third after 24 h of treatment. The electric conductivity of the supernatant was determined to explain the changes of membrane permeability and the release of the cellular material. The electric conductivity of the obtained supernatant was measured using a conductivity meter. The experiment was repeated three times.
Determination of ergosterol content in the cell membrane
Cellular ergosterol in the plasma membrane of U. virens was measured as it was described by Tian et al. (2011) and the content was counted as a percentage of the wet weight with some modifications. Briefly, about 100 μl which contains 105 spores/ml of U. virens spore suspension was inoculated in a PSA medium containing 1, 2, 3, and 4 μl/ml of the antifungal compound for one week at 28 °C. Samples without any antifungal compound were used as controls. After incubation, mycelia were harvested and washed twice using distilled water then the net weight of the harvested mycelia was determined. An aliquot of 10 ml 25% alcoholic potassium hydroxide solution was added into each sample and mixed for 2 min by vortex followed by incubation at 85 °C for 4 h then samples were allowed to cool to room temperature. Ergosterol extraction was done by adding 5 ml n-heptane into each sample, the mixture was mixed for 2 min by vortex, then it was allowed to stand for 1 h at room temperature for the layers to separate easily. The n-heptane layer was measured using a UV spectrophotometer and scanning was done between 200 and 300 nm and compared to a predetermined standard curve.
Antifungal activity of the metabolites on U. virens on susceptible rice seeds
The biocontrol potential of the antifungal compound extracted from S. morookaense against U. virens was evaluated using susceptible rice seeds. Prior to artificial infection by the pathogen, the seeds were surface sterilized by immersing in sodium hypochlorite (1%) solution for 10 min then washed three times with sterilized distilled water. First the healthy rice seeds were soaked in ethanol extract of the antifungal compound at different concentrations (1, 5, and 10%, v/v).Then the seeds were immersed for 5 h in the PSA U. virens spore suspension (1 × 105 spores ml−1). Uninoculated and sterilized seeds that were treated only with water and carbendazim were used as controls. Three replicates of 12 seeds per treatment were kept in Petri dishes that were lined with moist filter paper. After 7 days of incubation in the dark at 28 °C, the numbers of germinated seeds as well as healthy and diseased seedlings were recorded. In addition, seedling vigor (V) was also determined according to the method of Andresen et al. (2015) by measuring shoot and root lengths of 12 randomly selected seedlings.
Effect of temperature on stability of the antifungal metabolites
Temperature and heat stability of the extracted antifungal compound was determined by heating the compound at different temperature regimes (37, 50, 70, and 100 °C) for 1 h. Later all the treated samples were checked for their residual activity with respect to the untreated control against U. virens.
Phytotoxicity assay
For safety evaluation, phytotoxicity test was conducted by treating the sterilized susceptible rice variety (HXZ) seeds by the antifungal compound. The antifungal compound was replaced with water in the control. Then the treated seeds were sown in sterilized soil and data for important agronomic parameters including seed germination, seedling vigor and weight of plants were recorded after 14 days.
Data analysis
A statistical package (SPSS, version 17.0 for Windows, SPSS Inc.) was used for the data processing. The results were presented as mean ± standard error, and the significant difference was analyzed using the Duncan’s multiple range test at P = 0.05. Differences were considered significant when P ≤ 0.05.
Results
Antifungal activity of metabolites
The antifungal metabolites significantly inhibited the growth of the test fungi with inhibition zones in the range of 25–42 mm compared to 22–32 mm resulting from carbendazim (Table 1). The compound was more effective on U. virens and B. maydis respectively compared to carbendazim, a chemical control agent. The chemicals that were used for extraction in addition to 95% EtOH (Fig. 1) did not show any effect on the normal growth of the three pathogens (data not shown).
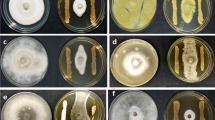
Microscopic photographs of (× 20) of antifungal effects of the metabolite on different pathogens. aU. virensbB. maydis and cR. solani (controls); dU. virenseB. maydisfR. solani (treated); gU. virenshB. maydis and iR. solani (with 95% EtOH); and j, k and l Hypha of the treated U. virens, B. maydis, and R. solani respectively; m, n and o Spores of the treated U. virens, B. maydis, and R. solani respectively
Effect of the antifungal compound on fungal morphology
Due to the significant effect of the antifungal metabolite showed by S. morookaense on the growth inhibition of the fungal pathogens, we decided to examine the effect of the compound on the fungal morphology of U. virens and B. maydis. Microscopic studies showed that different severe morphological abnormalities like hyphal coiling and swellings, excessive branching, thinning of hyphae, leakage of cellular materials that were caused by the antifungal metabolites were observed (Figs. 1 and 3).
Effect of the antifungal metabolite on spore germination
Spore germination of U. virens and B. maydis was affected by the antifungal metabolite which was isolated from the biocontrol agent. There is a direct relationship between the concentration of the antifungal compound and the germinated spore since the number of germinated spores decreased with increasing concentrations. At lower concentrations of 1% and 2%, the germination of B. maydis spores was not greatly affected as compared to control while there is a significant reduction of germinated spores of U. virens even at a lower concentration of 1% of the antifungal compound (Fig. 2). Concentrations of 4% were found to completely suppress spore germination in U. virens, and resulted in alteration of shapes of the U. virens spores. In the absence of the antifungal compound, we observed long, narrow, septate hyphae, with tear-drop shaped conidia. However, normal hyphal formation of U. virens was visibly affected in the presence of the antifungal compound with the formation of wide, short and crooked hyphae, which are not characteristic of the species.
Antifungal effects of S. morookaense on the wet mycelia weight
The effects of antifungal metabolites of S. morookaense on the wet mycelia weight of U. virens in liquid culture is shown in Table 2.The antifungal metabolites inhibited the growth of U. virens with a dose-dependent manner and almost all concentrations of the compound reduced the wet weight of mycelium of the pathogen. In the presence of 5% of the antifungal metabolite there was no growth (P ≤ 0.05) of U. virens mycelia as compared with the control (6.5 g).
Minimal inhibitory concentration (MIC)
Minimum inhibitory concentration of the antifungal metabolite was determined accordingly. The metabolite showed a significant antifungal activity among the tested pathogens with MIC values of 50, 100 and 150 μg/ml for B. maydis, R. solani, and U. virens respectively (Table 3). Ethanol did not inhibit the growth of the controls and all of the pathogens were found to grow in the absence of the antifungal compound, confirming the viability of the fungi.
Effect of the antifungal metabolite on the cell membrane permeability and its ergosterol content
The electric conductivity (Fig. 3) in the PSA culture media all significantly (P ≤ 0.05) increased with the incubation time (12 and 24 h) after the antifungal compound treatment compared to the untreated control. The result showed that the antifungal metabolite from S. morookaense brought a leakage of cellular electrolytes from the tested pathogen which might be due to the loss of cell membrane integrity.
In addition, ergosterol content was determined at 0, 1, 2, 3, and 4 μl/ml concentrations of the antifungal metabolite. The efficacy of the antifungal compound on the ergosterol content in the plasma membrane of U. virens is shown in Fig. 4. Ergosterol content (at 282 nm) in the plasma membrane of U. virens was inhibited significantly by the different concentrations of the antifungal compound in a dose-dependent fashion. A reduction in the percentage of ergosterol content in the plasma membrane compared with the control was observed at 21.6% for 1 μl/ml, 38.7% for 2 μl/ml, 44.8% for 3 μl/ml, and 72.9% for 4 μl/ml respectively.
Antifungal activity of a metabolite against U. virens on susceptible rice seedlings
The antifungal metabolite was further tested for its biological control efficacy on rice false smut disease in vitro and it showed its ability to control U. virens (Table 4). The antifungal compound reduced the negative effects of U. virens in the susceptible rice seeds when it was applied to the rice seeds 6 h prior to the pathogen inoculation and when it is compared with carbendazim, it caused a significant inhibition of the pathogen on the seeds and resulting in emergence of healthy seedlings. Only 23% of seeds treated with the pathogen germinated while 90% of seeds that were protected by the antifungal compound prior to infection with the pathogen successfully germinate. It is not only germination of seeds that was improved due to the presence of the antifungal compound but also seedling vigor was improved to 1826. In addition, the seeds that were treated by the antifungal metabolite showed a significantly higher (P ≤ 0.05) amount of fresh and dry weights compared to the control.
Heat stability of the antifungal metabolite against U. virens
There was no loss of the antifungal activity of the metabolite observed after it was exposed to a different tempearture gradient for 1 h except for the highest temperature (Table 5). Although the antifungal metabolite is thermostable, there was a decrease of 40.6% residual activity after the compound was boiled at 100 0 C for 1 h.
Phytotoxicity effects of the antifungal metabolite on rice seedlings
Results of germination percentage, seedling vigor traits of the studied rice cultivar are presented in Table 4. The antifungal compound did not show any toxicity on the germination and seedling vigor traits of the susceptible rice cultivar. Rather seeds that were treated with the antifungal compound gave rise to seedlings that showed increment in all the growth traits like shoot length, root length (Table 6) and seedling vigor compared to the control. The antifungal compound treated and emerged seedlings were also found to be healthier than the control plants as shown by their higher seedling weights (Table 4).
Discussion
Endophytes are defined as microbes that are able to colonize the internal tissues of their host plants without causing disease (Wilson 1995) and these microbes with bioactivities have been reported from various habitats and diverse environments. To our knowledge, this is the first report that shows the potential use of actinomycetes specifically Streptoverticillium morookaense as a biocontrol agent for controlling U. virens.
Here in the current study, extraction of metabolites from the culture extracts of S. morookaense resulted in compounds which potentially have a broad spectrum activity against different phytopathogenic fungi.
The extracted antifungal compounds from S. morookaense exhibited a higher activity against the tested pathogens particularly on U. virens and B. maydis when it is compared to the chemical fungicide, carbendazim. In the present study, the in vitro analysis showed the inhibition of mycelial growth and spore germination of U. virens and B. maydis in the presence of culture supernatant of S. morookaense. Suppression of both conidial germination and normal growth of mycelia increased in a dose dependent fashion and almost complete inhibition was observed at a concentration of 5%. Previous reports by Aremu et al. (2003), Yenjit et al. (2010), Li et al. (2011) and Manhas and Kaur (2016) showed that culture filtrates of different actinomycetes were able to show inhibitory effects on mycelia growth as well as germination of spores of different pathogens at different concentration rates. Basically the spore is an important structure for the survival and spread of a pathogenic fungus and reduction in percentage of spore germination increment is evident as the antifungal compound concentration increased. In addition to this, microscopic observations of the fungal mycelia from the margins of the inhibition zones of the treated samples using the antifungal compound showed that there was a severe structural alteration in vegetative cells and spores, which indicated that the metabolites probably attack the cell wall/cell membrane. Generally, antibiotic substances that were produced by actinomycetes are able to antagonize phytopathogenic fungi by inducing various morphological alterations such as swelling, stunting, distortion, hyphal protuberances in mycelial structure or the highly branched appearance of fungal germ tubes (Gunji et al. 1983). Similarly, Prapagdee et al. (2008) reported the absence of C. gloeosporioides conidia as one of the malformations caused by S. hygroscopicus and its sterile culture filtrates.
The low MIC values of the antifungal metabolite which varied from 50 to 150 μg/ml for B. maydis, R. solani, and U. virens respectively, based on the sensitivity of the test fungi further demonstrated its effectiveness to control the fungal plant pathogens that were under study. In addition, loss in integrity of cell wall/cell membrane by the antifungal metabolite was further confirmed by leakage of cellular materials which was indicated by changes in extracellular conductivity.
Ergosterol is specific to fungi and is the major sterol component of the fungal cell membrane and it is also responsible for maintaining cell function and integrity (Rodriguez et al. 1985). The effect of the antifungal metabolite on the amount of ergosterol was also assessed just to ensure the antifungal compound from S. morookaense target in the plasma membrane. In our study, it was confirmed that the antifungal compound can induce a considerable impairment of the ergosterol biosynthesis by U. virens. Hence, the plasma membrane is an important antifungal target of the antifungal compound. In relation to this, previous studies have exhibited that natural and synthetic drugs can cause a considerable reduction in the quantity of ergosterol (Arthington-Skaggs et al. 1999, 2000; Pinto et al. 2009).
The antifungal metabolites of S. morookaense were evaluated for their in vivo biocontrol potential against U. virens. The treatment of pathogen infested susceptible rice seeds with the metabolites leads to statistically significant (P ≤ 0.05) improvement in seed germination, seedling vigor and plant weight. In addition to disease control, the antifungal metabolite significantly enhanced vigor index and other agronomic parameters like fresh and dry weights when compared to the uninoculated control.
The application of carbendazim, a systemic fungicide which is usually used to protect rice plants from rice false smut fungus showed phytotoxicity by negatively affecting the plant biomass in Nicotiana tabacum (García et al. 2003). However, the antifungal compound which is extracted from S. morookaense in the present work did not show any phytotoxicity in the in vivo experiment. Rather, it enhanced the rate of seed germination and seedling vigor in the susceptible rice seeds compared to control plants.
The present study showed that the extraction of a new heat stable antifungal metabolite which has a plant growth promoting potential, from S. morookaense showed more promising activity against different fungal pathogens as compared to a standard chemical fungicide. The non-phytotoxic nature of the compound suggests that it might serve as a new, safe, and broad spectrum bio-fungicide to combat different plant diseases.
References
Agrios GN (2005) Plant pathology, 5th edn. Elsevier Acad Press, Amsterdam
Alivizatos AS, Pantazius S (1992) Preliminary studies on biological control of potato common scab caused by Streptomyces sp. In: Tjamos ES (ed) Biological control of plant diseases. Plenum Press, New York, pp 85–92
Andresen M, Wulff EG, Mbega ER, Stokholm MS, Glazowska SE, Zida PE (2015) Seed treatment with an aqueous extract of Agave sisalana improves seed health and seedling growth of sorghum. Eur J Plant Pathol 141:119–132. https://doi.org/10.1007/s10658-014-0530-6
Aremu EA, Furumai T, Igarashi Y, Sato Y, Akamatsu H, Kodama M, Otani H (2003) Specific inhibition of spore germination of Alternaria brassicicola by fistupyrone from Streptomyces sp. TP−A0569. J Gen Plant Pathol 69:211–217
Arthington-Skaggs BA, Jradi H, Desai T, Morrison CJ (1999) Quantitation of ergosterol content: novel method for determination of fluconazole susceptibility of Candida albicans. J Clin Microbiol 37:3332–3337
Arthington-Skaggs BA, Warnock DW, Morrison CJ (2000) Quantitation of Candida albicans ergosterol content improves the correlation between in vitro antifungal susceptibility test results and in vivo outcome after fluconazole treatment in a murine model of invasive candidiasis. Antimicrob Agents Chemother 44:2081–2085
Bagga PS, Kaur S (2006) Evaluation of fungicides for controlling false smut (Ustilaginoidea virens) of rice. Indian Phytopathol 59:115–117
Bauer AW, Kirby WM, Sherris JC, Turck M (1996) Antibiotic susceptibility testing by standardized single disc method. Am J Clin Pathol 44:493–496
Berdy J (2005) Bioactive microbial metabolites. J Antibiot 58:1–26
Bordoloi GN, Kumari B, Guha A, Thakur D, Bordoloi M, Roy MK (2002) Potential of a novel antibiotic, 2-methyl heptyl iso-nicotinate, as a biocontrol agent against fusarial wilt of crucifers. Pest Manag Sci 58:297–302. https://doi.org/10.1002/ps.457
Bressan W (2003) Biological control of maize seed pathogenic fungi by use of actinomycetes. BioControl 48:233–240
Chung EJ, Hossain MT, Khan A, Kim KH, Jeon CO, Chung YR (2015) Bacillus oryzicola sp. nov., an endophytic bacterium isolated from the roots of rice with antimicrobial, plant growth promoting, and systemic resistance inducing activities in rice. Plant Pathol J 31(2):152–164
Coleman TW, Grulke NE, Daly M, Godinez C, Schilling SL, Riggan PJ, Seybold SJ (2011) Coast live oak, Quercus agrifolia, susceptibility and response to goldspotted oak borer, Agrilus auroguttatus, injury in southern California. For Ecol Mang 261:1852–1865
Díaz-Dellavalle P, Cabrera A, Alem D, Larrañaga P, Ferreira F, Dalla-Rizza M (2011) Antifungal activity of medicinal plant extracts against phytopathogenic fungus Alternaria spp. Chilean J Agric Res 71:231–239
El-Tarabily KA, Sivasithamparam K (2006) Non-streptomycete actinomycetes as biocontrol agents of soil-borne fungal plant pathogens and as plant growth promoters. Soil Biol Biochem 38:1505–1520. https://doi.org/10.1016/j.soilbio.2005.12.017
Feng N, Ye W, Wu P, Huang Y, Xie H, Wei X (2007) Two new antifungal alkaloids produced by Streptoverticillium morookaense. J Antibiot 60(3):179–183
Fox JE, Gulledge J, Engelhaupt E, Burow ME, McLachlan JA (2007) Pesticides reduce symbiotic efficiency of nitrogen-fixing rhizobia and host plants. PNAS 104:10282–10287
García PC, Rivero RM, Ruiz JM, Romero L (2003) The role of fungicides in the physiology of higher plants: implications for defense responses. Bot Rev 69:162–172. https://doi.org/10.1663/0006-8101
Gunji S, Arima K, Beppu T (1983) Screening of antifungal antibiotics according to activities inducing morphological abnormalities. Agric Biol Chem 47:2061–2069. https://doi.org/10.1271/bbb1961.47.2061
Hwang BK, Lim SW, Kim BS, Lee JY, Moon SS (2001) Isolation and in vivo and in vitro antifungal activity of phenylacetic acid and sodium phenylacetate from Streptomyces humidus. Appl Environ Microbiol 67:3739–3745
Jayaprakashvel M, Mathivanan N (2011) Management of plant diseases by microbial metabolites. In: Maheshwari DK (ed) Bacteria in agrobiology: plant nutrient management. Springer-Verlag, Berlin, Heidelberg, pp 237–265
Kavitha A, Prabhakar P, Vijayalakshmi M, Venkateswarlu Y (2010) Purification and biological evaluation of the metabolites produced by Streptomyces sp. TK-VL_333. Res Microbiol 161:335–345. https://doi.org/10.1016/j.resmic.2010.03.011
Koiso Y, Morisaki N, Yamashita Y, Mitsui Y, Shirai R, Hashimoto Y, Iwasaki S (1998) Isolation and structure of an antimitotic cyclic peptide, ustiloxin F: chemical interrelation with a homologous peptide, ustiloxin B. J Antibiot 51:418–422
Law JW, Ser HL, Khan TM, Chuah LH, Pusparajah P, Chan KG, Goh BH, Lee LH (2017) The potential of Streptomyces as biocontrol agents against the rice blast fungus, Magnaporthe oryzae (Pyricularia oryzae). Front Microbiol 8:3. https://doi.org/10.3389/fmicb.2017.00003
Li Q, Jiang Y, Ning P, Zheng L, Huang J, Li G (2011) Suppression of Magnaporthe oryzae by culture filtrates of Streptomyces globisporus JK-1. Biol Control 58:139–148. https://doi.org/10.1016/j.biocontrol.2011.04.013
Loliam B, Morinaga T, Chaiyanan S (2012) Biocontrol of phytwophthora infestans, fungal pathogen of sedling damping off disease in economic plant nursery. Hindawi Publishing Corporation Psyche 12:1–6
Manhas K, Kaur T (2016) Biocontrol potential of Streptomyces hydrogenans strain DH16 toward Alternaria brassicicola to control damping off and black leaf spot of Raphanus sativus. Front Plant Sci 7:1869
Martinez JA (2012) Natural fungicides obtained from plants, fungicides for plant and animal diseases. In Dhanasekaran D (ed) ISBN: 978–953–307-804-5, InTech. https://doi.org/10.5772/26336
Mebeaselassie A, Luoye L, Aiqing F, Xiaoyuan Z, Jianxiong L (2015) Development of GFP-expressing Ustilaginoidea virens strain to study fungal invasion and colonization in rice spikelets. S Afr J Bot 97:16–24
Mebeaselassie A, Congyi Z, Yun Y, Jianxiong L (2017) Identification and evaluation of potential bio-control fungal endophytes against Ustilaginoidea virens on rice plants. World J Microbiol Biotechnol 33:120
Meij A, Worsley SF, Hutchings MI, Wezel GP (2017) Chemical ecology of antibiotic production by actinomycetes. FEMS Microbiol Rev 41:392–416
Moënne-Loccoz Y, Tichy HV, O'Donnell A, Simon R, O'Gara F (2001) Impact of 2,4-diacetylphloroglycenol-producing biocontrol strain Pseudomonas fluorescens F113 on intraspecies diversity of resident culturable fluorescent pseudomonads associated with the roots of field-grown sugar beet seedlings. Appl Environ Microbiol 67:3418–3425
Mohiddin FA, Bhat FA, Gupta V, Gupta D, Kalha CS (2012) Integrated disease management of false smut of rice caused by Ustilaginoidea virens. Trends in Biosci 5(4):301–302
Montesinos E (2003) Development, registration and commercialization of microbial pesticides for plant protection. Int Microbiol 6:245–252
Muniraju KM, Pramesh D, Mallesh SB, Mallikarjun K, Guruprasad GS (2017) Novel fungicides for the management of false smut disesases of rice caused by Ustilaginoidea virens. Int J Curr Microbiol App Sci 6(11):2664–2669
Nega A (2014) Review on concepts in biological control of plant pathogens. J Biol Agric Healthc 4(27):33–54
Olaf T, Song C, Dickschat JS, Vos M, Garbeva P (2017) The ecological role of volatile and soluble secondary metabolites produced by soil bacteria. Trends Microbiol 25(4):280–292
Ozaktan H, Bora T, Sukan S, Sargin S, Sukan FV (1999) Studies on determination on antagonistic potential and biopreparation of some bacteria against the fireblight pathogen. Acta Hortic 489:663–668
Pannu PPS, Thind TS, Goswami S (2010) Cultural studies on Ustilaginoidea virens, the incitant of false smut of rice (Oriza sativa). Indian J Agric Sci 85(7):28–31
Pinto E, Vale-Silva L, Cavaleiro C, Salgueiro L (2009) Antifungal activity of the clove essential oil from Syzygium aromaticum on Candida, aspergillus and dermatophyte species. J Med Microbiol 58:1454–1462
Prapagdee B, Kuekulvong C, Mongkolsuk S (2008) Antifungal potential of extracellular metabolites produced by Streptomyces hygroscopicus against phytopathogenic fungi. Int J Biol Sci 4:330–337. https://doi.org/10.7150/ijbs.4.330
Rodriguez RJ, Low C, Bottema CD, Parks LW (1985) Multiple functions for sterols in Saccharomyces cerevisiae. Biochim Biophys Acta 837:336–343
Saraf M, Pandya U, Thakkar A (2014) Role of allelochemicals in plant growth promoting rhizobacteria for biocontrol of phytopathogens. Microbiol Res 169:18–29
Sharma RC, Gill SS, Randhawa HS (1994) Vitavax-an effective seed dresser for combined control ofstripe disease and smuts of Barley. Seed Res 22(2):177–178
Sharma RR, Singh D, Singh R (2009) Biological control of postharvest diseases on fruits and vegetables by microbial antagonists: a review. Biol Control 50:205–221
Singh S, Pal V, Panwar M (1992) False smut of rice—its impact on yield components. Crop Research Hisar 5:246–248
Strobel GA (2003) Endophytes as sources of bioactive products. Microbes Infect 5:535–544
Tank N, Rajendran N, Patel B, Saraf M (2012) Evaluation and biochemical characterization of a distinctive pyoverdin from a Pseudomonas isolated from chickpea rhizosphere. Braz J Microbiol 639–648
Tian J, Ban XQ, Zeng H, He JS, Huang B, Wang YW (2011) Chemical composition and antifungal activity of essential oil from Cicuta virosa L. var latisecta Celak. Int J Food Microbiol 145:464–470
Tsuda M, Sasahara M, Ohara T, Kato S (2006) Optimal application timing of simeconazole granules for control of rice kernel smut and false smut. J Gene Plant Pathol 72:301–304
Vining LC (1990) Function of secondary metabolites. Annu Rev Microbiol 44:395–427. https://doi.org/10.1146/annurev.mi.44.100190.002143
Wang X, Wang J, Lai D, Wang W, Dai J, Zhou L, Liu Y (2017) Ustiloxin G, a new cyclopeptide mycotoxin from rice false smut balls. Toxins 9(2):54
Wilson D (1995) Endophytes—the evolution of the term, a clarification of its use and definition. Oikos 73:274–276. https://doi.org/10.2307/3545919
Yang LM, Chen L, Xu J, Liu JC, Ding KJ (2012) Estimation of yield loss caused by rice false smut. J Anhui Agric Univ 39:474–477
Yenjit P, Issarakraisila M, Intana W, Chantrapromma K (2010) Fungicidal activity of compounds extracted from the pericarp of Areca catechu against Colletotrichum gloesporioides in vitro and in mango fruit. Postharvest Biol Technol 55:129–132
Zhou YL, Xie XW, Zhang F, Wang S, Liu XZ, Zhu LH, Xu JL, Gao YM, Li ZK (2014) Detection of quantitative resistance loci associated with resistance to rice false smut (Ustilaginoidea virens) using introgression lines. Plant Pathol 63:365–372
Acknowledgements
This work was supported by the National Natural Sciences Foundation of China (30970627) and the fund for the Important Direction Research on Knowledge Innovation Project (KSCX2-EW-N-06) from CAS awarded to J.X. Li. M. Andargie was a recipient of the ‘Visiting Fellowship for Researchers from Developing Countries’ Award (2013FFSA0005) from Chinese Academy of Sciences. We would like to thank Prof Xiaoyi Wei for providing the metabolites that were extracted from S. morookaense.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest in this study.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 60 kb)
Rights and permissions
About this article
Cite this article
Andargie, M., Li, J. Antifungal activity against plant pathogens by compounds from Streptoverticillium morookaense. J Plant Pathol 101, 547–558 (2019). https://doi.org/10.1007/s42161-018-00234-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42161-018-00234-x