Abstract
The extracellular tannase from Emericela nidulans was immobilized on different ionic and covalent supports. The derivatives obtained using DEAE-Sepharose and Q-Sepharose were thermally stable from 60 to 75 °C, with a half life (t50) >24 h at 80 °C at pH 5.0. The glyoxyl-agarose and amino-glyoxyl derivatives showed a thermal stability which was lower than that observed for ionic supports. However, when the stability to pH was considered, the derivatives obtained from covalent supports were more stable than those obtained from ionic supports. DEAE-Sepharose and Q-Sepharose derivatives as well as the free enzyme were stable in 30 and 50 % (v/v) 1-propanol. The CNBr-agarose derivative catalyzed complete tannic acid hydrolysis, whereas the Q-Sepharose derivative catalyzed the transesterification reaction to produce propyl gallate (88 % recovery), which is an important antioxidant.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Tannases (EC 3.1.1.20) are found in prokaryotic and eukaryotic organisms. They catalyze the hydrolysis of ester and depsidic linkages in phenolic compounds such as tannins, the second most plentiful compounds in plants after lignin (Aguilar et al. 2007). Tannins have been classified into two groups: non-hydrolyzable and hydrolyzable tannins, which can be further subdivided into gallotannins and ellagitannins. Gallotannins have a mass of ~3 kDa and comprise a glucose nucleus and 6–9 galloyl groups that can be easily degraded by bacteria, yeast and filamentous fungi (Aguilar and Gutiérrez-Sánchez 2001; Mingshu et al. 2006). Ellagitannins have a molecular mass of 2–3 kDa and hexahydroxyphenic acid as the phenolic group. Their degradation by microbial action is not commonly observed. However, some bacteria and fungi found in rich ellagitannin soils, leaves, and tannin containing wastes—can produce tannases that hydrolyze these compounds (Mingshu et al. 2006) Esterase activity cleaves ester linkages between the aromatic ring and the glucose residue, while depsidic activity cleaves the ester linkage between aromatic rings. Most microorganisms, especially filamentous fungi such as Aspergillus niger, Penicillium sp., Paecylomyces variotii and Emericela nidulans (Mahendran et al. 2006; Gonçalves et al. 2011), produce tannases with biotechnological potential. E. nidulans tannase is hyper-tolerant to solvents and temperature, important characteristics for future applications. Tannases are extensively used in instant tea and wine production, juice clarification, feed additives, waste treatment (Aguilar and Gutiérrez-Sánchez 2001), production of antibacterial drugs (Sittig 1988), and propyl gallate synthesis for use as antioxidant (Lekha and Lonsane 1997).
Owing to its several advantages, such as the reuse of the enzyme for several cycles, increase in enzyme stability, easiness of separation from products, and the possibility of continuous use in bioreactors, enzyme immobilization is an important technique for biotechnological applications in different industrial sectors. Despite the reduced number of articles on the immobilization of microbial tannases, fungal enzymes have been immobilized using different supports such as Amberlite IR and alginate (Sharma et al. 2008), and agarose and chitosan (Sharma et al. 2002). Hence, this manuscript describes the immobilization of an extracellular tannase from E. nidulans on different ion-exchange (DEAE-Sepharose and Q-Sepharose) and covalent (glyoxyl-agarose and amino glyoxyl-agarose) supports as well as the synthesis of propyl gallate using a cyanogen bromide–agarose (CNBr) derivative.
Materials and methods
Microorganism and culture conditions
The filamentous fungus E. nidulans was isolated from Brazilian soil and identified by André Toselo Tropical Foundation, Campinas, São Paulo, Brazil. Submerged cultures were obtained by the addition of 1 ml spore suspension (105 spores/ml) obtained from BDA slants to 25 ml Khanna medium (Khanna et al. 1995), at an initial pH of 6.0, with 2 % (w/v) tannic acid as the only carbon source; the cultures were maintained under orbital agitation (100 rpm) for 72 h at 30 °C, as described by Gonçalves et al. (2011).
Obtainment of extracellular enzymes
After 72 h, cultures were harvested by vacuum filtration using Whatman No. 1 filter paper. The filtrate was used as source of tannase for the immobilization procedures as well as for the determination of enzymatic activity. The mycelium was discarded.
Preparation of supports
Crosslinked 6 % (w/v) agarose beads, CNBr-activated Sepharose 4B (CNBr-agarose), Q-Sepharose, and DEAE-Sepharose were purchased from GE-Healthcare (Uppsala, Sweden). Glyoxyl-agarose and amino-glyoxyl supports were prepared according to Guisán et al. (1993) and Fernandéz-Lafuente et al. (1999), respectively. Before use, Q-Sepharose and DEAE-Sepharose (5 g each) were added to 100 ml distilled water and maintained at room temperature for 12 h. Each support was then extensively washed with water to remove conservants, as described by the manufacturer. Usually, 1 g Sepharose 4B activated by CNBr was hydrated with 100 ml distilled water, and the pH was adjusted to 2, for 30 min under agitation, according to the manufacturer’s instructions.
Immobilization on ion-exchange supports
Immobilization was performed by adding 10 ml tannase solution to 5 mM potassium phosphate buffer, pH 7 (1:10; v/v) containing 1 g DEAE-Sepharose or Q-Sepharose. The mixture was then maintained overnight under agitation, harvested and washed with distilled water. The derivatives were maintained at 4 °C.
Immobilization on CNBr-agarose
Commercial CNBr-agarose (1 g) was added to a tannase-rich enzyme solution (10 ml) in 5 mM sodium phosphate buffer, pH 7 (1:10; v/v), maintained overnight under agitation, harvested and washed with distilled water. The derivative obtained was maintained at 4 °C.
Immobilization on glyoxyl-agarose and amino-glyoxyl-agarose
The immobilization on both supports was performed using 10 ml enzyme solution and 1 g glyoxyl-agarose or amino-glyoxyl-agarose. For glyoxyl-agarose, 100 mM bicarbonate buffer, pH 10.5, containing 50 mM DTT and 50 % (v/v) ethylene glycol was used. For amino-glyoxyl-agarose, the immobilization was carried out in 10 mM potassium phosphate buffer, pH 7, containing 50 % (v/v) ethylene glycol Both suspensions were agitated for 3 h and then the pH of the amino-glyoxyl-agarose suspension was increased to 10.5 and the agitation was maintained for 1 h After enzyme immobilization, linkages between glyoxyl and amine groups were reduced with sodium borohydride for 30 min at room temperature, the derivatives were washed with distilled water and maintained at 4 °C.
Determination of tannase activity and protein
The activity of free and immobilized tannase was quantified using 1 % (v/v) methyl gallate (Fluka) as substrate in 100 mM sodium acetate buffer, pH 4.5. The reaction was conducted at 40 °C for different time intervals. Gallic acid formed was quantified using a solution of methanolic rhodanine, as described by Sharma et al. (2000). Routinely, 0.7 g of each derivative in 1 ml distilled water was used, and samples, 100 μl, were used for gallic acid quantification. In addition, the derivatives (amino-glyoxyl-agarose, glyoxyl-agarose, DEAE-Sepharose and CNBr-agarose) were tested for successive hydrolytic cycles (1–6) as described above. After each hydrolytic cycle, the derivatives were separated from the reaction mixture, extensively washed with distilled water and re-used for a new cycle. One enzyme unit was defined as the amount of enzyme that produces 1 μmol gallic acid per min under the assay conditions. Proteins were quantified by the Lowry method using BSA as a standard.
Hydrolysis of tannic acid and synthesis of propyl gallate
The hydrolysis of 2 mM tannic acid (Sigma) was estimated using free enzyme (control) and CNBr-agarose derivatives. Usually, 0.5 g derivative in 100 mM sodium acetate buffer, pH 5, containing 30 % diglyme was used. Before analysis, samples of the reaction medium containing free enzyme were treated with ZnSO4 (5 %, w/v) and BaOH (4 %, w/v), agitated, and centrifuged at 23,000×g for 5 min to remove proteins. Hydrolysis products of tannic acid were quantified by HPLC using a 5 μm C18 column (150 × 4.6 mm) with 35 % (v/v) methanol/65 % (v/v) 100 mM ammonium phosphate buffer pH 8 as mobile phase at 25 °C. Detection was at 280 nm.
The synthesis of propyl gallate was performed using 2 mM tannic acid as substrate in 100 mM potassium phosphate buffer, pH 7, containing 50 % (v/v) 1-propanol. The reaction was conducted at room temperature, using the CNBr-agarose, Q-Sepharose and DEAE-Sepharose derivatives. The propyl gallate obtained was quantified by HPLC as described earlier. For both the quantifications, 1 mM gallic acid (Sigma) and 1 mM propyl gallate (Sigma) were used as standards.
Reproducibility
All experiments were performed as triplicates, and the values obtained were expressed as mean ± standard deviation.
Results and discussion
The extracellular tannase from E. nidulans was efficiently immobilized using both DEAE-Sepharose and Q-Sepharose ion-exchange supports, retaining its activity for long periods in the range 65–75 °C at pH 5. (Supplementary Fig. 1) Thus, the immobilization occurred in supports with either anionic or cationic properties. Considering this aspect, the clustered, positively-charged amino acids are responsible for protein linkage on Q-Sepharose, whereas the clustered, negatively-charged amino acids on DEAE-Sepharose, at the same pH value. At 80 °C, the enzyme immobilized on both supports maintained more than 60 % of its activity for 24 h. Under these conditions, the extracellular tannase from E. nidulans immobilized on DEAE-Sepharose and Q-Sepharose was more stable than the immobilized enzymes from P. variable (Sharma et al. 2008) and Paecylomyces variotii (Schons et al. 2011). The tannase from E. nidulans immobilized on CNBr-agarose showed reduced stability at all temperatures. When the pH of the suspension was elevated from pH 5 to 7, at 75 °C, the activity for both free and immobilized enzymes, in all supports, was drastically reduced after 4 h analysis (data not shown).
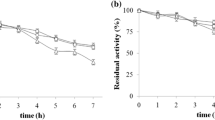
The stability of E. nidulans tannase immobilized on different supports in the presence 1-propanol was also studied (Fig. 1). The DEAE-Sepharose and Q-Sepharose derivatives were stable for 96 h with 30 % (v/v) and 50 % (v/v) 1-propanol at 25 °C. Under these conditions ~85 % of activity was maintained after 100 h incubation. In contrast, the activity of the enzyme immobilized on CNBr-agarose was severally inhibited in the presence of 1-propanol. The hyper-tolerance of the free enzyme to organic solvents, as for example 1-propanol, was previously reported (Gonçalves et al. 2011). On the other hand, it is the first time that tannase derivatives on ionic supports with hyper-tolerance to 1-propanol are reported. The remarkable stability in the presence of 1-propanol accounts for the use of the derivatives in propyl gallate synthesis.
Stability of the DEAE-Sepharose (filled circle), Q-Sepharose (filled square), CNBr (filled up-pointing triangle), glyoxyl-agarose (open square), and amino-glyoxyl agarose (open circle) derivatives in the presence of 30 % (a, c) or 50 % (b, d) 1-propanol at 25 °C. Activities were determined in triplicate and the results were presented as mean ± SD. Controls (100 % activity) corresponded to: 0.29 U/ml (filled circle), 0.25 U/ml (filled square), 0.20 U/ml (filled up-pointing triangle), 0.12 U/ml (open square) and 0.25 U/ml (open circle) (a, c); and 0.26 U/ml (filled circle), 0.23 U/ml (filled square), 0.28 U/ml (filled up-pointing triangle), 0.07 U/ml (open square) and (open circle) 0.18 U/ml (c, d)
Characterization of tannase immobilized on glyoxyl-agarose and amino-glyoxyl agarose
In the presence of 50 % (v/v) ethylene glycol, the enzyme immobilized on glyoxyl-agarose and amino-glyoxyl-agarose supports maintained ~75–95 % of activity at 75 and 80 °C for 3 h, at pH 7 (Fig. 2). The t50 values were obtained only with 25 h or more of incubation (data not shown). The thermal stability of the enzyme immobilized on both supports was higher than that observed for CNBr-agarose derivatives and for the derivatives obtained using ionic supports. In addition, the amino-glyoxyl-agarose and glyoxyl-agarose derivatives maintained ~50 % of the initial activity when incubated for 4 h in the presence of 30 % (v/v) and 50 % (v/v) 1-propanol, which was higher than observed for CNBr-agarose derivatives, although lower when compared with the derivatives with ionic supports (Fig. 1c, d). Both the amino-glyoxyl and glyoxyl derivatives were also more stable to pH than the CNBr-agarose derivative, but less stable than derivatives from ionic supports.
Thermal stability at 75 °C (a) and 80 °C (b) for glyoxyl-agarose (open square), amino-glyoxyl agarose (open circle)and CNBr (filled up-pointing triangle)derivatives in the presence of 50 % ethylene glycol at pH 7.0. Controls corresponded to 0.072 U/ml (open square),15 U/ml (open circle) and 0.17 U/ml (filled up-pointing triangle). Activities were determined in triplicate and the results were presented as mean ± SD
Stability of the CNBr-agarose derivative in the presence of protectors
The CNBr-agarose derivative was stable at pH 5 and 7 but its thermal stability decreased at higher pH values. Thus stabilizing agents may be used to protect the enzymatic activity of the derivative at alkaline pH. Among the stabilizing agents tested, the ethylene glycol preserved ~90 % of tannase activity for more than 15 h at pH 9 and 10 (Fig. 3). The results obtained for all protectors tested at 4 °C were better than those obtained at 25 °C. Considering the pH 10 at both temperatures, 50–55 % activity was maintained for 5 h in the presence of threalose, whereas at pH 9, glycerol had a similar or better effect, compared to threalose (Fig. 3). The encapsulated tannase from P. variotti was fully stable at pH 7.5 for 1 h, but only 40 % of its activity was maintained at pH 8.5–9.0 (Schons et al. 2011).
Stability of CNBr derivative at pH 9.0 (a, b) and 10.0 (c, d), maintained at 4 °C (a, c) and 25 °C (b, d), in the absence (filled square) and presence of threalose (filled circle), glycerol (filled up-pointing triangle), and ethylene-glycol (filled down-pointing triangle). For each condition, activities were determined in triplicate and the results were presented as mean ± SD. At pH 9, controls corresponded to 0.18 U/ml (filled square), 0.16 U/ml (filled circle) and 0.15 U/ml (filled up-pointing triangle, filled down-pointing triangle). At pH 10, controls corresponded to 0.15 U/ml (filled square), 0.13 U/ml (filled circle, filled down-pointing triangle) and 0.12 U/ml (filled up-pointing triangle)
The derivatives were also analyzed for successive hydrolytic cycles (data not shown). The amino-glyoxyl and glyoxyl-agarose derivatives retained ~82–87 % of activity at cycle 3. However, tannase activity of the glyoxyl-agarose derivative was drastically reduced at cycle 6, while the amino-glyoxyl derivative retained 38 % of activity. The CNBr-agarose derivative catalyzed six consecutive cycles, but 88 % reduction of the activity was observed for the last cycle, although ~55 % of activity was maintained at cycle 4. At cycle 2, 91–97 % of activity was observed for amino-glyoxyl-agarose, glyoxyl-agarose and CNBr-agarose derivatives. For the DEAE-Sepharose derivative, the activity decreased to 31 % at cycle 3.
Propyl gallate synthesis by the immobilized tannase
CNBr-agarose derivatives efficiently hydrolyzed tannic acid for 9 h at room temperature. The substrate was totally hydrolyzed after 10 h reaction and around 20 mM gallic acid was obtained from 2 mM tannic acid. Under the same conditions, the free enzyme hydrolyzed only 62 % of the tannic acid with a decrease of the enzymatic activity after 20 h. According to HPLC analysis, around 4.4 mM propyl gallate was obtained using CNBr-agarose derivative, which corresponded to 22 % of the theoretical maximal value (Fig. 4). Q-Sepharose and DEAE-Sepharose derivatives also hydrolyzed tannic acid, and about 4 and 2.7 mM gallic acid was obtained. These derivatives also catalyzed the transesterification reaction, resulting in the production of 88 and 23 % of the theoretical maximal value of propyl gallate, respectively (Fig. 4).
Time course of gallic acid production from tannic acid hydrolysis (filled square) and propyl gallate synthesis by transesterification reaction with 50 % 1-propanol (filled circle) using the CNBr-agarose (a), Q-Sepharose (b), and DEAE-Sepharose (c) derivatives The experiment was repeated three times with each support, activities were determined in triplicate and the results were presented as mean ± SD
The CNBr-agarose derivative was more efficient to hydrolyze tannic acid than Q-Sepharose and DEAE-Sepharose derivatives. On the other hand, the CNBr-agarose and DEAE-Sepharose derivatives showed similar efficiency to produce propyl gallate attaining ~22–23 % of the theoretical maximal value. The rate of conversion of gallic acid to propyl gallate using the Q-Sepharose derivative reached 88 % although the reduced hydrolytic activity on tannic acid. The yield of propyl gallate reported in this manuscript was higher than those observed when using the free enzymes from A. awamori BTMFW032 (Beena et al. 2011) and A. niger van Tieghen (Sharma and Gupta 2003).
Conclusion
The immobilization of an extracellular tannase on both ionic and covalent supports was effective thereby suggesting that DEAE-Sepharose, Q-Sepharose, glyoxyl-agarose, and amino-glyoxyl agarose may be considered attractive supports for tannase immobilization for industrial applications. In addition, the synthesis of propyl gallate using Q-Sepharose derivatives seems a promising approach for future industrial uses further studies for the optimization of reaction conditions using these derivatives for propyl gallate production should be conducted.
References
Aguilar CN, Gutiérrez-Sánchez G (2001) Review: sources, properties, applications and potential uses of tannin acyl hydrolase. Food Sci Techol Int 7:373–382
Aguilar CN, Rodriguez R, Gutiérrez-Sánchez G, Augur C, Favela-Torres E, Prado-Barragan LA, Ramírez-Cornel A, Contreas-Esquível AC (2007) Microbial tannases: advances and perspectives. Appl Microbiol Biotechnol 76(1):47–59
Beena PS, Basheer SM, Bhat SG, Bahkali AH, Chandrasekaran M (2011) Propyl gallate synthesis using acidophilic tannase and simultaneous production of tannase and gallic acid by marine Aspergillus awamori BTMFW032. Appl Biochem Biotechnol 164(5):612–628
Fernandéz-Lafuente R, Rodriguez V, Mateo C, Penzol G, Hernàndez-Justiz O, Irazoqui G, Villarino A, Ovsejevi K, Batista F, Guisán JM (1999) Stabilization of multimeric enzymes via immobilization and post-immobilization techniques. J Mol Catal B Enzym 7:181–189
Gonçalves HB, Riul AJ, Terenzi HF, Jorge JA, Guimarães LHS (2011) Extracellular tannase from Emericella nidulans showing hypertolerance to temperature and organic solvents. J Mol Catal B 71:29–35
Guisán JM, Alvaro G, Fernandéz-Lafuente R, Rosell CM, Garcia JL, Tagliani A (1993) Stabilization of heterodimeric enzyme by multipoint covalent immobilization: penicillin G acylase from Kluyvera citrophila. Biotechnol Bioeng 42(4):455–464
Khanna P, Sundari SS, Kumar NJ (1995) Production, isolation and partial purification of xylanases from Aspergillus sp. World J Microbiol Biotechnol 11(2):242–243
Lekha PK, Lonsane BK (1997) Production and application of tannin acyl hydrolase: state of the art. Adv Appl Microbiol 44:215–260
Mahendran B, Raman N, Kim DJ (2006) Purification and characterization of tannase from Paecilomyces variotii: hydrolysis of tannic acid using immobilized tannase. Appl Microbiol Biotechnol 70:444–450
Mingshu L, Kai Y, Qiang H, Dongying J (2006) Biodegradation of gallotannins and ellagitannins. J Basic Microbiol 46(1):68–84
Schons PF, Lopes FCR, Battestin V, Macedo GA (2011) Immobilization of Paecilomyces variotii tannase and properties of the immobilized enzyme. J Microencapsul 28(3):211–219
Sharma S, Gupta MN (2003) Synthesis of antioxidant propyl gallate using tannase from Aspergillus niger van Teighem in nonaqueous media. Bioorg Med Chem Lett 13:395–397
Sharma S, Bhat TK, Dawra RK (2000) A spectrophotometric method for assay of tannase using rhodanine. Anal Biochem 279(1):85–89
Sharma S, Bhat TK, Gupta MN (2002) Bioaffinity immobilization of tannase from Aspergillus niger on concanavalin A-Sepharose CL-4B. Biotechnol Appl Biochem 35:165–169
Sharma S, Agarwal L, Saxena RK (2008) Purification, immobilization and characterization of tannase from Penicillium variable. Bioresour Technol 99(7):2544–2551
Sittig M (1988) Trimethoprim. In: Pharmaceutical manufacturing encyclopedia. Noyes Publication, New Jersey, p. 282–284
Acknowledgments
This work was supported by grants from the Fundação de Amparo a Pesquisa do Estado de São Paulo (FAPESP) and Conselho de Desenvolvimento Científico e Tecnológico (CNPq). J. A. J. is a Research Fellow from e CNPq. The authors thank Maurício de Oliveira for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gonçalves, H.B., Jorge, J.A., Pessela, B.C. et al. Characterization of a tannase from Emericela nidulans immobilized on ionic and covalent supports for propyl gallate synthesis. Biotechnol Lett 35, 591–598 (2013). https://doi.org/10.1007/s10529-012-1111-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-012-1111-4