Abstract
The 67 kDa myosin-cross-reactive antigen (MCRA) is a member of the MCRA family of proteins present in a wide range of bacteria and was predicted to have fatty acid isomerase function. We have now characterised the catalytic activity of MCRAs from four LAB stains, including Lactobacillus rhamnosus LGG, L. plantarum ST-III, L. acidophilus NCFM and Bifidobacterium animalis subsp. lactis BB-12. MCRA genes from these strains were cloned and expressed in Escherichia coli, and the recombinant protein function was analysed with lipid profiles by GC–MS. The four MCRAs catalysed the conversion of linoleic acid and oleic acid to their respective 10-hydroxy derivatives, which suggests that MCRA proteins catalyse the first step in conjugated linoleic acid production. This is the first report of MCRA from L. rhamnosus with such catalytic function.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The 67 kDa myosin-cross-reactive antigen (MCRA) was first identified in Streptococcus pyogenes and was predicted to have polyunsaturated fatty acid (PUFA) isomerase function (Kil et al. 1994). MCRAs comprise a family of proteins that are present in a wide range of bacteria, especially lactic acid bacteria. They show high homology with linoleate isomerase from Lactobacillus reuteri PYR8 (Rosson et al. 2004), which transforms linoleic acid into conjugated linoleic acid (CLA). MCRAs have fatty acid hydratase activity and that the MCRAs from S. pyogenes (Volkov et al. 2010) and Bifidobacterium breve (Rosberg-Cody et al. 2011) are FAD-containing enzymes that act as hydratases on C9 bonds of C-18 free fatty acids, producing 10-hydroxy derivatives. Kishino et al. (2011) reported that MCRA from L. plantarum AKU 1009a is present in the cell membrane fraction and that it transforms linoleic acid into 10-hydyoxy-cis-12-octadecenoic acid (10-HOE).
The mechanism for the microbial production of CLA using L. acidophilus has been discussed and involves hydroxyl fatty acids as intermediates. The MCRA from L. plantarum (Kishino et al. 2011) is, with two other enzymes, a multi-component enzyme system for producing CLA, and CLA formation consists of three distinct steps: hydration of linoleic acid to 10-HOE, then isomerisation and dehydration of 10-HOE into CLA.
Previously, we confirmed the CLA production capacity of four bacterial strains (L. rhamnosus, L. plantarum, L. acidophilus, and B. animalis subsp. lactis), and all of them did indeed produce CLA with 10-HOE accumulation. The aim of present study was to investigate the MCRA proteins from these four lactic acid bacteria.
Materials and methods
Materials
Linoleic acid and oleic acid were purchased from Sigma–Aldrich. All other chemicals used were of analytical grade and are commercially available.
Microorganism cultivation and preparation of washed cells
Lactobacillus rhamnosus LGG, L. plantarum ST-III and L. acidophilus NCFM were aerobically cultured three times in de Man, Rogosa and Sharpe (MRS) culture medium consisting of 1 % Tryptone, 1 % meat extract, 0.5 % yeast extract, 2 % glucose, 0.1 % Tween 80, 0.2 % K2HPO4, 0.5 % sodium acetate, 0.2 % diammonium citrate, 0.02 % MgSO4·7H2O and 0.005 % MnSO4·H2O (pH 6.5) at 37 °C for 24 h. B. animalis subsp. lactis BB-12 was cultured in MRS supplemented with 0.05 % (w/v) l-cysteine·HCl. E. coli BL21 (DE3) carrying the plasmid pET28a was routinely cultured aerobically in Luria-Bertani (LB) medium (10 g Tryptone/l, 5 g yeast extract/l, 10 g NaCl/l) at 37 °C in the presence of kanamycin (50 μg/ml) as a selective marker.
DNA manipulations and plasmid construction
Genomic DNAs were isolated from the four strains as described by Hoffman and Winston (1987) with some modifications, adding lysozyme into the breaking system and treating the aqueous layer with protease K for 30 min after breaking. MCRA genes from B. animalis subsp. lactis BB-12 (GenBank: ADC85468), L. rhamnosus LGG (GenBank: YP_003170249.1), L. plantarum ST-III (GenBank: YP_003923433) and L. acidophilus NCFM (GenBank: AAV42528.1) were amplified from genomic DNAs with specific primers. PCR was performed with KOD-plus DNA polymerase (Toyobo, Japan) according to the manufacturer’s protocol. PCR conditions were as follows: 30 cycles of 45 s denaturation (94 °C), 30 s annealing (60 °C), and 2.5 min elongation (68 °C). For expression in E. coli, the MCRA ORF was amplified with the primers listed in Supplementary Table 1 and cloned into the pET28a expression vector (Novagen, USA), yielding plasmids pET28a–LGG, pET28a–ST-III, pET28a–NCFM and pET28a–BB12 (N-His-tagged version).
Production and purification of recombinant MCRA proteins
For protein production, E. coli BL21 Star strain (Invitrogen) separately harbouring pET28a–LGG, pET28a–ST-III, pET28a–NCFM and pET28a–BB12 plasmids were used. Bacteria were cultivated in LB medium supplied with kanamycin (50 μg/ml) at 37 °C until OD600 reached 0.6. At that point, IPTG was added at 0.1 mM, and the culture was placed at 20 °C for 20-h induction of protein expression.
For protein purification, cells were then harvested by centrifugation, washed with buffer A (0.1 M Tris/HCl, pH 6.5, 0.1 M NaCl), resuspended in the same buffer containing 1 mg lysozyme/ml, 1 mM PMSF, 1 μg DNase/ml and 10 mM MgCl2, and ultra-sonicated. The cell debris was removed by centrifugation. The His6-tagged fusion proteins were purified by nickel ion affinity chromatography with a chelating Sepharose Fast Flow column. Fusion proteins were eluted with 5 ml elution buffer (0.1 M Tris/HCl, pH 6.5, 0.1 M NaCl and 500 mM imidazole) and dialysed overnight against 0.1 M Tris/HCl buffer containing 20 % (v/v) glycerol (pH 6.5) at 4 °C. The protein concentration was determined from the supernatant by the Bradford method. Purified proteins were stored at −80 °C.
Immunoblotting
Protein, 10 μg, was subjected to SDS–PAGE (12 % gel) followed by protein transfer to a PVDF membrane; the immunoblots were developed with the use of anti-His antibody at a dilution of 1:1,000 (Tiangen, Beijing, China). Horseradish peroxidase (HRP)-conjugated anti-mouse IgG (Tiangen) secondary antibody diluted at 1:500 was used for the detection of specific antibody binding. The bands were visualised with enhanced chemiluminescence reagents (Kangwei, Beijing, China) according to the manufacturer’s instructions.
Activity assay and fatty acid analysis
To demonstrate the enzymatic activity of recombinant MCRA protein, 50 μg linoleic acid or oleic acid was mixed with 10 μg purified protein in 1 ml buffer A and incubated for 1 h at 25 °C. Lipid extraction and preparation of fatty acid methyl esters (FAMEs) were performed according to the procedures of Bligh and Dyer (1959). Nonadecanoic acid was used as the internal standard. Free fatty acids were dissolved in methanol and converted to corresponding methyl esters with (trimethylsilyl)-diazomethane. FAMEs were extracted with n-hexane and analysed by GC–MS on a Finnigan Trace gas chromatograph (Thermo, USA) equipped with a Finnigan Trace mass spectrometer (Thermo). Injections of 10 μl were performed automatically at a split ratio of 62:1. Hydrogen and helium were used as the carrier gases through an Agilent DB-WAX column (30 × 0.25 mm, i.d. 0.25 μm; Agilent, USA). The column temperature was set initially at 180 °C for 0.5 min, then increased to 230 °C in increments of 5 °C/min. The 230 °C was maintained for 13 min. The injector and detector were operated at 240 °C. Electron energy of 70 eV and ion source temperature of 230 °C were used.
Bioinformatics analysis
To analyse the four MCRA proteins, PSORTb, Conserved Domain Search, Pfam Search and Motif Scan were used. Homologs to the previously identified fatty acid hydratase from S. pyogenes M49 GenBank: ZP_00366513.1) and B. breve NCIMB 702258 (GenBank: ADY18551.1) were selected from BLAST analysis (Altschul et al. 1990). ClustalW2 (Larkin et al. 2007) was used for multiple sequence alignment, and protein phylogenetic trees were constructed with MEGA5 (Tamura et al. 2011).
Results and discussion
Sequence analysis and phylogenetic analysis
All four proteins were located in the cytoplasm. The calculated molecular weights were 64,444.08 Da (LGG), 64,751.24 Da (ST-III), 67,617.50 Da (NCFM) and 82,421.61 Da (BB12). Comparison with amino acid sequences in the database revealed that the four MCRA proteins showed more than 30 % homology with the MCRA from S. pyogenes M49 (LGG: 33 %, ST-III: 32 %, NCFM: 68 % and BB12: 47 %), and at least 29 % homology with the MCRA from B. breve NCIMB 702258 (LGG: 29 %, ST-III: 30 %, NCFM: 51 %, and BB12: 68 %). Figure 1 displays a phylogenetic analysis of the four MCRA proteins along with oleate hydratase homologs. The evolutionary relationship further supports the MCRAs as fatty acid hydratases.
Phylogenetic tree and multiple sequence alignment of FAD-binding domain of MCRA and oxidoreductases. On the left, the evolutionary relationships of MCRA proteins are shown. The scale bar represents an evolutionary distance of 0.2 amino acid substitutions per site. The four MCRAs (BB12, LGG, NCFM, ST-III) are underlined. Three MCRAs, reported to have fatty acid hydratase function, are indicated with a box (B. breve NCIMB 702258, L. plantarum AKU 1009a, S. pyogenes M49). On the right, alignment of the FAD-binding motif from these corresponding proteins is shown. Residues that are identical in all the sequences are highlighted. Positions of the start and the end of the protein region are shown for every sequence on either the left or right side of the alignment
The majority of amino acid residues that interact with FAD are conserved (Fig. 1). The detailed multiple sequence alignment of MCRAs presents a GxG(x)7L(x)17–23G motif consistent with FAD- and NAD(P)-binding Rossmann folds (Kleiger and Eisenberg 2002). The combined results of Pfam Search, Conserved Domain Search and Motif Scan showed that the four proteins contained a Strep_67 kDa_ant domain, which was the original domain of the MCRA family and an FAD-binding motif. Typically, the MCRAs of B. animalis subsp. lactis BB-12 and L. acidophilus NCFM have an extra FAD-dependent oxidoreductase domain, which is also found in the linoleate isomerase from Propionibacterium acnes (Liavonchanka et al. 2006) and might remove electrons from the PUFA substrate and transfer them to O2 or redox cofactors. According to previous reports (Volkov et al. 2010; Rosberg-Cody et al. 2011), MCRA proteins from S. pyogenes and B. breve are FAD-dependent; FAD is not involved in the catalysing reaction but plays a role in protein stability. There are other flavoenzymes that require FAD to maintain their proper protein architecture but without FAD being directly involved in the enzymatic reaction (Cromartie and Walsh 1976; Vargo et al. 1981; Liavonchanka et al. 2006). FAD in the four MCRAs might play the same role as previously reported.
Expression and purification of the recombinant MCRA proteins
The four MCRA proteins were overproduced in pET28a in E. coli and purified on the Ni2+–NTA column (Fig. 2a). Major bands with apparent molecular weights of 64 kDa (LGG), 64 kDa (ST-III), 67 kDa (NCFM) and 82 kDa (BB12) were visualised by SDS–PAGE after elution, and these corresponded to the expected molecular weights of the four proteins, which were absent before IPTG induction. Immunoblotting with anti-His-tag antibodies confirmed that each single band contained a 6×His tag (Fig. 2b).
Production of recombinant proteins in E. coli and enzymatic assays
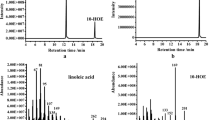
The four recombinant proteins converted linoleic acid and oleic acid into 10-HOE and 10-hydroxy-octadecanoic acid (10-HO), respectively (Fig. 3a, b). Both products showed two significant MS fragments at 169 m/z and 201 m/z, but there is a difference at 143 m/z (Fig. 4a, b). Combined with the fragmentation pattern (Fig. 4c, d), the 143 m/z peak distinguished the two products; 10-HOE lacked this significant peak. Two different oleic acids, cis-9-oleic acid and trans-9-oleic acid, were used as substrates, but the latter could not be converted (data not shown). All the results were in line with that of B. breve NCIMB 702258 (Rosberg-Cody et al. 2011), which can catalyse 10-HOE formation from linoleic acid and 10-HO from cis-9-oleic acid, respectively. Besides, no further products were transformed from hydroxyl derivatives by MCRA in our results was consistent with that of S. pyogenes (Volkov et al. 2010).
GC–MS total ion chromatograms of the products from linoleic acid and oleic acid upon incubation with the recombinant MCRA proteins. a Products formed from linoleic acid and its control. b Products formed from oleic acid and its control. Control: linoleic acid or oleic acid incubated in buffer A without protein extract for 1 h at 25 °C
MS fraction of the products of the reaction with recombinant MCRA protein with free linoleic acid and oleic acid. a Mass spectra of 10-HOE obtained after incubation of linoleic acid. b Mass spectra of 10-HO obtained after incubation of oleic acid. c The structure of 10-HOE and its fragmentation pattern. d The structure of 10-HO and its fragmentation pattern
The first attempt to identify the enzymatic activity of an MCRA from L. reuteri PYR8 was reported by Rosson et al. (2004) whose aim was to confirm its linoleic acid isomerase function, but no isomerase activity was detected. MCRA from S. pyogenes was heterologously expressed in E. coli and there was no CLA-forming isomerase activity (Feussner et al. 2008). We did not detect the linoleate isomerase activity from the MCRA proteins either but could confirm production of CLA in the four lactic acid bacteria; indeed, the four strains produced CLA with high concentrations of 10-HOE, although the accumulation of CLA was not at the same level in each of the four strains.
Our results were similar to a previous report (Ogawa et al. 2001) that the washed L. acidophilus AKU 1,137 cells accumulated CLA from linoleic acid via 10-HOE as an intermediate. According to Kishino et al. (2011), the MCRA protein from L. plantarum AKU 1009a converted linoleic acid to 10-HOE, and the product was finally transformed into CLA with two other enzymes. Our current results further illustrate the mechanism of CLA production in lactic acid bacteria, both Lactobacillus and Bifidobacterium, and suggest that the four lactic acid bacteria produce CLA with a multi-component enzyme system, similar to that of L. plantarum AKU 1009a. The remaining enzymes will be further investigated.
Conclusions
MCRAs from B. animalis subsp. lactis, L. rhamnosus, L. plantarum and L. acidophilus were cloned, expressed, and purified. Each protein can be classified as s fatty acid hydratases, converting linoleic acid and oleic acid to 10-HOE and 10-HO, which represents the first step in CLA production. This is the first report of the CLA production capacity of L. rhamnosus LGG and the MCRA function from L. rhamnosus.
References
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Cromartie TH, Walsh CT (1976) Escherichia coli glyoxalate carboligase. Properties and reconstitution with 5-deaza FAD and 1, 5-dihydrodeaza FADH2. J Biol Chem 251:329–333
Feussner I, Hornung E, Liavonchanka A (2008) International Patent WO2008 119735
Hoffman CS, Winston F (1987) A ten-minute DNA preparation from yeast efficiently releases autonomous plasmids for transformation of Escherichia coli. Gene 57:267–272
Kil KS, Cunningham MW, Barnett LA (1994) Cloning and sequence analysis of a gene encoding a 67-kilodalton myosin-cross-reactive antigen of Streptococcus pyogenes reveals its similarity with class II major histocompatibility antigens. Infect Immun 62:2440–2449
Kishino S, Park SB, Takeuchi M, Yokozeki K, Shimizu S, Ogawa J (2011) Novel multi-component enzyme machinery in lactic acid bacteria catalyzing C=C double bond migration useful for conjugated fatty acid synthesis. Biochem Biophys Res Commun 416:188–193
Kleiger G, Eisenberg (2002) GXXXG and GXXXA motifs stabilize FAD and NAD(P)-binding Rossmann folds through C(alpha)–H···O hydrogen bonds and van der Waals interactions. J Mol Biol 323(1):69–76
Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG (2007) Clustal W and Clustal X version 2.0. Bioinformatics 23(21):2947–2948
Liavonchanka A, Hornung E, Feussner I, Rudolph MG (2006) Structure and mechanism of the Propionibacterium acnes polyunsaturated fatty acid isomerase. Proc Natl Acad Sci USA 103(8):2576–2581
Ogawa J, Matsumura K, Kishino S, Omura Y, Shimizu S (2001) Conjugated linoleic acid accumulation via 10-hydroxy-12-octadecaenoic acid during microaerobic transformation of linoleic acid by Lactobacillus acidophilus. Appl Environ Microbiol 67(3):1246–1252
Rosberg-Cody E, Liavonchanka A, Göbel C, Ross RP, O’Sullivan O, Fitzgerald GF, Feussner I, Stanton C (2011) Myosin-cross-reactive antigen (MCRA) protein from Bifidobacterium breve is a FAD-dependent fatty acid hydratase which has a function in stress protection. BMC Biochem 12:9
Rosson RA, Ground AD, Deng MD, Sanchez-Riera F (2004). United States Patent US 6,743,609 B1
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739
Vargo D, Pokora A, Wang SW, Jorns MS (1981) Formation of epoxide intermediates in the reaction of enzyme-bound 5-deazaflavin with peroxides. J Biol Chem 256(12):6027–6033
Volkov A, Liavonchanka A, Kamneva O, Fiedler T, Goebel C, Kreikemeyer B, Feussner I (2010) Myosin cross-reactive antigen of Streptococcus pyogenes M49 encodes a fatty acid double bond hydratase that plays a role in oleic acid detoxification and bacterial virulence. J Biol Chem 285(14):10353–10361
Acknowledgments
This study was supported by the National Science Fund for Distinguished Young Scholars (31125021), the National High Technology Research and Development Program of China (2011AA100905), the National Natural Science Foundation of China (No. 31171636, 81071685 and 20836003), the National Basic Research Program of China 973 Program (2012CB720802), the 111 project B07029, Fundamental Research Funds for the Central Universities (JUSRP 11017, JUSRP31103), and SKLF-TS-201101.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yang, B., Chen, H., Song, Y. et al. Myosin-cross-reactive antigens from four different lactic acid bacteria are fatty acid hydratases. Biotechnol Lett 35, 75–81 (2013). https://doi.org/10.1007/s10529-012-1044-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-012-1044-y