Abstract
Baculoviral expression systems, including those of Autographa californica multiple nucleopolyhedrovirus Bombyx mori nucleopolyhedrovirus (BmNPV), are used for recombinant protein production. Four B. mori-derived (BmN4, Bm5, Bmc140, and Bme21) cell lines were infected with recombinant BmNPV viruses expressing firefly luciferase or EGFP as reporters under the control of a viral polyhedrin promoter. Bme21 exhibited significantly higher (100-fold) luciferase activity than BmN4 and Bm5. With the EGFP reporter protein, Bme21 cells showed a marked increase in the ratio of EGFP-positive cells, reaching 90 % on day 4 post-infection, while Bm5 and BmN4 cells had a slow increase in the ratio of their EGFP-positive population. The viral titer in a supernatant of Bme21 cell culture increased faster than those of Bm5 and BmN4 cells. This susceptibility indicates that the Bme21 cell line is useful for large-scale protein expression using BmNPV.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Baculoviridae family is infectious to arthropods, particularly to insects of the order Lepidoptera, Diptera, and Hymenoptera. The nucleopolyhedrovirus (NPVs), a major subgroup of baculoviruses, are characterized by enveloped rod-shaped virions containing circular double-stranded DNA (about 130 kbp). Baculoviruses, AcMNPV and BmNPV infecting Autographa californica and Bombyx mori, respectively have become popular as efficient vectors for the high-level expression of foreign proteins (Maeda 1989). The baculovirus expression vector system (BEVS) has many advantages over the Escherichia coli expression system, such as the capacity to insert large DNA fragments, eukaryotic protein modifications, and a high yield of large proteins (O’Reilly et al. 1992).
AcMNPV has a wider host range including 39 lepidopteran species (Bonning and Hammock 1992). In the AcMNPV expression system, however, only a few cell lines have been commercially available for use in BEVS. These include Sf21and Sf9 cells derived from Spodoptera frugiperda (Vaughn et al. 1977) and BTI-Tn-5B1-4 (Hi-5) cells derived from Trichoplusia ni (Granados et al. 1994). On the other hand, in the BmNPV expression system, several silkworm cell lines established from silkworm embryos (Pandharipande 1994; Pan et al. 2007; Imanishi et al. 2012), and larval and pupal ovaries (Sudeep et al. 2002; Khurad et al. 2006, 2009) are available, but only a few of them, such as Bm5 and BmN4, can be used in BEVS. The production of recombinant BmNPVs in BmN4 and Bm5 cells is low compared to recombinant AcMNPV in Sf9 or Sf21. A BmNPV bacmid system has been developed (Motohashi et al. 2005) and used for the production of recombinant protein using silkworm larvae or pupae (Miao et al. 2006; Kajikawa et al. 2009). However, B. mori cell lines supporting high-level expression of recombinant proteins using BmNPV have rarely been documented.
In the present study, four B. mori-derived cell lines were infected with recombinant BmNPVs to evaluate the efficiency of viral infection, replication, and recombinant protein expression.
Materials and methods
Cells and media
Spodoptera frugiperda (Sf21) and B. mori (BmN4, Bm5, Bmc140, and Bme21) cells were maintained in IPL-41 medium (GiBCO) supplemented with 10 % fetal bovine serum (FBS). Drosophila melanogaster (S2) cells were grown in Schneider’s Drosophila medium (GiBCO) supplemented with 10 % (v/v) FBS. All cell lines were grown at 27 °C.
The Bme21 cell line was established from embryos of a silkworm strain, t91 (previously e21), from the Institute of Genetic Resources, Graduate School of Agriculture, Kyushu University, Japan. Morphologically, Bme21 cells were small and round with clear cytoplasm measuring about 10–20 μm in diameter. The population doubling time of Bme21 cells was 48–96 h under optimal conditions. DNA fingerprinting profiles using simple-sequence repeat polymerase chain reaction (SSR-PCR) with four sets of SSR primers (Khurad et al. 2009) exhibited clear differences between the Bme21 cell line and the three B. mori (BmN4, Bm5, and Bmc140) cell lines (see supplement Fig. 1).
BmN4 (embryo-derived), Bm5 (embryo-derived), Bmc140 (pupa-derived), and Bme21 cells were split at a ratio of 1:2 every 4–5 days. Sf21 and S2 cells were split at a ratio of 1:5 every 4 days.
Construction of recombinant viruses
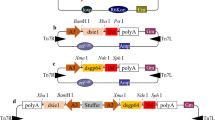
The reporter genes (luciferase or EGFP) were inserted into a multiple cloning site (MCS) of the donor plasmid pFastBac1 (Invitrogen). The resulting constructs were named pFBpoh-Luc and pFBpoh-EGFP, respectively. The recombinant donor plasmids were transformed into E. coli BmDH10Bac cells to generate a recombinant BmNPV bacmid (Motohashi et al. 2005). Through Tn7-mediated transposition in the bacterial cell, the reporter genes were transferred to a target site of the bacmid DNA. Large recombinant bacmid DNAs were isolated using the FlexiPrep kit (Amersham Pharmacia Biotech).
Recombinant bacmid DNA, 200 ng, was isolated and transfected into Bm5 cells using CellFectin transfection reagent (Gibco). After incubation for 5 days at 27 °C, the culture medium was harvested and centrifuged at 2,000×g for 5 min, followed by collection of the supernatant (P1 viral solution). Filter-sterilized P1 viral solutions of BmNPV/poh-Luc and BmNPV/poh-EGFP were stored at 4 °C. P1 viral stocks were further used to infect Bm5 cells to generate high-titer P2 stock. Correspondingly, P3 stocks were generated from the P2 stock. Virus stocks were maintained and titrated according to standard protocols (O’Reilly et al. 1992).
Assay of luciferase activity
105 cells of each cell line were infected with recombinant BmNPV at a multiplicity of infection (MOI) of 0.5. The infected cells were harvested at 12, 24, 72, and 96 h post-infection (hpi), washed with phosphate-buffer saline (PBS, pH 7.5), and lysed using 1 % (v/v) TritonX-100 for 30 min. The luciferase activity in the resulting solutions was assayed by a Luminometer (Bio-Orbit) using PicaGene kit (Toyo Ink). Each infection experiment was carried out in duplicate and repeated more than two times independently. Luciferase activity was quantified as relative light units (RLU) using the protein, determined by Lowry’s method according to the procedure described previously (Wang and Smith 1975).
Fluorescence observations
Fluorescence of EGFP in cultured cells of all the cell lines was observed with a fluorescence microscope (TMD 300, Nikon, Japan) at 48, 72, and 96 hpi respectively.
Results
Expression of luciferase in four B. mori-derived cell lines infected with recombinant BmNPV
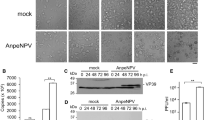
To compare the abilities to support the production of recombinant protein of all six cell lines, namely, B. mori-derived cell lines BmN4, Bm5, Bme21, Bmc140, the S. frugiperda-derived cell line Sf21 and the D. melanogaster-derived cell line S2, recombinant BmNPV expressing luciferase was generated using the Bac-to-Bac baculovirus expression system (Invitrogen) according to the manufacturer’s protocol. The Bm5 and BmN4 cell lines were obtained from public cell banks for replication of BmNPV. As shown in Fig. 1, luciferase activity was detected in the lysates of all four B. mori cell lines, whereas no activity was detected in Sf21 cells or S2 cells. Among the four B. mori cell lines, Bme21 was the most productive; this cell line exhibited significantly higher (100-fold) luciferase activity than BmN4 and Bm5 cells. Although Bmc140 cells generated luciferase activity, the level was quite low and slightly above the baseline. These results suggest that the Bme21 cell line is the most suitable for BEVS using BmNPV.
Expression of luciferase in cell lines infected with BmNPV/polh-Luc at an MOI of 0.5. Cells were harvested at the indicated time post-infection and luciferase activity in the cell lysates was assayed. The average value was calculated from four data and expressed in relative light units (RLU) per microgram of total cellular protein with a standard deviation
EGFP expression in BmNPV-infected B. mori-derived cell lines
To confirm the remarkable ability of Bme21 in the production of recombinant protein, another reporter protein, EGFP, was employed. All six insect cell lines were infected with BmNPV/polh-EGFP at an MOI of 0.5, and the emission of green fluorescence in the cells was observed at day 4 post-infection (pi) (Fig. 2a). In agreement with the results obtained using luciferase-expressing BmNPV, the green fluorescence in the Bme21 cell line was also higher than those in BmN4 and Bm5 cells; only a few Bmc140 cells fluoresced. The other cells, Sf21 and S2, did not fluoresce at all. A large amount of protein synthesis by recombinant BmNPV would require rapid replication of viral DNA and maintenance of gene expression, both of which promise a steady mass production of protein. Therefore, we investigated the temporal pattern of green fluorescence expression in the hyper-sensitive Bme21 cell line, in comparison with those in the Bm5 and BmN4 cell lines. The cell lines were infected with BmNPV/polh-EGFP, and the emission of green fluorescence was observed at 48, 72, and 96 hpi (Fig. 2b). On day 2 pi, green fluorescence was observed in all three B. mori cell lines tested. Thereafter, the Bme21 cell line showed a marked increase in the ratio of EGFP-positive cells, reaching 90 % at day 4 pi, while Bm5 and BmN4 cells underwent a very slow increase in the ratio of the positive population.
Expression of EGFP in cell lines infected with BmNPV/polh-EGFP. a B. mori-derived cell lines (Bm5, BmN4, Bme21, and Bmc140) and the other cell lines (Sf21 and S2) were infected with BmNPV/polh-EGFP at an MOI of 0.5 and photographed on day 4 post-infection. b B. mori-driven cell lines (Bm5, BmN4, and Bme21) were infected with BmNPV/polh-EGFP at an MOI of 1, and observed at 48, 72, and 96 hpi after infection. The upper figure in each panel shows the fluorescent image, and the lower figure, the bright-light image
EGFP expression in Bme21 cells infected with BmNPV at different MOIs
In general, high-MOI infection is preferred for stable and assured production of recombinant proteins, but it is costly to prepare high-titer virus stocks. As shown in Fig. 2b, the Bme21 cell line appears to support rapid replication of viral DNA. If this is indeed the case, then this feature of Bme21 could be of great advantage for large-scale protein production. Later on, the Bme21 cells were infected at different MOIs of BmNPV/polh-EGFP (0.001, 0.01, 0.1, 1, and 10) to determine the minimum required MOI (Fig. 3). The expression of EGFP was monitored by fluorescence microscopy at 48, 72, and 96 hpi. At MOIs of 0.1, 1, and 10, cells showed intense fluorescence within 48 hpi, as expected, with the fluorescence intensity reaching a maximum at 72 hpi (Fig. 3, lane c, d, e). Surprisingly, even at low MOIs (0.001 and 0.01), expression of EGFP was first detected at 48 hpi and rapidly increased with time, reaching a maximum at 96 hpi. Taken together, all the data indicate that BmNPV can replicate quickly and efficiently in Bme21 cells.
Discussion
AcMNPV can infect different types of cells, including a variety of mammalian cells (Airenne et al. 2011; Hitchman et al. 2011); however, replication of AcMNPV requires host cells established from insect origin. BmNPV also requires B. mori-derived cell lines for viral genome replication. BmN4 and Bm5 cells are available from public cell banks, but the replication of BmNPV in these cells seems to be less effective when compared with the combination of AcMNPV and S. frugiperda-derived cell lines, although silkworm BEVS may be more beneficial than the AcMNPV-S. frugiperda system because of the distinctive features of B. mori (see "Introduction" section).
No host genes are involved in the susceptibility of silkworm cells and larvae to baculovirus. However, several viral genes, such as antiapoptotic factor p35 (Schwartz et al. 2002) and iap (Chen et al. 2012), viral DNA helicase p143 (Yu and Carstens 2012), host cell-specific factor hcf-1 (Wilson et al. 2005) and host range factor hrf-1 (Ishikawa et al. 2004), have been identified as host range effectors in certain insect cell lines or insect larvae. The differences in BmNPV susceptibility among the silkworm cell lines tested in this study suggest the existence of host genes involved in BmNPV replication.
To construct a large-scale industrial protein expression system, a B. mori cell line that is highly permissive of recombinant BmNPV replication is required. In this report, we identified the Bme21 cell line (derived from embryos of B. mori strain e21) as by far the most promising candidate when compared to some of the commonly used B. mori-derived cell lines, such as BmN4 and Bm5. The rapid amplification of recombinant viruses from a low-titer stock demonstrates that Bme21 cells would be very useful in producing large amounts of high-titer viral stocks for industrial use.
References
Airenne KJ, Makkonen KE, Mähönen AJ, Ylä-Herttuala S (2011) Baculoviruses mediate efficient gene expression in a wide range of vertebrate cells. Methods Mol Biol 737:279–301
Bonning BC, Hammock BD (1992) Development and potential of genetically engineered viral insecticides. Biotechnol Genet Eng Rev 10:455–489
Chen TH, Lo YP, Yang CF, Chen WJ (2012) Additive protection by antioxidant and apoptosis-inhibiting effects on mosquito cells with dengue 2 virus infection. PLoS Negl Trop Dis 6:e1613
Granados RR, Li GX, Derksen ACG, McKenna KA (1994) A new insect-cell line from Trichoplusia ni (BTI-TN-5B1-4) susceptible to Trichoplusia ni single enveloped nuclear polyhedrosis-virus. J Invertebr Pathol 64:260–266
Hitchman RB, Murguía-Meca F, Locanto E, Danquah J, King LA (2011) Baculovirus as vectors for human cells and applications in organ transplantation. J Invertebr Pathol 107:S49–S58
Imanishi S, Kobayashi J, Sekine T (2012) Serum-free culture of an embryonic cell line from Bombyx mori and reinforcement of susceptibility of a recombinant BmNPV by cooling. In Vitro Cell Dev Biol Anim 48:137–142
Ishikawa H, Ikeda M, Alves CA, Thiem SM, Kobayashi M (2004) Host range factor 1 from Lymantria dispar nucleopolyhedrovirus (NPV) is an essential viral factor required for productive infection of NPVs in IPLB-Ld652Y cells derived from L. dispar. J Virol 78:12703–12708
Kajikawa M, Sasaki K, Wakimoto Y, Toyooka M, Motohashi T, Shimojima T, Takeda S, Park EY, Maenaka K (2009) Efficient silkworm expression of human GPCR (nociceptin receptor) by a Bombyx mori bacmid DNA system. Biochem Biophys Res Commun 385:375–379
Khurad AM, Kanginakudru S, Qureshi SO, Rathod MK, Rai MM, Nagaraju J (2006) A new Bombyx mori larval ovarian cell line highly susceptible to nucleopolyhedrovirus. J Invertebr Pathol 92:59–65
Khurad AM, Zhang MJ, Deshmukh CG, Bahekar RS, Tiple AD, Zhang CX (2009) A new continuous cell line from larval ovaries of silkworm, Bombyx mori. In Vitro Cell Dev Biol Anim 45:414–419
Maeda S (1989) Expression of foreign genes in insects using baculovirus vectors. Annu Rev Entomol 34:351–372
Miao Y, Zhang Y, Nakagaki K, Zhao T, Zhao A, Meng Y, Nakagaki M, Park EY, Maenaka K (2006) Expression of spider flagelliform silk protein in Bombyx mori cell line by a novel Bac-to-Bac/BmNPV baculovirus expression system. Appl Microbiol Biotechnol 71:192–199
Motohashi T, Shimojima T, Fukagawa T, Maenaka K, Park EY (2005) Efficient large-scale protein production of larvae and pupae of silkworm by Bombyx mori nuclear polyhedrosis virus bacmid system. Biochem Biophys Res Commun 326:564–569
O’Reilly DR, Miller LK, Luckow VA (eds) (1992) Baculovirus expression vectors. A laboratory manual. WH Freeman, New York
Pan MH, Xiao SQ, Chen M, Hong XJ, Lu C (2007) Establishment and characterization of two embryonic cell lines of Bombyx mori. In Vitro Cell Dev Biol Anim 43:101–104
Pandharipande TN (1994) Two new continuously growing cell lines from Bombyx mori (Lepidoptera: Bombycidae). Appl Entomol Zool 29:604–607
Schwartz PS, Chen CS, Waxman DJ (2002) Enhanced bystander cytotoxicity of P450 gene-directed enzyme prodrug therapy by expression of the antiapoptotic factor p35. Cancer Res 62:6928–6937
Sudeep AB, Mishra AC, Shouche YS, Pant U, Mourya DT (2002) Establishment of two new cell lines from Bombyx mori (L.) (Lepidoptera: Bombycidae) and their susceptibility to baculoviruses. Indian J Med Res 115:189–193
Vaughn JL, Goodwin RH, Tompkins GJ, McCawley P (1977) The establishment of two cell lines from the insect Spodoptera frugiperda (Lepidoptera: Noctuidae). In Vitro Cell Dev Biol Plant 13:213–217
Wang C, Smith RL (1975) Lowry determination of protein in the presence of Triton X-100. Anal Biochem 63:414–417
Wilson JA, Forney SD, Ricci AM, Allen EG, Hefferon KL, Miller LK (2005) Expression and mutational analysis of Autographa californica nucleopolyhedrovirus HCF-1: functional requirements for cysteine residues. J Virol 79:13900–13914
Yu M, Carstens EB (2012) Choristoneura fumiferana multiple nucleopolyhedrovirus LEF-3-P143 complex can complement DNA replication and budded virus in an AcMNPV LEF-3-P143 double knockout bacmid. J Gen Virol 93:383–388
Acknowledgments
This work was supported in part by KAKENHI Nos. 22248003, 22248004 and 23580077 from the Japan Society for the Promotion of Science. The cost of publication was supported in part by the Research Grant for Young Investigators of Faculty of Agriculture, Kyushu University. Cultured silkworm cell lines used in this study were kindly provided by Dr. Aoki, Kyushu University.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lee, J.M., Kawakami, N., Mon, H. et al. Establishment of a Bombyx mori nucleopolyhedrovirus (BmNPV) hyper-sensitive cell line from the silkworm e21 strain. Biotechnol Lett 34, 1773–1779 (2012). https://doi.org/10.1007/s10529-012-0971-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-012-0971-y