Abstract
We established the first continuous cell line that uses a serum-free culture from the embryo of Bombyx mori (Lepidoptera: Bombycidae), designated as NIAS-Bm-Ke17. This cell line was serially subcultured in the SH-Ke-117 medium. The cells adhere weakly to the culture flask, and most cells have an oval shape. The cell line was subcultured 154 times, and the population doubling time is 83.67 ± 5.22 h. Random amplification of polymorphic DNA-polymerase chain reaction with a tenmar single primer for discrimination of insect cell lines recognized the NIAS-Bm-Ke1 cell line as B. mori. This cell line does not support the growth of the B. mori nuclear polyhedrosis virus (BmNPV) in the absence of the heat-inactivated hemolymph of B. mori. However, the heat-inactivated hemolymph in 1% volume of the medium supported a high level of susceptibility to BmNPV. In addition, the cooling treatment of the cells at 2.5°C also enhanced the susceptibility. We report a new serum-free culture system of the B. mori cell line for the baculovirus expression vector system.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Molecular biology techniques have progressed rapidly in recent years, and insect cell lines have been valuable tools in the production of useful proteins by gene expression. Insect cell lines have been researched in the propagation of specific pathogens in invertebrate pathology since they were first established by Grace (1962). Insect viruses, particularly the baculovirus, have also been used in genetic studies and for recombinant protein production in research and pharmaceutical applications. The Sf9, Sf21 (Vaughn et al. 1992), and BTI-TN-5B1-4 (Granados et al. 1994) cell lines are the most popular cell lines for the baculovirus expression vector system (BEVS), particularly because they do not require fetal bovine serum (FBS) as a growth factor. However, there is no serum-free Bombyx mori cell line for the BEVS. Lynn (2001) reported that more than 500 insect cell lines have been established from 120 different insect species. In addition, we have established many insect cell lines because of the need for molecular study (Imanishi et al. 2006; Takahashi et al. 2006; Suzuki et al. 2008; Cui et al. 2009; Fujita et al. 2009; Furukawa et al. 2009; Iwanaga et al. 2009; Tanaka et al. 2009a, b, c, 2010; Sagisaka et al. 2010; Suzuki et al. 2010). In particular, we established the first continuous cell lines using a serum-free culture from the embryo of B. mori (Lepidoptera: Bombycidae), designated as NIAS-Bm-Ke1; NIAS-Bm-Ke17 is its cloned cell line. Because B. mori BEVS has two convenient systems that express the foreign gene by using the cell line and the larvae, this B. mori BEVS system will be a more multipurpose gene expression system. In this study, we report that BmNPV susceptibility to serum-free cultured cells was highly improved by using cell cooling and using the heat-inactivated hemolymph of B. mori.
Materials and Methods
NIAS-Bm-Ke1 (the parent cell line of the cloned NIAS-Bm-Ke17 cell line) and NIAS-Bm-Ke17 cloned cell lines were established using the following methods: first, the primary culturing of the embryonic tissues of B. mori was started in June 1985. This was followed by subculturing in December 1986; we designated this cell line as NIAS-Bm-Ke1. As a medium, MGM-448 (Mitsuhashi 1984) that contained 10% volume of FBS (Company South Logan, UT) was supplied to the cells of the primary culture. Then, to decrease FBS volume, MGM-448 was gradually substituted by MM (Mitsuhashi and Maramorosch 1964). Next, FBS content was decreased to 1% volume. Finally, FBS from the MM medium was eliminated completely. The NIAS-Bm-Ke17 cloned cell line was established from the cloning (Tomita et al. 1999) of the NIAS-Bm-Ke1 cell by forming clonal colonies on SeaPlaque agarose (FMC Bio-Products, Rockland, ME) gel. The SH-Ke117 of a serum-free medium was developed for culturing NIAS-Bm-Ke17 cells.
We confirmed the NIAS-Bm-Ke1 cell line by random amplification of polymorphic DNA-polymerase chain reaction (RAPD-PCR) as a continuous cultured cell line derived from B. mori. RAPD-PCR, with a tenmar single primer for discrimination of NIAS-Bm-Ke1, was performed in a total volume of 25 μl, containing 2.5 μl of TaKaRa Ex Taq buffer (TaKaRa Co., Shiga, Japan), 1 μl of 2.5 mM dNTP, 0.5 μl of 10 pM each primer, 0.125 μl of TaKaRa Ex Taq polymerase (TaKaRa Co.), 2 μl of 0.05 μg/μl of template DNA, and 18.375 μl of Milli-Q water. Six primers were used to amplify the DNA of all 13 cell lines. The base sequences of the primers were 5′-GGTGCGGGAA-3′ (1), 5′-GTTTCGCTCC-3′ (2), 5′-GTAGACCCGT-3′ (3), 5′-AAGAGCCCGT-3′ (4), 5′-AACGCGCAAC-3′ (5), and 5′-CCCGTCAGCA-3′ (6). The program used polymerase chain reaction (PCR) amplification in a DNA thermal cycler (TP3000; TaKaRa Co.). The PCR was first performed at 95°C for 5 min (denaturation step), followed by 35 cycles, each consisting of 94°C for 30 s, 50°C for 30 s (annealing step), and 72°C for 1 min (extension step); after that, it was terminated at 72°C for 7 min.
Recombinant luciferase assay.
BmPTLNPV (Tomita et al. 1995) that was replaced by the firefly recombinant luciferase gene was inoculated to 30,000 cells at 1MOI for 96 h at 27°C. Also, HylucNPV (Mori et al. 1992) that has the same susceptibility to both B. mori cells and Spodoptera furgiperda cells was inoculated to NIAS-Bm-Ke1 and Sf9 cell line. Harvested cells were then measured against recombinant luciferase activity by using a Luminometer (Lumat LB9501, Berthold Technologies GmbH, Bad Wildbad, Germany).
Results and Discussion
MGM-448 medium is suitable for primary culture. However, the continuous cultured-cell lines are not able to culture by this medium that almost FBS volume was eliminated. However, NIAS-Bm-Ke1 cell line was finally succeeded to culture by serum-free MM medium. In MM medium, two protein substances of lactalbumin hydrolysate and TC-yeastolate are a part of ingredients. The former protein has high content of essential amino acids. And the latter protein is a mixture of peptides, amino acids, carbohydrates, simple and complex as well as vitamins. We consider that these two substances played a role as a standby of FBS.
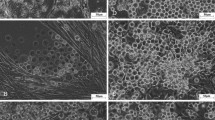
The NIAS-Bm-Ke17 (Fig. 1) cells were selected from five cloned cell lines of the NIAS-Bm-Ke1 cell line by considering cell growth and cell shape. The cells were around 20 μm in size and oval in shape. The adhesion against the cell culture flask was weak, and pipetting can easily detach the cells from the flask. For large-scale culture, the NIAS-Bm-Ke17 cells can be cultivated by not only stationary culture but also suspension culture for the weak anchorage-dependent cells. However, the poly-d-lysine cell matrix supports cell adhesion. This matrix-coated well plate (BD BioCoat, Bedford, MA) was used for the NIAS-Bm-Ke17 cell cultures; moreover, 4% (v/v) of the heat-inactivated hemolymph of B. mori was added to the medium. The heat-inactivated hemolymph ensured that the NIAS-Bm-Ke17 cells adhered to the cell culture flask strongly. The NIAS-Bm-Ke17 cell line can also be used for virus cloning using the plaque assay technique. Furthermore, two cell lines of NIAS-Bm-Ke1 and NIAS-Bm-Ke17 were not contaminated with mycoplasma.
Differentiation of the NIAS-Bm-Ke1 cell line by primers 4 and 6.
The primers 1, 2, 3, 4, 5, and 6 of all cell lines of NIAS-Bm-Ke1, NIAS-Bm-M1, NIAS-Bm-oyanagi2, NIAS-Bm-aff3, NIAS-Pss-5, NIAS-Cuox-11, and NIAS-Hc-2 were used for DNA amplification (Fig. 2a and b ). Among them, two primers showed polymorphism: 5′-AAGAGCCCGT-3′ (4) and 5′-CCCGTCAGCA-3′ (6). DNA fragment patterns of the NIAS-Bm-Ke1 cell line were markedly different from other insect cell lines of Plautia stali Scott (NIAS-Pss-5), Culicoides oxystoma (NIAS-Cuox-11), and Herse convolvuli (NIAS-Hc-2). However, the DNA fragment of the NIAS-Bm-Ke1 cell line was especially amplified at 400 bp similar to other B. mori cell lines. The NIAS-Bm-Ke1 cell line was clearly identified by the DNA fragment pattern as B. mori. Furthermore, the NIAS-Bm-Ke17 cell line is a cloned cell line of NIAS-Bm-Ke1, and we recognized that the NIAS-Bm-Ke17 cell line was derived from B. mori tissues.
(a) RAPD-PCR analysis (RAPD-P4, 5′-AAGAGCCCGT-3′). Bombyx mori: BmN4, NIAS-Bm-aff3, NIAS-Bm-Ke1, NIAS-Bm-M1, NIAS-Bm-F1, NIAS-Bm-oyanagi2, NIAS-Bm-ao1; Bombyx mandarina: NIAS-Boma-529b; Plautia stalli Scott: NIAS-Pss-5; Polypedilum vanderplanki: NIAS-Pv-210, NIAS-Pv-1; Culicoides oxystoma: NIAS-Cuox-11; Herse convolvuli: NIAS-Hc-2. NIAS-Bm-M1 was derived from the male embryo of the autosexing race of B. mori; NIAS-Bm-oyanagi2 from the embryo of the hybrid race of both Japanese No. 137 and Chinese No. 145; NIAS-Bm-aff3 from the larva fat body of the hybrid race of (Japanese No. 509 × Japanese No. 500) and (Chinese No. 509 × Chinese No. 502); NIAS-Pss-5 from the embryo of P. stali Scott, NIAS-Cuox-11 from C. oxystoma, and NIAS-Hc-2 from H. convolvuli. (b) RAPD-PCR analysis (RAPD-P6, 5′-CCCGTCAGCA-3′).
The serum-free culture is applicable to the cultivation of NIAS-Bm-Ke17 cells. SH-Ke117 of a new medium was produced by modifying the MM medium from the SHIMA Laboratories Co., LTD. (Tokyo, Japan). The medium accelerated cell growth; population doubling time was 83.67 ± 5.22 h.
The NIAS-Bm-Ke17 cell line did not express the susceptibility of BmPTLNPV when culturing with a serum-free medium. However, the heat-inactivated hemolymph of B. mori was able to improve the BmPTLNPV susceptibility when the NIAS-Bm-Ke17 cells were cultured by the serum-free medium. The increase in heat-inactivated hemolymph from 0.5 to 2.5% volume promoted the susceptibility of BmPTLNPV. The heat-inactivated hemolymph increased the amount of expression of recombinant luciferase in the cells (Fig. 3). When the cells were cultured by the medium with 2.5% volume of the heat-inactivated hemolymph, the recombinant luciferase amount was 16 times compared to that with the serum-free medium. A heat-inactivated hemolymph volume of more than 5% did not increase the amount of recombinant luciferase; this is because high concentration of the heat-inactivated hemolymph disturbed cell growth.
Effect of hemolymph on BmPTLNPV infection. Heat-inactivated hemolymph of Bombyx mori: this was obtained from the fourth day of the fifth larvae and heated to 56°C for 30 min. It was centrifuged for 30 min at 1,000×g, and then the supernatant was filtered for elimination of microorganisms by using 0.22 μm (Sartorius Stedium Biotech GmbH, Goettingen, Germany). The heat-inactivated hemolymph was stocked in a freezer at −30°C.
In addition to the heat-inactivated hemolymph of B. mori, we improved the susceptibility of BmPTLNPV to the NIAS-Bm-Ke17 cells by using a new technology of cooling the cells. The NIAS-Bm-Ke17 cells in the growth phase were preserved at 2.5, 5, 10, 20, and 25°C for 24 h. Then, 2.5% volume of the heat-inactivated hemolymph was added to the medium. Moreover, BmPTLNPV was inoculated at 1 MOI at 27°C for 96 h. The NIAS-Bm-Ke17 cells that were preserved at 2.5°C expressed a high amount of recombinant luciferase. The NIAS-Bm-Ke17 cells showed a recombinant luciferase amount that was 32 times compared to that obtained with the cells preserved at 25°C (Fig. 4). This means that BmPTLNPV was infected into the NIAS-Bm-Ke17 cells in high susceptibility at low temperature. Next, the cooling period was checked. The NIAS-Bm-Ke17 cells were preserved at 2.5°C for a period of 0, 6, 12, 24, 48, 72, 96, or 120 h. The NIAS-Bm-Ke17 cells preserved for 48 h produced higher amounts of recombinant luciferase than the cells preserved for other periods. In addition, when BmPTLNPV was inoculated before the NIAS-Bm-Ke17 cells were preserved for 48 h at 2.5°C, two times the recombinant luciferase amounts were produced (data not shown). On the other hand, the NIAS-Bm-Ke17 cells cultured by the medium without the heat-inactivated hemolymph produced a small amount of recombinant luciferase (Fig. 5).
Effect of incubation temperature on BmPTLNPV infection. The NIAS-Bm-Ke17 cells in a culture flask (Sumilon, MS-21050; Sumitomo Bakelite Co., Ltd.) were preserved in incubators at 2.5, 5, 10, 20, and 25°C for 24 h. As soon as the cells were removed from the refrigerator, a 2.5% (volume/medium) of heat-inactivated hemolymph of Bombyx mori was added to the medium. Then, the recombinant virus was inoculated to the cells for 96 h.
Both the heat-inactivated hemolymph and the cooling of 2.5°C for 48 h had a positive effect on the susceptibility of BmPTLNPV. As a result, the susceptibility for BmPTLNPV was enhanced remarkably.
Hyluc-NPV susceptibility to the NIAS-Bm-Ke1 cells was compared with BmN4, Sf9, and NIAS-Bm-Ke17 cells. Despite cooling or non-cooling for these cell lines, the Sf9 cells produced the highest amount of recombinant luciferase. Compared to the preservation of Sf9 cells at 25°C, the amount of recombinant luciferase produced was 1/163 times for NIAS-Bm-Ke1 cells, 1/214 times for NIAS-Bm-Ke17 cells, and 1/22.9 times for BmN4 cells. On the other hand, at 2.5°C preservation, compared to the same Sf9 cells, recombinant luciferase production was 1/293 times for NIAS-Bm-Ke1 cells, 1/55 times for NIAS-Bm-Ke17 cells, and 1/110 times for BmN4 cells (Fig. 6a ). Thus, the Sf9 cells had superior expression ability. However, Hyluc-NPV susceptibility induced by a cooling effect against 25°C was indicated as follows: NIAS-Bm-Ke1 cells showed a value that was 4/5 times, NIAS-Bm-Ke17 cells 8.7 times, BmN4 cells 3/10 times, and Sf9 cells 1.4 times (Fig. 6b ). This indicates that the NIAS-Bm-Ke17 cells have a strong susceptibility to Hyluc-NPV recombinant virus in a cooling environment.
(a) Effect of temperature on Hyluc-NPV in four cell lines. Hyluc-NPV (Mori et al. 1992), which has the same susceptibility to both Bombyx mori cells and Spodoptera furgiperda cells, was inoculated to the NIAS-Bm-Ke1, NIAS-Bm-Ke17, BmN4, and Sf9 cell lines. (b) Ratios of Hyluc-NPV (2.5 against 25°C) in four-cell lines.
The heat-inactivated hemolymph accelerated both cell proliferation and BmNPV susceptibility in vitro (Imanishi et al. 1993); however, there are no reports about the acceleration of BmNPV susceptibility when the cells were cooled at 2.5°C. Matsubara et al. (1984) reported that the B. mori larva of the fifth instar showed high susceptibility by the peroral administration of polyhedral of BmNPV after cold treatment (5°C, 24 h). This was discussed because that type of BmNPV easily increases in the membrane of the mid-gut or easily penetrates the membrane. When a B. mori larva is preserved at low temperature, the protease that inactivates the BmNPV stops working (Funakoshi and Aizawa 1989). However, both the NIAS-Bm-Ke1 and NIAS-Bm-Ke17 cell lines are cultured cells derived from embryonic tissues. Okazaki et al. (1995) reported that the fifth instar first-day larva of B. mori shows a high death rate when the larvae that inoculated with BmNPV after encountered with 2.5°C for 24 h. This phenomenon appears to be almost the same as the result in this paper. In order to elucidate the BmNPV infection mechanisms at the molecular level, it is important to analyze the BmNPV-responsive silkworm genes that may be involved in viral infection. However, the genome-wide host gene expression profile in response to BmNPV infection has not yet been analyzed clearly. We reported in the previous paper (Sagisaka et al. 2010) that the regulated-host genes in B. mori cells upon BmNPV infection by using a 44 K oligonucleotide microarray (Agilent Technologies, Inc., Santa Clara, CA) derived from the silkworm EST database, KAIKO cDNA system (http:kaikocdna.dna.affrc.go.jp/) was examined. Microarray analysis showed that 35 genes were up-regulated by BmNPV infection. Meanwhile, 23 clones had no significant homology with known genes. 17 clones were significantly down-regulated. Eight of them were unknown genes with no or very low similarity with known genes, whereas some clones were predicted to encode enzyme, homologs of transitional endoplasmic reticulum ATPase, NADH dehydrogenase and aspartate aminotransferase. We elucidated that luciferase activity reaches its highest level in cells within 1 h from cooling (data not shown). We estimate the following: even if some of the host genes that were predicted to be the resistant genes against BmNPV infection expressed to produce some kinds of protein, the cell body is very cold. The products do not activate as a resistant factor against the early gene of ie-1. Sagisaka et al. (2010) reported that we could not confirm significant regulation of host genes by microarray analysis, probably because of differences in silkworm cell line and infection period. Nobiron et al. (2003) found only a host Hsp 70 cognate (Hsc70) gene was up-regulated in AcMNPV-infected S. furgiperda Sf9 cells. However, Sagisaka et al. (2010) indicated that the expression level of the silkworm Hsc70 ortholog gene was not increased in response to BmNPV. In this study, probably the expression level of recombinant luciferase differs between AcMNPV-infected Sf9 and BmNPV-infected NIAS-Bm-Ke17 by the regulation of each host gene. Microarray analysis is very useful tool to identify BmNPV-responsive host genes. Although the products of the unknown gens are expected to be related to viral infection, the molecular functions of these products still remain to be elucidated. Functional analysis of these gene products may lead to the discovery of new mechanism for BmNPV infection in the insect cells.
References
Cui X.; Urita S.; Imanishi S.; Nagasawa T.; Suzuki K. Isolation and characterization of a 41 kDa serin from the wild silkworm Antheraea yamamai. J. Insect Biotechnol. Sericol 78(1): 11–16; 2009.
Fujita K.; Sagisaka A.; Tomimoto K.; Ishibashi J.; Imanishi S.; Yamakawa M.; Tanaka H. DNA vector-based RNA interference in cell lines derived from Bombyx mori. Biosci. Biotechnol. Biochem. 73(9): 2026–2031; 2009.
Funakoshi M.; Aizawa K. Anti-viral substance in the silkworm gut juice against a nuclear polyhedrosis virus of the silkworm. Bombyx mori. J. Invertebr. Pathol. 53: 135–136; 1989.
Furukawa S.; Tanaka H.; Ishibashi J.; Imanishi S.; Yamakawa M. Functional characterization of a Cactus homolog from the silkworm Bombyx mori. Biosci. Biotechnol. Biochem. 73(12): 2665–2670; 2009.
Grace T. D. C. Establishment of four strains of cells from insect tissues grown in vitro. Nature 195: 788–789; 1962.
Granados R. R.; Guoxun L.; Derksen A. C. G.; McKenna K. A. A new insect cell line from Trichoplusia ni (BTI-Tn-5B1-4) susceptible to Trichoplusia ni single enveloped nuclear polyhedrosis virus. J. Invertebr. Pathol. 64: 260–266; 1994.
Imanishi S.; Inoue H.; Kobayashi J.; Kadono K.; Koumoto H.; Belloncik S.; Kuwabara N.; Sakamoto H.; Tomita S. In vitro multiplication of Bombyx mori nuclear polyhedrosis virus. Bull. Natl. Inst. Seric. Entomol. Sci. 7: 9–29; 1993.
Imanishi S.; Yan S.; Kimura H. Development of baculovirus gene expression system using Bombyx mori culture cell line (NIAS-Bm-Ke1) that adjusted to serum-free media. J. Seric. Sci. Jpn. 75(1): 45–51; 2006.
Iwanaga M.; Arai R.; Shibano Y.; Kawasaki H.; Imanishi S. Establishment and characterization of the Bombyx mandarina cell line. J. Invertebr. Pathol. 101(2): 124–129; 2009.
Lynn D. E. Novel techniques to establish new insect cell lines. In Vitro Cell. Dev. Biol. Anim. 37: 319–321; 2001.
Matsubara F.; Wu Y. L.; Mori H.; Oogashira J. Difference in susceptibility of germ free silkworm at each instar to NPV after cold treatment. J. Seric. Sci. Jpn. 53(3): 205–209; 1984.
Mitsuhashi J. Isolation of a continuous cell line from larval fat bodies of an Arctiid moth, Spilarctia seriatopunctata (Insecta, Lepidoptera, Arctiidae). Zool. Sci. 1: 415–419; 1984.
Mitsuhashi J.; Maramorosch K. Leafhopper tissue culture: embryonic, nymphal, and imaginal tissue from aseptic insects. Contr. Boyce Thompson Inst. 22: 435–460; 1964.
Mori H.; Nakazawa H.; Shirai N.; Shibata N.; Sumida M.; Matsubara F. Foreign gene expression by a baculovirus vector with an expanded host range. J. Gen. Virol. 73: 1877–1880; 1992.
Nobiron I.; O’reilly D. R.; Olszewski J. A. Autographa californica nucleopolyhedrovirus infection of Spodoptera furgiperda cells: a global analysis of host gene regulation during infection, using a differential display approach. J. Gen. Virol. 84: 3029–3039; 2003.
Okazaki H.; Kanaya T.; Nishimura S.; Ogawa K.; Watanabe H. Inoculation of Baculovirus vector to Bombyx mori larvae treated at low temperature. J. Seric. Sci. Jpn. 64: 504–508; 1995.
Sagisaka A.; Fujita K.; Nakamura Y.; Ishibashi J.; Noda H.; Imanishi S.; Mita K.; Yamakawa M.; Tanaka H. Genome-wide analysis of host gene expression in the silkworm cells infected with Bombyx mori nucleopolyhedrovirus. Virus Res. 147(2): 166–175; 2010.
Suzuki M. G.; Imanishi S.; Dohmae N.; Asanuma M.; Matsumoto S. Identification of a male-specific RNA binding protein that regulates sex-specific splicing of Bmdsx by increasing RNA binding activity of BmPSI. Mol. Cell. Biol. 30(24): 5776–5786; 2010.
Suzuki M. G.; Imanishi S.; Dohmae N.; Nishimura T.; Shimada T.; Matsumoto S. Establishment of a novel in vivo sex-specific splicing assay system to identify a trans-acting factor that negatively regulates splicing of Bombyx mori dsx female exons. Mol. Cell. Biotechnol. 28(1): 333–343; 2008.
Takahashi T.; Murakami H.; Imanishi S.; Miyazaki M.; Kamiie K.; Suzuki K.; Taira H.; Yamashita T. Calreticulin is transiently induced after immunogen treatment in the fat body of the silkworm Bombyx mori. J. Insect Biotechnol. Sericol. 75(2): 79–84; 2006.
Tanaka H.; Fujita K.; Sagisaka A.; Tomimoto K.; Imanishi S.; Yamakawa M. shRNA expression plasmids generated by a novel method efficiently induce gene-specific knockdown in a silkworm cell line. Mol. Biotechnol. 41(2): 173–179; 2009a.
Tanaka H.; Sagisaka A.; Fujita K.; Kaneko Y.; Imanishi S.; Yamakawa M. Lipopolysaccharide elicits expression of immune-related genes in the silkworm. Bombyx mori. Insect Mol. Biol. 18(1): 71–75; 2009b.
Tanaka H.; Sagisaka A.; Nakajima Y.; Fujita K.; Imanishi S.; Yamakawa M. Correlation of differential expression of silkworm anitimicrobial peptide genes with different amount of Rel family proteins and their gene transcriptional activity. Biosci. Biotechnol. Biochem. 73(3): 599–606; 2009c.
Tanaka H.; Suzuki N.; Nakajima Y.; Sato M.; Sagisaka A.; Fujita K.; Ishibashi J.; Imanishi S.; Mita K.; Yamakawa M. Expession profiling of novel bacteria-induced genes from the silkworm. Bombyx mori. Arch. Insect Biochem. Physiol. 73(3): 148–162; 2010.
Tomita S.; Kanaya T.; Kobayashi J.; Imanishi S. Isolation of p10 gene from Bombyx mori nuclear polyhedrosis virus and study of its promoter activity in recombinant baculovirus vector system. Cytotechnology 17: 65–70; 1995.
Tomita S.; Tamura T.; Imanishi S. Stable transformation of Lepidoptera cultured cells with novel cloning method. In Vitro Cell. Dev. Biol 35: 311–313; 1999.
Vaughn J. L.; Goodwin R. H.; Thomkins G. J.; McCawley P. Screening of insect cell lines for the production of recombinant proteins and infectious virus in the baculovirus expression system. Biotechnol. Prog. 8: 391–396; 1992.
Acknowledgements
We thank Dr. S. Tomita (Transgenic Silkworm Research Unit, National Institute of Agrobiological Sciences) for his kind advice. This work was supported by grants for research from the Research and Development Program for New Bio-industry Initiatives.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: T. Okamoto
Rights and permissions
About this article
Cite this article
Imanishi, S., Kobayashi, J. & Sekine, T. Serum-free culture of an embryonic cell line from Bombyx mori and reinforcement of susceptibility of a recombinant BmNPV by cooling. In Vitro Cell.Dev.Biol.-Animal 48, 137–142 (2012). https://doi.org/10.1007/s11626-011-9465-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11626-011-9465-9