Abstract
Suspension-cultured hazel cells were ultrasonicated at power densities of 4 and 455 mW for 4–40 min. Both treatments stimulated the production of major taxanes: Taxol, 10-deacetylbaccatin, and baccatin III. The highest amounts of these taxanes (0.46, 0.26, and 0.07 mg/l, respectively) were obtained at 8 and 20 min of the treatment at 455 mW. Ultrasound had no adverse effects on cell viability, growth, or membrane integrity. Increased release of taxanes by ultrasound resulted not from increased membrane permeability but more likely from stimulation of taxanes biosynthesis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ultrasound is a physical tool for manipulation of plant and animal cells and organs. Early studies about ultrasound were mostly focused on its hazardous effects on living organisms. However, more recently, increasing attention has been paid to the beneficial effects and potential applications of ultrasound in plant biotechnology based on its intensity and exposure time as well as on the physiological conditions of plant tissues (Lin and Wu 2002; Pong et al. 2006). Low-intensity ultrasound can produce a variety of effects on biological materials, such as alteration of enzyme activity, cell growth, cell membrane and organelle structure (Bochu et al. 1998). Ultrasound has also been applied to improve the extraction of natural products such as for Taxol from cultured cells (Wu and Ge 2004). However, the mechanism(s) by which ultrasound exerts its effects is a matter of debate. Taxol (paclitaxel) is a diterpenoid alkaloid and is currently one of the best drugs for the treatment of various cancers. Yew trees produce very low yields of Taxol and grow very slowly. However, hazel (Corylus avellana), an angiosperm that produces Taxol and other taxanes (Otaggio et al. 2008; Rezaei et al. 2011), is widely available and hazel cells grow faster in vivo and are easier to cultivate in vitro than yew, thus proving to be more beneficial than yew. The aim of the present study was evaluation of ultrasound-induced physiological responses and taxane production in hazel cells. Possible effects of ultrasound exposure on alteration of cell membrane integrity are also discussed.
Materials and methods
Cell culture and ultrasound treatments
A rapidly growing cell line of hazel (Corylus avellana L. cv. Gerd Eshkevar) was used, and the cells were cultured in a modified MS medium without glycine and supplemented with 3 mg NAA/l, 3 mg IAA/l, and 1 mg kinetin/l (Sahebjamei et al. 2007). Suspension cultures were kept in dark at 25°C with shaking at 110 rpm and were subcultured every 7 days. The growth curve of the cells was plotted, and the cells in the mid-growth phase (days 6–11) were exposed to ultrasound by a piezoelectric ultrasound device (Barber et al. 1997). Power was supplied to the sonicator through a voltage stabilizer to offset any fluctuations in the supply. The pressure amplitude of the sound waves was measured using the hydrophone technique as previously described (Rezaei et al. 2011). The frequencies of this ultrasonic unit was 29 kHz and the spatial peak intensities were 4 and 455 mW/cm2 for fundamental frequency based on calibration certification test results. After the treatment, the cells were filtered and transferred into fresh media and kept for a further 1 week until harvesting.
Biochemical analysis
The growth of hazel cells was monitored by their dry wt Viability of the cells was assessed with Evans Blue (aqueous, 0.05% w/v) as described by Smith et al. (1984), and damage to membranes was evaluated by measuring the lipid peroxidation rate (De Vos et al. 1991) and leakage of electrolytes from membranes (Chen et al. 2008).
Intra- and extracellular taxanes were extracted with methylene chloride/water (1:1 v/v) and were detected by HPLC (Rezaei et al. 2011). Quantification of Taxol, baccatin III, and 10-deacetylbaccatin was accomplished by comparing the retention time and peak area with reference standards.
Free proline was extracted from frozen cells in 3% (v/v) aqueous sulfosalicylic acid and estimated using ninhydrin reagent (Vendruscolo et al. 2007). Fructan content was measured using a modified anthrone method with 0–30 μg inulin/ml standards (Jermyn 1956). The peroxidase activity was assessed using guaiacol as an electron donor (Sahebjamei et al. 2007).
Statistical analysis
SPSS (version 16, Chicago, IL, USA) were used for statistical analysis. An LSD test was calculated for multiple means comparisons at a significance level of p ≤ 0.05.
Results
Cell growth and viability
Ultrasound exposure of low power (4 mW) for 8 and 20 min increased the biomass by 11 and 7%, respectively. However, exposure for 40 min decreased the dry mass by 5% of that of the control cells (Fig. 1). Ultrasound exposure of higher power (455 mW) for 8 min significantly increased dry mass of the cells by 15%, but 20-min and 40-min exposure significantly decreased dry mass by 9% of that of the control cells (Fig. 1). Cell viability did not change with up to 8-min ultrasound exposure, but decreased to 19% of that of the control cells after exposure for 20–40 min (Fig. 1).
Viability and growth of suspension-cultured hazel cells treated with ultrasound. Six-day-old cells were exposed to ultrasound at power densities of 4 and 455 mW for 4–40 min. Cell dry weight (DW) and viability were measured after 7 days. Data are presented as mean ± SD with n = 3. Bars with different letters in each graph are significantly different at p ≤ 0.05 according to LSD test
Membrane integrity
Ultrasound exposure of 4 mW for up to 20 min had no significant effect on lipid peroxidation of membrane of hazel cells, but 40-min exposure increased the lipid peroxidation rate by 7% of that of the control cells (Fig. 2). However, ultrasound exposure of 455 mW for 20–40 min increased the lipid peroxidation rate by 16–19% of that of the control cells (Fig. 2). Changes in electrolyte leakage through hazel cell membranes showed tendencies similar to those of lipid peroxidation rate of membrane (Fig. 2).
Membrane integrity of hazel cells monitored by measuring malondialdehyde (MDA) as the final product of lipid peroxidation and by electrolyte leakage. Six day old cells were exposed to ultrasound at power densities of 4 and 455 mW for 4–40 min. Lipid peroxidation rate was measured after 7 days and electrolyte leakage was measured immediately after the treatments. Data are presented as mean ± SD with n = 3. Bars with different letters in each graph are significantly different at p ≤ 0.05 according to LSD test
Accumulation of proline and fructans and peroxidase activity
Fructan content of ultrasound-treated cells was greater (14–68%) than that of the control cells (Fig. 3a). Proline content of all ultrasound-treated hazel cells was significantly greater (9–30%) than that of the control cells (Fig. 3b).
Ultrasound stimulated peroxidase activity by 51–228% of that of the control cells (Fig. 4). The enzyme activity increased with increasing exposure time up to 20 min. An increase of exposure time to 40 min, however, decreased the peroxidase activity, although it was still greater than that of the control cells (Fig. 4).
Accumulation of taxanes
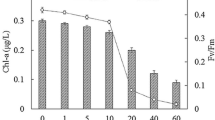
Major taxanes, i.e., Taxol, 10-deacetylbaccatin, and baccatin III, were detected both inside the hazel cells (intracellular) and in the media (extracellular). As Table 1 shows, Taxol was the major taxane of hazel cells. Taxol content of ultrasound-treated cells was 6-times greater than that of the control cells (0.46 ± 0.01 vs. 0.08 ± 0.01 mg/l). Treatment of hazel cells with ultrasound also increased the production of 10-deacetylbaccatin (maximum 0.26 ± 0.01 mg/l) and baccatin III (maximum 0.07 mg/l), which were 5- and 7-times greater, respectively, than those in the control cells (Table 1). Detailed changes in the percentage release of all three detected taxanes are shown in Table 1.
Discussion
Ultrasound increases the release of secondary metabolites of suspension-cultured plant cells into their media (Liu et al. 2003; Wang et al. 1998). This was attributed more to the increase of membrane permeability rather than physiological response of the cells to ultrasound. Several aspects of the effects of ultrasound on the physiology of suspension-cultured hazel cells have been considered in our study. Our study demonstrated the stimulatory effects of ultrasound on the growth of hazel cells in short exposure periods. Similar stimulatory effects of ultrasound have been reported in suspension-cultured cells of rice and carrot and solid cultured callus of aloe (Liu et al. 2003; Wang et al. 1998). Acoustic cavitation and changes of membrane fluidity have been suggested as the most possible causes for the various physiological effects of ultrasound (Liu et al. 2003). Adverse effects of ultrasound on plant cells have also been attributed to the changes in structure and properties of membrane unsaturated fatty acids, resulting in enhanced free-radical formation and increased membrane permeability (Chen et al. 2008). However, our results showed that the treatment of hazel cells with an appropriate dosage of ultrasound does not change the lipid peroxidation rate of membrane or solute leakage of the cells but provides the cells with increased proline and fructans contents. Fructans stabilize membranes by direct hydrogen bonding to the phosphate and choline groups of membrane lipids (Garcia et al. 2011) and accumulation of proline protects the plant cells against singlet oxygen production during stress conditions, thus maintaining membrane integrity (Hong et al. 2000; Vendruscolo et al. 2007). Activation of peroxidase was another noteworthy effect of ultrasound in hazel cells. Considering that peroxidase is an antioxidant enzyme acting specifically on peroxide, ultrasound exposure again resulted in stabilization of membrane integrity by increasing the peroxidase activity and limiting the damage caused by peroxide radicals (Zhou et al. 2011). Coincident with these results was low membrane leakage of ultrasound-treated cells. However, the mechanisms by which ultrasound triggers such responses in cells remain to be clarified.
The taxane release ratio of cells exposed to ultrasound for short periods was very similar to that of the control cells. However, the total amount of each taxane markedly increased, implying again that ultrasound stimulated the biosynthesis of taxanes and not increase their release through the membrane.
References
Barber BP, Hiller RA, Lofstedt R, Putterman SJ, Weninger KR (1997) Defining the unknowns of sonoluminescence. Phys Rep 28:143–165
Bochu W, Yoshikoshi A, Sakanishi A (1998) Carrot cell growth response in a stimulated ultrasonic environment. Colloid Surface B 12:89–95
Chen B, Huang J, Wang J, Huang L (2008) Ultrasound effects on the antioxidative defense systems of Porphyridium cruentum. Colloids Surf B 16:88–92
De Vos CHR, Schat H, DeWaal MDA, Vooijs R, Ernst WHO (1991) Increased resistance to copper-induced damage of the root cell plasmalemma in copper tolerant Silene cucubalus. Physiol Plant 82:523–528
Garcia PM, Asega AF, Silva EA, Carvalho MA (2011) Effect of drought and re-watering on fructan metabolism in Vernonia herbacea (Vell.) Rusby. Plant Physiol Biochem 49:664–670
Hong Z, Lakkineni K, Zhang Z, Verma DP (2000) Removal of feedback inhibition of delta(1)-pyrroline-5-carboxylate synthetase results in increased proline accumulation and protection of plants from osmotic stress. Plant Physiol 122:1129–1136
Jermyn MA (1956) A new method for determining ketohexoses in the presence of aldohexoses. Nature 177:38–39
Lin LD, Wu JY (2002) Enhancement of shikonin production in single- and two-phase suspension cultures of Lithospermum erythrorhizon cells using low-energy ultrasound. Biotechnol Bioeng 78:81–88
Liu Y, Takatsuki H, Yoshikoshi A, Wang B, Sakanishi A (2003) Effects of ultrasound on the growth and vacuolar H+-ATPase activity of Aloe arborescens callus cells. Colloids Surf B B32:105–116
Otaggio L, Bestoso F, Armirotti A, Balbi A, Damonte G, Mazzei M, Sancandi M, Miele M (2008) Taxanes from shells and Leaves of corylus avellana. J Nat Prod 71:58–60
Pong M, Umchid S, Guarino AJ, Lewin PA, Litniewski J, Nowicki A, Wrenn SP (2006) In vitro ultrasound-mediated leakage from phospholipid vesicles. Ultrasonics 45:133–145
Rezaei A, Ghanati F, Behmanesh M, Mokhtari-Dizaji M (2011) Ultrasound-potentiated salicylic acid-induced physiological effects and production of Taxol in hazel (Corylus Anellana L.) cell culture. Ultrasound Med Biol 37:1938–1947
Sahebjamei H, Abdolmaleki P, Ghanati F (2007) Effects of magnetic field on the antioxidant enzyme activities of suspension-cultured tobacco cells. Bioelectromagnetics 28:42–47
Smith MAL, Palta JP, McCown BH (1984) The measurement of isotonicity and maintenance of osmotic balance in plant protoplast manipulations. Plant Sci Lett 33:249–258
Vendruscolo ECG, Schuster I, Pileggi M, Scapim CA, Molinari HB, Marur CJ, Vieira LG (2007) Stress-induced synthesis of proline confers tolerance to water deficit in transgenic wheat. J Plant Physiol 164:1367–1376
Wang BC, Yoshikoshi A, Sakanishi A (1998) Carrot cell growth response in a stimulated ultrasonic environment. Colloids Surf B 12:89–95
Wu J, Ge X (2004) Oxidative burst, jasmonic acid biosynthesis, and Taxol production induced by low-energy ultrasound in Taxus chinensis cell suspension cultures. Biotechnol Bioeng 85:714–721
Zhou R, Li Y, Yan L, Xie J (2011) Effect of edible coatings on enzymes, cell-membrane integrity, and cell-wall constituents in relation to brittleness and firmness of Huanghua pears (Pyruspyrifolia Nakai, cv. Huanghua) during storage. Food Chem 124:569–575
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Safari, M., Ghanati, F., Hajnoruzi, A. et al. Maintenance of membrane integrity and increase of taxanes production in hazel (Corylus avellana L.) cells induced by low-intensity ultrasound. Biotechnol Lett 34, 1137–1141 (2012). https://doi.org/10.1007/s10529-012-0865-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-012-0865-z