Abstract
TGF-β1 plays a necessary and important role in the induction of chondrogenic differentiation of bone marrow stromal cells (BMSCs). In this study, porcine BMSCs were infected with a replication-deficient adenovirus expression vector carrying the hTGF-β1 gene. The transduced BMSCs were cultured as pelleted micromasses in vitro for 21 days, seeded onto disk-shaped PGA scaffolds for 3 days and subsequently implanted into the subcutaneous tissue of mice. BMSCs transduced with AdhTGF-β1 expressed and secreted more hTGF-β1 protein in vitro than those of the control group. Histological and immunohistological examination of the pellets revealed robust chondrogenic differentiation. Tissues made from cells transduced with AdhTGF-β1 exhibited neocartilage formation after 3 weeks in vivo. The neocartilage occupied 42 ± 5% of the total tissue volume which was significantly greater than that of the control group. Furthermore, there was extensive staining for sulfated proteoglycans and type II collagen in the AdhTGF-β1 group compared to controls, and quantification of GAG content showed significantly greater amounts of GAG in experimental groups. The results demonstrate that transfer of hTGF-β1 into BMSCs via adenoviral transduction can induce chondrogenic differentiation in vitro and enhance chondrogenesis in vivo.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cartilage damage remains a major concern in orthopedics. Current methods of treatment often cause injury to other areas of the joint and cannot prevent further cartilage degeneration, synovitis or chronic malfunction of the joint. Tissue engineering strategies that employ cell-scaffold constructs to repair cartilage defects represent great advances towards alleviating these problems but the lack of a sufficient source of seed cells continues to impede the adequate restoration of joint function (Vacanti and Upton 1994).
Recently, numerous studies have demonstrated that bone marrow stromal cells (BMSCs) can be induced to chondrocytes when cultured in the presence of members of the transforming growth factor β superfamily (Uoth et al. 2002; Xia et al. 2004) and other growth factors. Among these growth factors, researchers have shown that TGF-β1 plays a necessary and important role in the induction of chondrogenic differentiation of BMSCs in vitro. The addition of the recombinant exogenous growth factor TGF-β1 can upregulate the expression of type II collagen and glycosaminoglycan. It has also been shown that TGF-β1 can stimulate proteoglycan synthesis and stabilize the chondrogenic phenotype (Johnstone et al. 1998; Han et al. 2005). When chondrogenically-induced BMSCs were seeded onto biodegradable scaffolds and implanted subcutaneously, some studies have failed to obtain mature cartilage formation (Chen et al. 2005; Jenner et al. 2007; Xu and Xu 2008). One possibility for these findings is that apparent dedifferentiation of the induced cells may be caused by failure upon in vivo implantation to maintain the high concentration of hTGF-β1 used in vitro to induce chondrogenesis. The genetic transfer of the hTGF-β1 gene into BMSCs could compensate for the loss by promoting the BMSCs to secrete the main inductive factor to stimulate chondrogenesis through autocrine and paracrine pathways. TGF-β1 bioactivity, when transferred into BMSCs by genetic transfection, can be maintained for 3 weeks and chondrocyte-specific extracellular matrix components, such as aggrecans and collagens, are synthesized (Xia et al. 2007). Adenoviral-mediated transfer of TGF-β1 can also induce chondrogenic differentiation of human mesenchymal stem cells in vitro (Saraf and Mikos 2006).
In the present study, we applied adenoviral transfer of hTGF-β1 into BMSCs to induce chondrogenic differentiation of BMSCs in vitro. An in vivo study was also performed to determine whether this mode of growth factor delivery might also enhance chondrogenesis in vivo, thereby laying a foundation for the use of hTGF-β1 gene-transfected BMSCs in cartilage tissue engineering.
Materials and methods
Animals
A total of 10 infantile Changfeng pigs (6–8 kg) and 10 5- to 8-week-old athymic mice (BALB/cA-nu/nu) were used. The experimental protocol was approved by the Animal Experiment Committee of Shanghai Jiao Tong University.
Isolation and culture of BMSCs
Pigs were anesthetized via intramuscular injection of xylazine (2 mg/kg) and ketamine (20 mg/kg). In brief, 6–8 ml bone marrow was aspirated from the iliac crest and washed twice with phosphate-buffered saline (PBS). The cells were then resuspended in low glucose Dulbecco’s Modified Eagle Medium (DMEM); supplemented with 10% (v/v) fetal bovine serum (FBS), l-glutamine (300 µg/ml), ascorbic acid (50 µg/ml), penicillin (100 U/ml), and streptomycin (100 µg/ml), layered onto a Ficoll gradient (1.077 g/ml), and centrifuged for 30 min at 500g. The nucleated cell fraction was collected from the 1.077 g/ml interface, plated on to 100-mm dishes and cultured at 37°C and 5% CO2. After 10–14 days, adherent colonies of cells were trypsinized and counted. These cells were then plated at 2 × 106 cells per 100-mm dish and grown at 37°C and 5% CO2.
Adenoviral transduction in vitro
The replication-deficient adenovirus carrying human TGF-β1 complementary DNA (AdhTGF-β1) was constructed by gene recombination (Xia et al. 2006). When the BMSCs reached 70–80% confluence, the low-glucose DMEM was replaced with serum-free media containing AdhTGF-β1 at multiplicities of infection (MOI) of 200. As a control, each cell was transduced with Ad-LacZ (BD Biosciences, K1651-1) at 200 MOI. After 4 h incubation with the virus, the viral media were removed and the cells were washed twice with PBS. Low glucose DMEM media supplemented with 10% FBS were then added and the cells were incubated in this media for 72 h.
Preparation of tBMSC pellet cultures
The transduced BMSCs (tBMSCs) and the corresponding control cells were trypsinized, counted, and 500,000 cells were collected by centrifugation for 5 min at 500g in 15-ml conical tubes. The cells were then incubated in high-glucose DMEM defined medium supplemented with 2% FBS, 0.1 μM dexamethasone, 10 mg transferrin/l, 7.2 mg bovine insulin/l, 100 mg sodium pyruvate/l, and 50 mg ascorbic acid/ml at 37°C and 5% CO2 for 21 days (Mackay et al. 1998). The media were changed every 3–4 days and samples were analyzed for the presence of hTGF-β1.
Preparation of tBMSC-scaffold constructs and implantation
Disc-shaped PGA (Albany International Research, Albany, NY) scaffolds were prepared by pressing 15 mg of PGA fibers in a 2 ml syringe. These scaffolds measured 8 mm diameter and 2 mm thick and were sterilized in 75% (v/v) ethanol for 30 min followed by three washes in PBS and air-drying. Transduced BMSCs were seeded onto the scaffolds by dropping 0.4 ml cell suspension of 50 × 106 cells/ml in DMEM onto each scaffold. The tBMSC-scaffold constructs were then incubated in high glucose DMEM defined media supplemented with 2% FBS, 0.1 μM dexamethasone, 10 mg transferrin/l, 7.2 mg bovine insulin/l, 100 mg sodium pyruvate/l and 50 mg ascorbic acid/ml at 37°C and 5% CO2 for 3 days. The tBMSC-PGA constructs were then implanted into the subcutaneous tissue of 10 mice at one side of the dorsa. Each mouse also received one tBMSC-PGA construct transduced with Ad-LacZ at contralateral side of the dorsa to serve as a control. Each implant was placed within a distinct tissue pouch to avoid communication between experimental and control groups. At 3 weeks post-implantation, the mice were sacrificed, and the constructs were removed and analyzed by gross observation, histological examination, immunohistochemical localization of type II collagen and GAG quantification.
ELISA for hTGF-β1 protein production
Samples taken from the media during in vitro pellet culture were quantitatively analyzed for the production of hTGF-β1 using a commercial enzyme-linked immunosorbent assay kit (ELISA; R&D Systems, Minneapolis, MN, USA). Following the manufacturer’s protocol, the concentration of hTGF-β1 protein was determined after activation with 0.2 M HCl (Annes et al. 2003).
Histological analysis of pellets and tissue formed in vivo
Samples were prepared from the pellets after 21 days of in vitro culture, and tissues were harvested from the mice after 21 days following in vivo implantation. The samples were fixed in 10% phosphate-buffered formalin for 24 h and then embedded in paraffin. Transverse sections of 5 μm thickness of the specimens were stained with hematoxylin and eosin (H&E) and Safranin O. The volumes of neocartilage of the harvested specimens relative to the total tissue volume were determined using Image-Pro Plus analysis software (Version 4.5.0.19, Media Cybernetics Inc).
Immunohistochemical examination of pellets and of tissue formed in vivo
Tissue sections of the pellets after 21 days of culture or of tissues harvested from the mice after 21 days in vivo were analyzed for the presence of type II collagen by staining with mouse antihuman type II collagen monoclonal antibody (Chemicon International, United Kingdom). Sections were rehydrated and treated with 0.4% (w/v) pepsin in 0.1 M HCl at room temperature for 30 min and washed twice with PBS. The sections were then incubated in PBS containing 10% (v/v) goat serum for 20 min to reduce non-specific binding. The sections were then incubated in a humidified chamber overnight at 4°C with 1:100 diluted mouse anti-human type II collagen monoclonal antibody in PBS. The sections were thoroughly rinsed with PBS three times and incubated with a peroxidase-conjugated rabbit anti-mouse immunoglobulin (Dako, USA) for 1 h at room temperature, followed by three washes with PBS. The staining was visualized by incubation for 5 min at room temperature with diaminobenzidinetrahydrochloride and counterstained with hematoxylin. As a negative control, untreated porcine skin sections were also used. As a blank control, some samples were processed without the primary antibodies.
GAG quantification of the tissues formed in vivo
The GAG content of the harvested tissues following in vivo implantation was determined by the specific binding of Alcian Blue to GAG in the tissue extracts at a low pH in the presence of a nonionic detergent and a high salt concentration (Xia et al. 2004). The GAG-dye complexes formed precipitates, which were isolated by centrifugation. The precipitated complexes were dissolved in solutions of 33% propan-1-ol in 4 M guanidine-HCl, and the absorbance was measured in a microplate reader at 600 nm. In this assay, five tissue samples (50 mg each) were randomly selected from the experimental group, the control group and native porcine articular cartilage. A standard curve was constructed using chondroitin 4-sulfate solutions of known values (Sigma). All samples and standards were tested in duplicate. GAG content is given as mg/g harvested tissue.
Statistical analysis
All values were presented as mean ± standard deviation. The mean concentrations of active hTGF-β1 protein from in vitro culture were compared between two groups by Student’s t-test (n = 5). Student’s t-test was also used to analyze the differences in cartilage volume between the experimental and control groups. Analysis of variance (ANOVA) was used to determine differences in GAG content in harvested tissues among the experimental group, the control group and native cartilage. An a priori significance level of α = 0.05 was used to determine significance between groups.
Results
Production of hTGF-β1
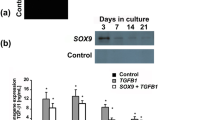
The amount of hTGF-β1 produced by the transduced cells was determined by ELISA analysis of samples taken from the media at 7, 14, and 21 days of pellet culture. The cells transduced with AdhTGF-β1 synthesized 3340 ± 435, 5123 ± 607, and 1179 ± 174 pg/ml of active hTGF-β1 at 7, 14, and 21 days, respectively. The maximum concentration of active hTGF-β1 in the media was at 14 days of pellet culture and gradually decreased with time. However, even after 21 days, the transduced cells produced significantly more hTGF-β1 than the control groups (Fig. 1).
Active hTGF-β1 synthesis by pellet culture of transduced cells. The highest concentration of hTGF-β1 was at day 14. Values were expressed as means, and error bars represent standard deviation (SD), n = 5. The media conditioned by the cells in pellet were collected at 7, 14 and 21 days and used for hTGF-β1 determination by ELISA
Histological and immunohistochemical analysis of the pellets
Histological examination of sections of the pellets from BMSCs transduced with AdhTGF-β1 after 21 days of culture revealed considerable chondrogenic differentiation. H&E staining showed elongated, perichondrium-like cells lining the surface of the pellets, with some nests of cartilage throughout the depth of the pellets. In these sections, chondrocytes were embedded in lacunae (Fig. 2). Staining with Safranin O showed abundant sulfated proteoglycans in the extracellular matrix surrounding chondrocyte-like cells (Fig. 3). In contrast, the control pellets, made from cells that were transduced with Ad-LacZ, did not have any chondrocyte-like cells or evidence of chondrogenic differentiation (Fig. 2). Safranin O staining showed no evidence of sulfated proteoglycans in the control pellets (Fig. 3).
Histological analysis of pellets made from transduced cells. (a) AdhTGF-β1 (b) AdhTGF-β1 (c) Ad-LacZ (d) Ad-LacZ. All pellets were stained with H&E. Pellets made from cells transduced with AdTGF-β1 showed robust chondrogenic differentiation. There was no obvious chondrogenic differentiation in any of the pellets of cells transduced with Ad-LacZ (Bar = 100µm)
Safranin O staining of pellets made from transduced cells. (a) AdhTGF-β1 (b) AdhTGF-β1 (c) Ad-LacZ (d) Ad-LacZ. Pellets made from cells transduced with AdhTGF-β1 stained strongly for the presence of sulfated proteoglycans in the ECM of the chondrogenesis region. There was no evidence of the presence of sulfated proteoglycans in the ECM in any pellets transduced with Ad-LacZ (Bar = 100 µm)
The expression of type II collagen in hTGF-β1 transduced cells was detected by immunohistochemical staining. There was robust type II collagen staining in the cartilage sections of these pellets, but none whatsoever in the pellets that were made from cells transduced with Ad-LacZ (Fig. 4).
Detection of type II collagen expression in cells by immunohistochemistry. (a) AdhTGF-β1 (b) AdhTGF-β1 (c) Ad-LacZ (d) Ad-LacZ. Immunohistochemical staining revealed the presence of type II collagen in the neocartilage region of pellets made from cells transduced with AdhTGF-β1. In contrast, there was no type II collagen staining in any pellets of cells transduced with Ad-LacZ (Bar = 100 µm)
Histological and immunohistochemical analysis of the tissues formed in vivo
After 3-weeks in vivo implantation, the transduced BMSC-scaffold constructs were relatively firm and resistant to compression and were easily separated from the adherent fibrous capsule. Tissues made from cells transduced with AdhTGF-β1 exhibited neocartilage formation, although the tissue was not homogeneous. Rather, the tissues were comprised of nests of cartilage separated by strips of fibrous tissue. Mature chondrocytes were embedded in lacunae in the regions of cartilage (Fig. 5). Tissues made from cells transduced with Ad-LacZ showed only a small quantity of neocartilage surrounded by abundant fibrous tissue and remnants of undegraded PGA scaffolds. The neocartilage volume, expressed as a percent of the total tissue volume, for the experimental group and the control group were 42 ± 4.6% and 17.5 ± 2.9%, respectively, according to the Image-Pro Plus analysis, and this difference was statistically significant (Fig. 5). Furthermore, Safranin O staining confirmed the presence of a great deal of sulfated proteoglycans in the ECM of the regions of cartilage in the experimental group. There was much more extensive staining for sulfated proteoglycans in the AdhTGF-β1 group than those in the Ad-LacZ group (Fig. 6).
Histological analysis of engineered tissue made from transduced cells at 3 weeks post-implantation using H&E staining. (a) AdhTGF-β1 (Bar = 200 µm) (b) AdhTGF-β1 (Bar = 100 µm) (c) Ad-LacZ (Bar = 200 µm) (d) Ad-LacZ (Bar = 100 µm). Tissues made from cells transduced with AdTGF-β1 showed some nests of cartilage separated by strips of fibrous tissue. Mature chondrocytes were embedded in lacuna in the chondrogenesis region. Tissues made from cells transduced with Ad-LacZ showed only a small quantity of neocartilage surrounded by large amounts of fibrous tissue, as well as undegraded PGA scaffolds
Safranin O staining of tissues made from transduced cells at 3 weeks post-implantation. (a) AdhTGF-β1 (b) Ad-LacZ. Tissues made from cells transduced with AdTGF-β1 confirmed the presence a great deal of sulfated proteoglycans in the ECM of chondrogenesis region. Tissues made from cells transduced with Ad-LacZ showed only a few sulfated proteoglycans in the ECM of the cartilage region. There was more extensive staining for sulfated proteoglycans in AdhTGF-β1 groups than in Ad-LacZ groups (Bar = 100 µm)
Immunohistochemical staining showed a great deal of particles of type II collagen in the cartilaginous regions in the tissues made from cells transduced with AdhTGF-β1. Tissues made from cells transduced with Ad-LacZ showed few particles of type II collagen in cartilaginous regions, much less than in the tissues made from cells transduced with AdhTGF-β1 (Fig. 7).
Immunohistochemistry of tissues made from transduced cells at 3 weeks post-implantation. (a) AdhTGF-β1 (b) Ad-LacZ. Tissues made from cells transduced with AdTGF-β1 showed a great deal of brown particles of type II collagen in the cartilage region. Tissues made from cells transduced with Ad-LacZ showed few brown particles of type II collagen in chondrogenesis region. There was more extensive staining for type II collagen in AdhvTGF-β1 group than in the Ad-LacZ group (Bar = 100 µm)
GAG quantification of the tissues formed in vivo
The mean GAG contents of native articular cartilage, the tissues made from cells transduced with AdhTGF-β1, and the control tissues harvested at 3 weeks post-implantation were 11.4 ± 0.7, 7.2 ± 1.7, and 3.3 ± 0.2 mg/g, respectively, and these differences were statistically significant (P < 0.05). The GAG levels in the tissues in the experimental group reached 63% of the GAG amount found in native articular cartilage.
Discussion
Cartilage damage has become a major problem in orthopedics. The limited availability of autologous chondrocytes that can be harvested without a negative impact has led to a focus on the use of stem cells, which have already been seeded in scaffolds, implanted into damaged areas, and successfully grown into new healthy tissues. Numerous studies have demonstrated that BMSCs can serve as an alternate source of cells for cartilage tissue engineering because of their ability to differentiate into the chondrogenic phenotype and produce cartilage-specific extracellular matrix under appropriate conditions (Worster et al. 2000; Valcourt et al. 2002; Wang et al. 2003). Members of the TGF-β superfamily play an important role in the induction of chondrogenic differentiation of BMSCs. TGF-β1 stimulated upregulation of expression of type II collagen and proteoglycans, and exerts a strong induction of chondrogenesis (Hiraki et al. 1998; Johnstone et al. 1998). TGF-β1 also stabilizes the chondrogenic phenotype (Galera et al. 1992; Han et al. 2005). Recombinant exogenous inductive factor hTGF-β1 must be added in a relatively continuous manner. When induced BMSCs were implanted in the subcutaneous tissue, the chondrogenic ability of induced BMSCs was limited, possibly because of the pivotal role of hTGF-β1 in stabilizing the chondrogenic phenotype. When implanted in vivo, the mostly differentiated BMSCs de-differentiate and no longer secrete cartilage-specific matrix, most likely as a result of the removal of the high concentration of hTGF-β1 from the extracellular environment.
The present study was designed to assess the potential of adenoviral transduction of hTGF-β1 to induce chondrogenic differentiation of the BMSCs in vitro and enhance chondrogenesis in vivo. The results demonstrate that hTGF-β1 gene transduction causes BMSCs to express and secrete hTGF-β1 protein. Although the cells transduced with AdhTGF-β1 synthesized low levels of hTGF-β1 at day 21, the cells still differentiated into chondrocytes. These data suggested that sustained production of hTGF-β1, albeit at lower levels, was sufficient for the induction of the chondrogenic differentiation of BMSCs.
PGA is a resorbable polymer that has been well characterized in our previous studies and undergoes degradation mainly through hydrolysis (Xia et al. 2005; Liu et al. 2007). In this study, the fibrous PGA scaffolds were seeded with transduced cells at a high density. The resulting constructs were then implanted into the subcutaneous tissue of mice dorsa. After 3 weeks of implantation, the tissue made from cells transduced with AdhTGF-β1 was comprised of nests of cartilage separated by strips of fibrous tissues. The volume of neocartilage as a percent of the total tissue volume was greater than the tissue made from cells transduced with Ad-LacZ. Immunohistochemical staining showed extensive staining of type II collagen in the cartilaginous regions in the tissues made from cells transduced with AdhTGF-β1, much more so than that in the control Ad-LacZ-transduced group. These results show that gene transfer of the main inductive factor hTGF-β1 into BMSCs markedly improves the formation of neocartilage over control groups when implanted in vivo.
Proteoglycan is one of the primary components of the cartilage extracellular matrix, and plays a major role in providing the osmotic resistance necessary for cartilage to resist compressive loads. GAG chains are covalently attached to the protein cores of individual proteoglycan molecules (Xia et al. 2004). In this study, the mean GAG content for the tissue made from cells transduced with AdhTGF-β1 was significantly higher than that of the tissue made from cells transduced with Ad-LacZ; however, native cartilage contained significantly more GAG than both experimental and control groups. These results indicated that gene transfer of hTGF-β1 into BMSCs increased the production of cartilaginous matrices over control groups but not at concentrations similar to those of native cartilage. This is likely due to the early (3 week) timepoint used for final analyses in the present study, and is also possibly because of the complex milieu of growth factors and extracellular signaling molecules present in native tissue. Additionally, the engineered cartilage was grown heterotrophically, possibly depriving it of such molecules as found in the normal cartilaginous environment.
The chondrogenic differentiation of the BMSCs in vitro and the enhanced chondrogenesis in vivo suggested that cartilage tissue engineering based on genetically modified BMSCs might be more successful than other tissue engineering strategies, possibly due to the continued release of hTGF-β1. The process of chondrogenic differentiation of BMSCs is a complicated one with multiple influential factors, so further studies are required to elucidate the role of hTGF-β1 on in vivo chondrogenic differentiation. It was also unclear if gene transfer of hTGF-β1 could support chondrogenic differentiation of BMSCs to hypertrophic chondrocytes during the process of endochondral ossification. Nonetheless, this study could influence the design of a more suitable microenvironment to encourage chondrogenic differentiation of BMSCs.
References
Annes JP, Munger JS, Rifkin DB et al (2003) Making sense of latent hTGF-β activation. J Cell Sci 116:217–224
Chen J, Wang C, Lü S et al (2005) In vivo chondrogenesis of adult bone-marrow-derived autologous mesenchymal stem cells. Cell Tissue Res 319:429–438
Galera P, Vivien D, Pronost S et al (1992) TGF-β up-regulation of collagen type II in primary cultures of rabbit articular chondrocytes(RAC) involves increased mRNA level without affecting mRNA stability and procollagen processing. J Cell Physiol 3:596–606
Han F, Adams CS, Tao Z et al (2005) Transforming growth factor-beta1 (TGF-beta1) regulates ATDC5 chondrogenic differentiation and fibronectin isoform expression. J Cell Biochem 95:750–762
Hiraki Y, Inoue H, Hirai R et al (1998) Effects of transforming growth factor beta on cell proliferation and glycosaminoglycan synthesis by rabbit growth-plate chondrocytes in culture. Biochem Biophys Acta 969:91–99
Jenner JM, Eijk F, Saris DB et al (2007) Effect of transforming growth factor-beta and growth differentiation factor-5 on proliferation and matrix production by human bone marrow stromal cells cultured on braided poly lactic-co-glycolic acid scaffolds for ligament tissue engineering. Tissue Eng 13:1573–1582
Johnstone B, Herring TM, Caplan AI et al (1998) In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res 38:265–272
Liu X, Zhou G, Liu W et al (2007) In vitro formation of lacuna structure by human dermal fibroblasts co-cultured with porcine chondrocytes on a 3D biodegradable scaffold. Biotechnol Lett 29:1685–1690
Mackay AM, Beck SC, Murphy JM et al (1998) Chondrogenic differentiation of cultured human mesenchymal stem cells from marrow. Tissue Eng 4:415–428
Saraf A, Mikos AG (2006) Gene delivery strategies for cartilage tissue engineering. Adv Drug Deliv Rev 58:592–603
Uoth U, Tuli R, Osyczka AM et al (2002) In vitro engineered cartilage constructs produced by press-coating biodegradable polymer with human mesenchymal stem cells. Tissue Eng 1:131–143
Vacanti CA, Upton J (1994) Tissue-engineered morphogenesis of cartilage and bone by means of cell transplantation using synthetic biodegradable polymer matrices. Plast Reconstr Surg 21:445–454
Valcourt U, Gouttenoire J, Moustakas A et al (2002) Functions of transforming growth factor-beta family type I receptors and Smad proteins in the hypertrophic maturation and osteoblastic differentiation of chondrocytes. J Biol Chem 37:545–558
Wang WG, Lou SQ, Ju XD et al (2003) In vitro chondrogenesis of human bone marrow-derived mesenchymal progenitor cells in monolayer culture: activation by transfection with TGF-beta 2. Tissue Cell 35:69–77
Worster AA, Nixon AJ, Brower-Toland BD et al (2000) Effect of transforming growth factor betal onchondrogenic differentiation of culture equine mesenchymal stem cells. Am J Vet Res 9:1003–1010
Xia WY, Liu W, Cui L et al (2004) Tissue engineering of cartilage with the use of chitosan–gelatin complex scaffolds. J Biomed Mater Res 71B:373–380
Xia WY, Liu W, Cao YL et al (2005) In vitro engineering tubular cartilage using polyglycolic acid and bone marrow stroma cells. Chin J Microsurg 28:241–246
Xia WY, Ding WL, Liu W et al (2007) Bone marrow stromal cells transfected with adeno-hTGF-β1 and expression hTGF-β1. Shanghai Jiao Tong Univ (Medical Science) 27:1185–1188
Xu S, Xu Y (2008) Recent progress of BMSCs acting as seeding cell for tissue engineered cartilage. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 22:163–166
Acknowledgements
This study was supported by the Natural Basic Research Program of China and the “973” Program (2005CB52702).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xia, W., Jin, YQ., Kretlow, J.D. et al. Adenoviral transduction of hTGF-β1 enhances the chondrogenesis of bone marrow derived stromal cells. Biotechnol Lett 31, 639–646 (2009). https://doi.org/10.1007/s10529-009-9930-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-009-9930-7