Abstract
Growth factors have a pivotal role in chondrogenic differentiation of stem cells. The differential effects of known growth factors involved in the maintenance and homeostasis of cartilage tissue have been previously studied in vitro. However, there are few reported researches about the interactional effects of growth factors on chondrogenic differentiation of stem cells. The aim of this study is to examine the combined effects of four key growth factors on chondrogenic differentiation of mesenchymal stem cells (MSCs). Isolated and expanded rabbit bone marrow-derived MSCs underwent chondrogenic differentiation in a micromass cell culture system that used a combination of the following growth factors: transforming growth factor beta 1 (TGF-β1), bone morphogenetic protein 2 (BMP2), parathyroid hormone related protein (PTHrP), and fibroblast growth factor 2 (FGF2) according to a defined program. The chondrogenic differentiation program was analyzed by histochemistry methods, quantitative RT-PCR (qRT-PCR), and measurement of matrix deposition of sulfated glycosaminoglycan (sGAG) and collagen content at days 16, 23, and 30. The results showed that the short-term combination of TGF-β1 and BMP-2 increased sGAG and collagen content, Alkaline phosphates (ALP) activity, and type X collagen (COL X) expression. Application of either PTHrP or FGF2 simultaneously decreased TGF-β1/BMP-2 induced hypertrophy and chondrogenic markers (at least for FGF2). However, successive application of PTHrP and FGF2 dramatically maintained the synergistic effects of TGF-β1/BMP-2 on the chondrogenic differentiation potential of MSCs and decreased unwanted hypertrophic markers. This new method can be used effectively in chondrogenic differentiation programs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Articular cartilage is an avascular, aneural tissue comprised of only one cell type, which would appear to be a simple, unobtrusive task for tissue engineering programs. However, articular cartilage development is mediated by a tightly regulated symphony of growth factors whose interactions result in the establishment of a well-designed architecture.
There are numerous reports that discuss the in vivo and in vitro research on growth factors and their probable impact on chondrogenesis. Members of the transforming growth factor beta family (TGFβ) [1], bone morphogenetic proteins (BMPs) [2], insulin-like growth factors (IGFs) [3], fibroblast growth factors (FGFs) [4, 5], and different isoforms of parathyroid hormone related protein (PTHrP) [6] have been investigated for chondrogenic differentiation of mesenchymal stem cells (MSCs).
Studies of the combined effect of growth factors on regulation of chondrocyte proliferation and differentiation reported contradictory findings [7,8,9]. TGF-β1 pre-treated chondrocytes derived from different zones of the growth plate were exposed to parathyroid hormone (1–34) (PTH) [10]. The results showed that these TGF-β1 pre-treated chondrocytes responded to PTH and induced cell proliferation/differentiation and alkaline phosphatase (ALP) expression in a cell maturation-dependent manner. Further studies of the molecular mechanisms involved in cell proliferation demonstrated that TGF-β1 and PTHrP synergistically stimulated chondrocyte proliferation by using the same cis-acting regulatory element of the Cyclin D1 gene [11]. These findings indicated that a few genes acted as integrators of different growth factor related stimuli in chondrocytes.
Members of the TGFβ family are considered potent chondrogenic factors. TGFβ promoted chondrogenesis is usually accompanied by induction of a hypertrophic phenotype in MSCs [12]. The combination of TGFβs with BMP growth factors leads to enhanced chondrogenic differentiation [13] and amplified expression of hypertrophic markers such as ALP and COL X [14]. However, Shintani et al. have reported that the combination of TGF-β1 with BMP2 for chondrogenic differentiation of synovial explants had an enhanced effect on BMP2 induced chondrogenesis and conversely arrested the early stage of hypertrophy promoted by BMP2 [15].
Research on a mouse model of cartilage-specific loss of function of the TGFβ/BMP2 signaling pathway as well as a chondrogenic cell line indicated that TGFβ and BMP acted differently during the in vivo and in vitro experiments. There was an antagonistic effect on in vivo chondrogenesis; however, TGFβ activated BMP signaling during chondrogenesis of the cell line in vitro [16]. In support of these observations, short-term exposure to TGFβ reduced anti-chondrogenic effects of BMPs in a mesenchymal culture of a mouse early limb [17]. This study concluded that TGFβ inhibited the BMP mediated anti-chondrogenic action by modulating expression of the GATA genes, in particular GATA5. Recent studies that investigated the combined effect of TGFβ and BMP signaling on chondrogenesis proposed a central role for Msx2 and Dlx5 transcription factors in the regulation of BMP osteoinductive activity [14, 18, 19]. In line with these results, exogenous co-expression of SRY-box containing gene 9 (SOX9) and BMP2 in a cell line transduced with recombinant vectors showed that SOX9, a master transcription factor for chondrogenesis, inhibited BMP2 induced osteogenic markers and potentiated BMP2-induced chondrogenic differentiation [20].
Cross-talks between TGFβ and other signaling pathways may further complicate the role of growth factor signaling in chondrogenic differentiation program of MSCs. For example, TGF-β1/3 mediated chondrogenic differentiation of human MSCs was enhanced by inhibition of glycogen synthase kinase 3-beta, a member of the protein kinase family in the Wnt signaling pathway [21,22,23].
There are a number of different mechanisms that appear to be involved in the orchestration of the TGFβ/BMP2 signaling pathway in chondrogenesis. FGF2 is a potent mitogen for various cell types [24]. However, the chondrogenic potential of this growth factor on MSCs is controversial. MSCs cultured in expansion medium that contained FGF2 have shown increased chondrogenic gene expression [25] and enhanced osteogenic or chondrogenic potential, depending on the culture medium (osteogenic or chondrogenic-inducing) [26]. Simultaneous application of FGF2 and TGF-β1 in a micromass culture system for chondrocyte differentiation of either adipose or bone marrow derived MSCs has shown a significant increase in expressions of chondrogenic markers [27]. However, the adverse effect of FGF2 on the incremental effect of combined BMP-6 and TGFβ on chondrogenesis of MSCs has been reported [28].
Overall, these findings show that interactions of growth factors and their effects on chondrogenic differentiation is a rather different story from that of individual growth factors. This study aims to investigate the results of interaction between four key growth factors (TGF-β1, BMP2, FGF2, and PTHrP) involved in chondrogenic differentiation of MSCs and tries to describe the impact of them on expression of chondrogenic markers.
Materials and Methods
Mesenchymal Stem Cells (MSCs) Isolation and Expansion
MSCs were isolated from rabbit bone marrow according to principles (animal care, use, medication, anesthesia, operation and scarification) approved by the Royan Institute Ethics Committee (EC/93/1001) and a previously described method [29]. Briefly, aspirated and heparinized bone marrow was washed once with basal medium that consisted of 85% (v/v) DMEM (Sigma) and 15% FBS (Invitrogen), expanded in a T150 flask, and cultured in humidified incubators at 37 °C and 5% CO2 for 12 days. The culture media was changed every 3 days and unattached cells were discarded. The adherent cells were harvested using trypsin-EDTA digestion and further passaged one to two more times. The proliferated cells were then aliquoted and cryopreserved in 1 ml medium that contained 90% (v/v) FBS and 10% (v/v) DMSO by slow-cooling in a freezer at −80 °C for subsequent experiments.
Mesenchymal Stem Cells (MSCs) Characterization by Multi-Lineage Differentiation
The passage-3 expanded cells were trypsin-EDTA digested from the culture flask and collected after centrifugation. Next, the cells were resuspended in either 2 ml osteogenic inducing medium [basal DMEM supplemented with dexamethasone (10 nM), β-glycerol phosphate (10 mM), ascorbic acid (50 μg/ml), and 10% FBS] or adipogenic inducing medium [basal DMEM supplemented with insulin (10 μg/ml), dexamethasone (100 nM), indomethacin (50 μg/ml), ascorbic acid (50 μg/ml) and 10% FBS]. The resuspended cells were counted and cultured in 6-well plates at 2 × 104 cells/well. The culture plate was then incubated in humidified incubator at 37 °C and 5% CO2 for 14 days, and the culture medium was changed every 3 days.
The osteocyte differentiated cells were washed once with PBS, fixed with 100% methanol for 10 min, and covered with1% (w/v) alizarin red solution for 5 min. The adipocyte-committed cells were washed once with PBS, fixed with 4% paraformaldehyde for one hour and stained with 0.3% (w/v) oil red in isopropanol for 15 min.
Chondrogenic Differentiation
Chondrogenic differentiation of MSCs was carried out according to a previously reported procedure with minor modifications [30]. Briefly, rabbit bone marrow derived passage-3 MSCs were expanded and trypsinized, and then collected via centrifugation. The cells were resuspended in a chondrogenic inducing medium [basal DMEM supplemented with ascorbic acid (50 μg/ml), sodium pyruvate(100 μg/ml), L-proline (40 μg/ml), linoleic acid (5.33 μg/ml), dexamethasone (100 nM), insulin (6.25 μg/ml), transferrin (6.25 μg/ml), selenious acid (6.25 μg/ml), and BSA (1.25 μg/ml)] that contained 10 ng/ml TGF-β1 (R&D Systems, 240-B). The resuspended cells were counted and pelleted in a non-adherent 96-well V-shape bottom plate (Corning Life Sciences, 3956) at 2.5 × 105 cells/well by centrifugation at 1000g for 5 min. The plates were then incubated in a humidified CO2 incubator at 37 °C and the micromasses were covered with 200 μl chondrogenic medium, which was replaced every 2 days. The cells were differentiated into chondrocytes according to a well-defined chondrogenic program in which 200 ng/ml BMP2 (Sigma, H4791), 1 ng/ml PTHrP, and 100 ng/ml FGF2 were used in a time-dependent manner (Fig. 1). At the end of the differentiation program, the micromasses were collected and stored at −80 °C until extraction.
Chondrogenic differentiation program designed for investigation of combinatorial effect of growth factors on chondrogenesis of rabbit bone marrow derived MSCs. The arrangement and duration of micro-mass treatment with four growth factors [TGFβ1 (T), BMP2 (B), PTHrP (P) and FGF2 (F)] were tuned in a way that 12 experimental groups and three harvesting time points (Day 16, 23 and 30) were defined. TGFβ1 was used as a basic growth factor throughout the course while BMP2 was transiently applied from day 4 to day 16. PTHrP and FGF-2 were used after BMP2 treatment for either one or two weeks individually or together. Up: 96 well plate was used for chondrogenic differentiation in a micromass culture system
Multivalent Extraction of DNA, RNA, Sulfated Glycosaminoglycan (sGAG), and Collagen
We performed multivalent extraction of the main micromass biomolecules according to a previously reported method with some modifications [31]. Briefly, 20 mg of frozen samples were ground in liquid nitrogen using a mortar and pestle, followed by the addition of 1 ml of 4 M GuHCl buffer that contained 50 mM Tris-HCl (pH 7.5) and 1 mM EDTA. The samples were continually ground until the consistency of a fine homogenate powder. The mixture was continuously vortexed, incubated on ice for 30 min, and centrifuged at 15,000g for 10 min at 4 °C. The supernatant was collected and used for the DNA content assay. The pellet was further extracted by using 0.25 ml of GITC extraction buffer [4 M guanidine isothiocyanate, 50 mM Tris-HCl (pH 7.5), and 1 mM EDTA] and vortexed for 5 min at 4 °C. The resuspended mixture was then centrifuged at 21,000g for 10 min at 4 °C, and the pellet was stored at −80 °C until subsequent extraction of GAG and collagen according to a described papain digestion method [32]. The supernatant that contained RNA was further purified by successive precipitation in 0.5 ml isopropanol, followed by centrifugation at 12,000g for 10 min, resuspension in 1 ml 75% ethanol, and centrifugation of the RNA sample at 7500g for 10 min. The RNA was solubilized in DEPC-treated water and used for cDNA synthesis.
Measurement of DNA Content
We used the PicoGreen assay to quantify DNA content of the micromasses. Briefly, 10 μl of PicoGreen reagent (Quant-iT™ PicoGreen ® dsDNA Reagent and Kit, Invitrogen P7589) was diluted in 2 ml of TE buffer (10 mM Tris-HCl, 1 mM sodium EDTA, pH 8.0) and used to prepare the serial dilution of the λDNA standard (5 to 20 ng/ml) and the unknown samples. In standard PCR tubes, 5 μl of the unknown samples and a proper volume of the standards were brought to 20 μl with addition of TE buffer as well as 10 μl of diluted PicoGreen reagent. The DNA content of the samples and standards were read in duplicate using a Corbett RotorGene device at 480 nm excitation and 520 nm emission under the following PCR conditions: incubation at 50 °C for 2 min followed by 10 cycles at 50 °C for 5 s. The readings were blanked against the reading from the vials which contained no samples and used for normalization of sGAG and collagen content quantification.
Sulfated Glycosaminoglycan (sGAG) and Collagen Content Quantification
We performed colorimetric assessment to determine the amount of the sGAG using a Blyscan Sulfated Glycosaminoglycan Assay Kit (Biocolor B1000 Std.) according to the manufacturer’s instructions. Briefly, 0.25 ml of 1,9-dimethyl-methylene blue (DMMB) dye reagent was added to 80 μl of papain digested extracts and incubated for 30 min at room temperature. The unbonded dye was removed by centrifugation. The sGAG-bonded dye pellet was dissolved in 0.25 ml of dissociation reagent buffer. A serial dilution of chondroitin 4-sulfate (200 to 1500 ng) was prepared to generate a standard curve according to the same procedure used for sample preparation. The absorbance was read at 656 nm using a microplate reader (Multiskan spectrum, Thermo Scientific).
Collagen content of the micromasses was measured by a Hydroxyproline Assay Kit (Sigma, MAK008) following the manufacturer’s instructions. In brief, papain digested extracts were acid hydrolyzed in 12M HCl and the absorbance was determined by a spectrophotometer using a standard curve of hydroxyproline.
Histochemical and Immunohistochemical Analysis
Micromass pellets were incubated in Bouin’s solution for 6 h, then fixed overnight at 4 °C in PBS buffer that contained 2% paraformaldehyde. The pellets were dehydrated using a tissue processor device (Tissue processor DS 2080/H, Did Sabz Co.), embedded in paraffin, and cut into 5 μm sections for histological analysis.
Immunohistochemistry analysis was performed on the 5 μm sections of the micromasses after paraffin removal and rehydration. The sections were then incubated with 0.01 M sodium citrate buffer (pH 6.0) for 15 min at 100 °C for COL II detection or with 0.25% trypsin/1 mM EDTA for 5 min at 37 °C for COL X antigen retrieval. The primary antibody incubation was performed overnight at 4 °C using 1:100 anti-collagen type II antibody (Abcam, ab34712) and 1:300 anti-collagen type X antibody (Abcam, ab58632) as the primary antibodies. The secondary antibody was applied to the sections for 2 h at room temperature using 1:200 goat anti-rabbit IgG (Abcam, ab6721) and DAB substrate for detection of the reaction.
Histochemical analysis of alkaline phosphatase (ALP) activity was conducted using a qualitative method. Briefly, 10 μm sections of the fresh/frozen micromass were prepared using a microtome cryostat (Leica CM1850) and stained with a Leukocyte Alkaline Phosphatase Kit (Sigma, 86R) according to the manufacturer’s instructions.
Quantitative Analysis of Gene Expression Using Real-Time PCR
RNA of the micromasses extracted with GuHCl/GITC buffers was used for cDNA synthesis using First Strand cDNA synthesis Kit (Takara, 6110A), according to the manufacturer’s instructions. Expression levels of type II collagen (COL II), COL X, ALP, matrilin-1 (MATN1) and SRY-box containing gene 9 (Sox9) genes were measured by quantitative RT-PCR (qRT-PCR; Step one RT PCR Applied Biosystems, USA) using a SYBR® Green PCR Master Mix (Invitrogen). The real time-PCR conditions were as follows: incubation at 95 °C for 2 min, followed by 35 cycles at 95 °C for 15 s, and 60 °C for 60 s. All data were normalized to an endogenous control (GAPDH) and the untreated calibrator samples. The quantitative results were derived by the 2−∆∆Ct method. Table 1 lists the primer sequences.
Statistical Analysis
Statistical analyses were carried out using Prism 5 software. Each experiment was performed in triplicate and analyzed by one-way ANOVA followed by post hoc analyses. The results were expressed as mean ± SD. In all cases, a p-value of <0.05 was considered to be statistically significant.
Results
Evaluation of Bone Marrow-Derived Mesenchymal Stem Cells (MSCs)
The bone marrow-derived cells contained a large population of non-adherent cells that could be removed by media exchange and consecutive cellular passages. The remaining, attached fibroblastic-like cell colonies were subsequently passaged. By passage-3, they comprised a single phenotype population (Fig. 2a). These passage-3 cells were trypsinized and characterized for their multilineage differentiation potential as shown in Fig. 2b and c. We confirmed the MSCs phenotype by the adipogenic and osteogenic potency of the fibroblastic-like cells using Oil red and alizarin red staining of the differentiated cells. In order to further confirm pluripotency of the isolated cells, we used qRT-PCR to analyze the expression changes of the mRNA level during multilineage differentiation (Fig. 2). Relative expression of key genes involved in adipogenic differentiation of MSCs significantly increased by more than 10-fold and showed at least a one-fold increase for osteogenic differentiation.
Morphological appearance and multilineage differentiation of rabbit bone marrow-derived MSCs. Rabbit bone marrow-derived cells showed a homogenous morphology with a typical fibroblast-like cell shape at passage 3 and effectively attached to the culture plates (a). Adipogenic (b) and osteogenic-induced differentiation (c) exhibited strong metachromatic staining in bone marrow-derived cells stained with oil red and alizarin red and were further confirmed with qRT-PCR analysis of adipocyte/osteocyte markers respectively
Histological Analysis of Growth Factor-Promoted Chondrogenesis
In order to determine the net effects of growth factor on differentiation of micromasses we have used the same chondrogenic media that contained all of the necessary additives in all test groups. Members of TGFβ family, in particular TGF-β1 and TGF-β3, are considered to be basic growth factors in most chondrogenic programs. Therefore, we used TGF-β1 in all of the comparative groups and designed a defined program in which the three other growth factors were employed to study the combined effects on TGF-β1-mediated chondrogenesis of MSCs (Fig. 1). Groups G1, G2, and G3 only used TGF-β1 and were considered to be the control groups for the other groups on days 16, 23, and 30, respectively.
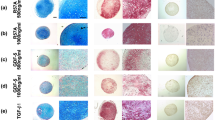
We observed no differences in metachromatic staining between groups, with the exception of G1, G10, and G11 (Fig. 3). It appeared that only the TGF-β1 growth factor was adequate for a proper metachromatic reaction at least 23 days after initiation of differentiation. However, we observed considerable differences between the groups in the number and shape of lacunas when other growth factors, in addition to TGF-β1, were used in the chondrogenic program.
Metachromasia reaction of sGAG in micromass sections stained with safranin O (top) and toluidine blue (bottom). Chondrogenesis of mesenchymal stem cells (MSCs) in the micromass culture were performed in the presence of transforming growth factor beta (TGF-β1), bone morphogenetic protein 2 (BMP2), fibroblast growth factor 2 (FGF2), and parathyroid hormone related protein (PTHrP) according to a well-defined program and histologically investigated on days 16 (a), 23 (b), and 30 (c). TGF-β1-treated micromasses resulted in the deposit of an appropriate metachromatic matrix at least on days 23 and 30. BMP2 or PTHrP combined with TGF-β1, in contrast to FGF2, improved the metachromasia reaction in chondrogenic differentiated micromasses
Quantification of Sulfated Glycosaminoglycan (sGAG) Content of the Micro-Masses
In addition to the metachromatic reaction, we used a quantitative method to measure the amount of sGAG deposited in the extracellular matrix (ECM) of the micromasses (Fig. 4a). DNA content of each micromass was also determined to avoid any unwanted errors from the cell counts and various experimental conditions. We normalized sGAG content to DNA content of the micromasses and used it to evaluate the chondrogenic differentiation process between the test groups.
Evaluation of chondrogenic differentiation of marrow derived mesenchymal stem cells (MSCs). sGAG and collagen content in micromasses that underwent growth factor-mediated chondrogenic differentiation (a). sGAG content normalized by the amount of DNA and differentially measured in the only treated TGF-β1 (T) groups (G1, G2, and G3), TGF-β1 and BMP2 (T/B) treated groups (G6, G5, and G4), TGF-β1; BMP2 and PTHrP (T/B/P) treated groups (G7 and G8), TGF-β1; BMP2 and FGF2 (T/B/F) treated groups (G10 and G11), TGF-β1; BMP2; PTHrP and FGF2 (T/B/P/F) treated groups (G9), or TGF-β1; BMP2; FGF2 and PTHrP (T/B/F/P) (G12) test groups at days 16, 23, and 30. Histological analysis of alkaline phosphatase (ALP) activity in micromass sections on days 23 (B1) and 30 (B2). ALP activity was depicted as a blue color in the border zone of the micromasses. The dark points represent lacunas in the micromasses. The higher activity of ALP enzyme was observed in the TGFβ1/BMP-2 treated group at day 30 (G4 group) while the least activity was found in groups treated with PTHrP or FGF2 (G8 and G11). The combination of PTHrP and FGF2 (G9 and G12 groups) moderately decreased ALP activity (especially in G12) compared to PTHrP or FGF2 alone. Immunohistochemical analysis of the combined effect of growth factor on deposition of type II collagen (COL II) and type X collagen (COL X) in chondrogenic MSCs (c). Application of BMP2 in combination with transforming growth factor beta (TGF-β1) resulted in improved expression of COL II as well as COL X (G4) which was modified by the use of either PTHrP or FGF2
No significant changes were found in sGAG deposition between all groups at days 16 and 23. However, the results showed a considerable increase with BMP2 and a significant decrease with FGF2 in sGAG content compared to the control TGF-β1 group (G3) at day 30 (p < 0.05). PTHrP in combination with TGF-β1 and BMP2 did not significantly change the sGAG content of the micromasses despite at least a 10% increase in sGAG compared to the control group at day 30. The order of application of PTHrP and FGF2 in the third or fourth week of differentiation in combination with TGF-β1 and BMP2 (G9 or G12) had a profound influence on sGAG deposition, so that GAG content significantly decreased in the G12 group compared to the G9 group (p < 0.05).
Measurement of Collagen Content in the Micromasses
Measurement of the collagen content provided useful information about the chondrogenesis condition of the micromasses. We normalized the collagen content of each of the micromasses to its DNA content, as μg of collagen to μg of ds DNA (Fig. 4a). There were no significant changes in the level of collagen content between the control groups and each of the other groups at days 16 and 30. Although not significant (p < 0.05), the combination of BMP2 with TGF-β1 resulted in at least a greater than 30% increase in collagen content in group G4 compared to the control (G3) group at day 30.
Addition of FGF2, like sGAG, resulted in a decrease of the combined positive effects of TGF-β1 and BMP2 on collagen content. The combination of PTHrP with the TGF-β1/BMP2 complex did not have any detectable impact on collagen deposition in comparison with TGF-β1 alone, although it decreased the synergistic effect of TGF-β1 and BMP2. Additionally, the use of PTHrP in the third week followed by addition of FGF2 in the fourth week (G9) was more effective for collagen deposition compared to G12, in which FGF2 was added prior to PTHrP in the third week.
Evaluation of Alkaline Phosphatase (ALP) Activity
In vitro chondrogenesis of MSCs usually induces expression of hypertrophic markers. ALP activity is an important marker of hypertrophic chondrocytes that can be traced by measurement of its gene expression or enzyme activity. In order to study ALP activity, we have employed a qualitative method to trace enzyme activity during chondrogenic differentiation of the micromasses. By this method, ALP activity formed a blue color due to enzymatic action on the proper substrate (Fig. 4b). We did not evaluate ALP activity in the day 16 groups (G1 and G6) due to technical limitation.
There was no ALP activity on days 23 and 30 in the groups (G2 and G3) that were only treated with TGF-β1. However, ALP had some activity in the TGF-β1/BMP2 treated group (G5), which drastically increased at day 30 (G4). Addition of PTHrP had minor effects on TGF-β1/BMP2-induced ALP activity at day 23 (G7) in contrast to addition of FGF2 (G10). However, PTHrP showed a greater effect on reduction of ALP activity at day 30 (G8).
Immunohistochemical Analysis of Growth Factors Mediated Chondrogenesis
Immunohistochemistry analysis was carried out with collagen type II (COL II) and collagen type X (COL X) antibodies to confirm the obtained results (Fig. 4c). COL II is the major component of cartilage collagen content and an important marker of chondrogenesis. COL X is mainly expressed in pre-hypertrophic and hypertrophic zones of the cartilage growth plate and is considered a hypertrophic marker.
We performed immunohistochemistry staining only on the day 30 groups because the collagen content did not significantly change between the comparative groups at days 16 and 23. BMP2 combined with TGF-β1 (G4) resulted in noticeable deposition of COL II, whereas PTHrP had a negative influence on this combination in the G8 group. However, the addition of FGF2 just after PTHrP (G9) modified the negative effect of PTHrP on the combined action of BMP2 and TGFβ1. FGF2 combined with TGF-β1/BMP2 (G11) or added just prior to PTHrP (G12) reduced COL II deposits compared to the other groups.
Quantitative Real-Time PCR (qPCR) Analysis of Growth Factor Mediated Chondrogenesis
Differential expressions of a number of important genes involved in chondrogenesis (COL2A1, COL10A1, ACAN, ALPL, MATN1, and SOX9) were analyzed by qPCR (Fig. 5).
Combined effect of transforming growth factor beta (TGF-β1), bone morphogenetic protein 2 (BMP2), fibroblast growth factor 2 (FGF2), and parathyroid hormone related protein (PTHrP) growth factors on relative gene expression of bone marrow derived mesenchymal stem cells (MSCs) that underwent chondrogenic differentiation. Expressions of typical chondrocyte markers were normalized to the expression of a housekeeping gene (GAPDH) and quantitatively compared to the reference sample using the CT method. Only treated TGF-β1 groups at days 16, 23, and 30 (G1, G2, and G3, respectively) were used as the reference samples (control) for gene expression analysis and the data were displayed as ln (2^(∆∆Ct)). Mean values for n = 3 samples together with the SEM. *: p < 0.05 vs. control; **: p < 0.01 vs. control; ***: p < 0.001 vs. control
The expression of SOX9, a master regulator of chondrogenesis, increased in a time-dependent manner from days 16 to 30. However, the addition of FGF2 significantly reduced the expression of SOX9 in the FGF2 groups (G11 and G12), but not group G9 in which FGF2 was added after PTHrP.
Aggrecan differentially expressed in the 12 test groups compared to their TGF-β1 control groups. Aggrecan greatly reduced when PTHrP or FGF2 were used in combination with TGF-β1/BMP2 for two weeks (G8 or G11). However, application of PTHrP for one week followed by addition of FGF2 increased ACAN expression at day 30 (G9).
The combination of BMP2 and TGF-β1 positively affected COL2A1 gene expression in the G5 and G4 groups compared to the related TGF-β1 treated control groups (G2 and G3). Addition of PTHrP to TGF-β1/BMP2 for one week improved the effect of their interaction (G7). Extending this period to two weeks significantly decreased the expression of COL II compared to the other test groups at day 30. FGF2 adversely affected the combined effect of TGF-β1/BMP2 on COL2A1 expression (G10 and G11). However, the addition of PTHrP in the fourth week to the combination of TGF-β1/BMP2/FGF2 had the same incremental effect as TGF-β1/BMP2 on COL2A1 expression.
Relative expression of ALP and COL X continuously increased with the addition of BMP2 at days 16, 23, and 30 (G6, G5, and G4) compared to the control groups. PTHrP combined with TGF-β1/BMP2 significantly decreased ALAP expression after two weeks (G8). However, FGF2 was more potent than PTHrP in inhibiting BMP2 induced ALP activity and expression of the COL10A1 gene so that it effectively reduced the expression of ALAP and Col10A1at day 23.
MATN1, the coding gene of a non-collagenous protein, expression gradually increased in the TGF-β1/BMP2 treated groups (G6, G5 and G4) compared with the TGF-β1 treated control groups (G1, G2, and G3). The results showed that FGF2 limited the increased effect of TGF-β1/BMP2 on expression of MATN1 (G10 and G11). In contrast, PTHrP slightly, but not significantly, increased TGF-β1/BMP2 promoted induction of MATN1 expression (at least for G8).
Discussion
In vitro chondrogenic differentiation is usually initiated by the condensation of MSCs and the use of a high-density micromass cell culture system. In addition to cell-cell interactions, several growth factors effectively impact the commitment and differentiation of MSCs to a chondrocyte lineage. Here, we have investigated the combined effects of four previously studied key growth factors [15, 17, 33, 34] on chondrogenic differentiation of rabbit bone marrow derived MSCs. We employed histological and histochemistry methods, and measured sGAG and collagen content to evaluate the impact of these growth factors and their interactions on chondrogenesis of MSCs. Relative gene expression and immunohistochemistry analysis of the chondrogenic markers were performed to verify our findings.
Measurement of sGAG showed a gradual increase in micromasses with the maximum deposition in the fourth week. BMP2 combined with TGF-β1 further accelerated sGAG synthesis at the fourth week, such that it reached more than 7 times at day 16 compared to an almost 4-fold increase in the TGF-β1 control group.
Collagen content increased with the use of BMP2, although it was not significant for G4 compared to G3. Previous studies reported a similar interaction of TGF-β1/BMP2 on the dramatic increase in deposition of sGAG and collagen content [14, 15]. In our study however, relative gene expressions of COL2A1, ACAN, and SOX9 decreased in the presence of BMP2, at least until the end of day 16.
BMP2 simultaneously has chondrogenic or osteogenic effects in osteochondral axis [20]. A previous study reported that TGF-β1 enhances BMP2 induced chondrogenic characteristics of explants [15]. Additionally it has been shown that overexpression of exogenous SOX9 potentiates BMP2-induced chondrogenic differentiation of MSCs in vitro [20]. Therefore, we concluded that short-term treatment of MSCs with TGF-β1 followed by BMP2 might increase chondrogenic markers due to the ability of TGF-β1 to trigger expression of the SOX9 transcription factor. However, our results showed that four days of pre-treatment with TGF-β1 followed by BMP2 plus TGF-β1 for 12 days caused an initial, sharp reduction in COL2A1, ACAN, and SOX9 expressions compared to the control groups which noticeably increased when post-treatment with TGF-β1 continued for one or two weeks after day 16.
Although the combination of BMP2 with TGF-β1 appears to be promising in terms of chondrogenesis, it simultaneously induces expression of undesirable hypertrophic markers [14]. Our results have shown that BMP2 considerably increased expressions of ALP and COL X, especially by day 30.
We included PTHrP in our research because multiple studies previously reported the effectiveness of PTHrP in chondrogenesis [10, 35] and inhibitory function on chondrocyte hypertrophy [33, 36]. In the current study, the combination of PTHrP with TGF-β1 was used over one (G7) or two weeks (G8) after the end of treatment with BMP2 to avoid any potentially adverse effects.
Analysis of the amount of sGAG and collagen revealed that the one-week application of PTHrP did not considerably affect micromasses pretreated with TGF-β1/BMP2. However, we observed a nonsignificant decrease with a two-week application of PTHrP. qRT-PCR analysis showed that SOX9 expression was not affected by PTHrP and followed the same trend as the TGF-β1/BMP2 treated group (G5 and G4). However, gene expression analysis of COL2A1, ACAN and MATN1 showed that PTHrP tended to decrease TGF-β1/BMP2 induced chondrogenic markers and promotion of maturation.
PTHrP and Indian hedgehog (Ihh) have been shown to form a negative feedback loop responsible for regulation of hypertrophy during osteochondral ossification [35, 36]. In this study the application of PTHrP might have caused a decrease in Ihh expression and related chondrogenic markers through the Ihh signaling pathway.
FGF2 is a member of the FGF family. Its role in chondrogenesis has been reported by several studies [25, 37]. We used FGF2 during the third or fourth week of the differentiation program and studied its combined effect on TGF-β1/BMP2 pre-treated micromasses. Our data showed that application of FGF2 severely reduced chondrogenic marker as well as decreased expression of COL X and ALP. Our findings contrasted those by Bosseti et al. [37] who investigated the combined effects of TGF-β2 and FGF2 on chondrogenesis of MSCs. However, Hildner et al. [28] reported a decreased effect of FGF2 on TGF-β3/BMP-6 promoted chondrogenesis, which was similar to the current study.
Our results indicated that FGF2 decreased the expression of ALP and COL X as well as chondrogenic markers that are usually increased in TGFβ/ BMP promoted in vitro chondrogenesis of MSCs.
In this study FGF2, unlike BMP2, had an antagonistic effect on GAG and collagen synthesis during chondrogenesis. FGF2 and BMP2 work in different ways and employ various signaling pathways. BMP2 uses the Smad pathway, whereas FGF2 exerts its function via the Erk signaling pathway. FGF2 activated Erk pathway inhibits phosphorylation of Smad1 [38] and Smad2 [26], which is used by the TGFβ signaling pathway and therefore inhibits development of TGF-β1/BMP2 mediated chondrogenic characteristics.
We also assessed the impact of the combined effect of PTHrP and FGF2 on differentiation potential of TGF-β1/BMP2. We defined two groups (G9 and G12), in which PTHrP and FGF2 were successively and separately applied. There were no differences between G9 and G12 with respect to metachromatic staining. However, they varied in micromass structure. G9, like G11, received FGF2 at the fourth week and it seemed that the observed similarity in lacuna shape and size was due to treatment with FGF2 during the last week. G9, unlike G11, received PTHrP for at least one week and it possibly resulted in loss of the inhibitory effect of FGF2 on TGF-β1/BMP2 promoted chondrogenesis. Deposition of GAG and collagen in G12, like G11, significantly decreased compared to G4 and G9. Therefore, application of PTHrP could not limit the decreasing action of FGF2 on ECM synthesis in the G12 group.
Expressions of SOX9, ACAN, COL2A1, and MATN1 were affected differently by the order of application of FGF2 and PTHrP in the G9 and G12 groups. The expression of SOX9 and ACAN in G9, where PTHrP was applied prior to FGF2 on TGF-β1/BMP2 pre-treated micromasses were considerably increased whereas, SOX9 and ACAN expressions were drastically decreased in G12 group in which application of FGF2 was prior to PTHrP. However, the expression of COL2A1 and MATN1 in G9 unlike G12 were noticeably decreased compared to G3 control group and were not in the same direction with SOX9 and ACAN. Another regulatory factor appears to be involved in the regulation of COL II expression in addition to SOX9. This finding was previously reported in a number of studies [39, 40].
MATN1 expression significantly decreased using FGF2 compared to the TGF-β1/BMP2 pre-treated group. Surprisingly, application of PTHrP rather than FGF2 caused an increase in the expression of MATN1 in the G8 group and could inhibit the negative impact of FGF2 on the expression of MATN1 in the G12 group. The incremental effect of PTHrP on the expression of cartilage matrix protein (CMP/MATN1), a marker of mature chondrocytes [41], demonstrated the impact of PTHrP on chondrocyte maturation. It possibly leads to chondrocytes pre-maturation by an inhibitory effect on the expression of Ihh in proliferating chondrocytes.
In conclusion, this study demonstrated that transient application of BMP2 in combination with TGF-β1 was more effective in inducing chondrogenic differentiation than TGF-β1 alone. However, increased expression of a hypertrophy phenotype was observed in differentiated MSCs using BMP2. Application of PTHrP or FGF2 on TGF-β1/BMP2 pre-treated micromasses showed the pros and cons of either expression of hypertrophic and chondrogenic markers. Thus, application of TGF-β1/BMP2 on micromasses followed by successive combinations of PTHrP/FGF2 (G9 group) showed an incremental effect, at least in part, on chondrogenic markers, yet effectively decreased expressions of ALP and COL X.
References
Chimal-Monroy, J., & de Leon, L. D. (2003). Differential effects of transforming growth factors beta 1, beta 2, beta 3 and beta 5 on chondrogenesis in mouse limb bud mesenchymal cells. International Journal of Developmental Biology, 41, 91–102.
Sekiya, I., Larson, B. L., Vuoristo, J. T., Reger, R. L., & Prockop, D. J. (2005). Comparison of effect of BMP2,-4, and-6 on in vitro cartilage formation of human adult stem cells from bone marrow stroma. Cell and Tissue Research, 320, 269–276.
Wang, L., & Detamore, M. S. (2009). Insulin like growth factor I improves chondrogenesis of predifferentiated human umbilical cord mesenchymal stromal cells. Journal of Orthopaedic Research, 27, 1109–1115.
Moore, E., Bendele, A., Thompson, D., et al. (2005). Fibroblast growth factor-18 stimulates chondrogenesis and cartilage repair in a rat model of injury-induced osteoarthritis. Osteoarthritis and Cartilage, 13, 623–631.
Handorf, A. M., & Li, W.-J. (2011). Fibroblast growth factor-2 primes human mesenchymal stem cells for enhanced chondrogenesis. PLoS One, 6, e22887.
Lee, J.-M., & Im, G.-I. (2012). PTHrP isoforms have differing effect on chondrogenic differentiation and hypertrophy of mesenchymal stem cells. Biochemical and Biophysical Research Communications, 421, 819–824.
Indrawattana, N., Chen, G., Tadokoro, M., et al. (2004). Growth factor combination for chondrogenic induction from human mesenchymal stem cell. Biochemical and Biophysical Research Communications, 320, 914–919.
Fukumoto, T., Sperling, J., Sanyal, A., et al. (2003). Combined effects of insulin-like growth factor-1 and transforming growth factor-β1 on periosteal mesenchymal cells during chondrogenesis in vitro. Osteoarthritis and Cartilage, 11, 55–64.
Kim, H.-J., & Im, G.-I. (2008). Combination of transforming growth factor-beta2 and bone morphogenetic protein 7 enhances chondrogenesis from adipose tissue-derived mesenchymal stem cells. Tissue Engineering Part A, 15, 1543–1551.
Nasatzky, E., Azran, E., Dean, D., Boyan, B. D., & Schwartz, Z. (2000). Parathyroid hormone and transforming growth factor-β1 coregulate chondrocyte differentiation in vitro. Endocrine, 13, 305–313.
Datta, N. S., Pettway, G. J., Chen, C., Koh, A. J., et al. (2007). Cyclin D1 as a target for the proliferative effects of PTH and PTHrP in early osteoblastic cells. Journal of Bone and Mineral Research: the Official Journal of the American Society for Bone and Mineral Research, 22, 951–964.
Mueller, M. B., Fischer, M., Zellner, J., et al. (2010). Hypertrophy in mesenchymal stem cell chondrogenesis: effect of TGF-β isoforms and chondrogenic conditioning. Cells, Tissues, Organs, 192, 158–166.
Toh, W. S., Liu, H., Heng, B. C., Rufaihah, A. J., Ye, C. P., & Cao, T. (2005). Combined effects of TGFβ1 and BMP2 in serum-free chondrogenic differentiation of mesenchymal stem cells induced hyaline-like cartilage formation. Growth Factors, 23, 313–321.
Mehlhorn, A., Niemeyer, P., Kaschte, K., et al. (2007). Differential effects of BMP2 and TGF-β1 on chondrogenic differentiation of adipose derived stem cells. Cell Proliferation, 40, 809–823.
Shintani, N., Siebenrock, K. A., & Hunziker, E. B. (2013). TGF- β1 enhances the BMP2-induced chondrogenesis of bovine synovial explants and arrests downstream differentiation at an early stage of hypertrophy. PLoS One, 8, e53086.
Bettina, K., Tao, Y., & Elda, M. (2011). Interraction of TGF-B and BMP signaling pathways during chondrogenesis. PLoS One, 6, 1–9.
Karamboulas, K., Dranse, H. J., & Underhill, T. M. (2010). Regulation of BMP-dependent chondrogenesis in early limb mesenchyme by TGFβ signals. Journal of Cell Science, 2010(123), 2068–2076.
Lee, M.-H., Kim, Y.-J., Kim, H.-J., et al. (2003). BMP2-induced Runx2 expression is mediated by Dlx5, and TGF-β1 opposes the BMP2-induced osteoblast differentiation by suppression of Dlx5 expression. Journal of Biological Chemistry, 278, 34387–34394.
Lee, R. H., Kim, B., Choi, I., et al. (2004). Characterization and expression analysis of mesenchymal stem cells from human bone marrow and adipose tissue. Cellular Physiology and Biochemistry, 14, 311–324.
Liao, J., Hu, N., Zhou, N., et al. (2013). Sox9 potentiates BMP2-induced chondrogenic differentiation and inhibits BMP2-induced osteogenic differentiation. PLoS One, 9, e89025.
Eslaminejad, M. B., Karimi, N., & Shahhoseini, M. (2013). Chondrogenic differentiation of human bone marrow-derived mesenchymal stem cells treated by GSK-3 inhibitors. Histochemistry and Cell Biology, 140, 623–633.
Eslaminejad, M. B., Karimi, N., & Shahhoseini, M. (2011). Enhancement of glycosaminoglycan-rich matrix production in human marrow-derived mesenchymal stem cell chondrogenic culture by lithium chloride and SB216763 treatment. Cell Journal (Yakhteh), 13, 117.
Eslaminejad, M. B., & Fallah, N. (2014). Small molecule-BIO accelerates and enhances marrow-derived mesenchymal stem cell in vitro chondrogenesis. Iranian Journal of Medical Sciences, 39, 107.
Asano, A., Kimura, K., & Saito, M. (1999). Cold-induced mRNA expression of angiogenic factors in rat brown adipose tissue. Journal of Veterinary Medical Science, 61, 403–409.
Kabiri, A., Esfandiari, E., Hashemibeni, B., Kazemi, M., Mardani, M., & Esmaeili, A. (2012). Effects of FGF-2 on human adipose tissue derived adult stem cells morphology and chondrogenesis enhancement in Transwell culture. Biochemical and Biophysical Research Communications, 424, 234–238.
Ito, T., Sawada, R., Fujiwara, Y., & Tsuchiya, T. (2008). FGF-2 increases osteogenic and chondrogenic differentiation potentials of human mesenchymal stem cells by inactivation of TGF-β signaling. Cytotechnology, 56, 1–7.
Solchaga, L. A., Penick, K., Porter, J. D., Goldberg, V. M., Caplan, A. I., & Welter, J. F. (2005). FGF-2 enhances the mitotic and chondrogenic potentials of human adult bone marrow derived mesenchymal stem cells. Journal of Cellular Physiology, 203, 398–409.
Hildner, F., Peterbauer, A., Wolbank, S., et al. (2010). FGF-2 abolishes the chondrogenic effect of combined BMP-6 and TGF-β in human adipose derived stem cells. Journal of Biomedical Materials Research Part A, 94, 978–987.
Johnstone, B., Hering, T. M., Caplan, A. I., Goldberg, V. M., & Yoo, J. U. (1998). In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Experimental Cell Research, 238, 265–272.
Solchaga, L. A., Penick, K. J., & Welter, J. F. (2011). Chondrogenic differentiation of bone marrow-derived mesenchymal stem cells: tips and tricks. Mesenchymal Stem Cell Assays and Applications, 698, 253–278.
Hoemann, C. D., Sun, J., Chrzanowski, V., & Buschmann, M. D. (2002). A multivalent assay to detect glycosaminoglycan, protein, collagen, RNA, and DNA content in milligram samples of cartilage or hydrogel-based repair cartilage. Analytical Biochemistry, 300, 1–10.
Hoemann, C. D. (2004). Molecular and biochemical assays of cartilage components. In F. De Ceuninck, M. Sabatini, P. Pastoureau (Eds.), Cartilage and Osteoarthritis. Methods in Molecular Medicine, vol 101. New Jersey: Humana Press.
Kim, Y. J., Kim, H. J., & Im, G. I. (2008). PTHrP promotes chondrogenesis and suppresses hypertrophy from both bone marrow-derived and adipose tissue-derived MSCs. Biochemical and Biophysical Research Communications, 373, 104–108.
Khan, I. M., Francis, L., Theobald, P. S., et al. (2013). In vitro growth factor-induced bio engineering of mature articular cartilage. Biomaterials, 34, 1478–1487.
Minina, E., Wenzel, H. M., Kreschel, C., et al. (2001). BMP and Ihh/PTHrP signaling interact to coordinate chondrocyte proliferation and differentiation. Development, 128, 4523–4534.
Minina, E., Kreschel, C., Naski, M. C., Ornitz, D. M., & Vortkamp, A. (2002). Interaction of FGF, Ihh/Pthlh, and BMP signaling integrates chondrocyte proliferation and hypertrophic differentiation. Developmental Cell, 3, 439–449.
Bosetti, M., Boccafoschi, F., Leigheb, M., Bianchi, A. E., & Cannas, M. (2012). Chondrogenic induction of human mesenchymal stem cells using combined growth factors for cartilage tissue engineering. Journal of Tissue Engineering and Regenerative Medicine, 6, 205–213.
Kretzschmar, M., Doody, J., & Massague, J. (1997). Opposing BMP and EGF signalling pathways converge on the TGF-beta family mediator Smad1. Nature, 389, 618.
Aigner, T., Gebhard, P. M., Schmid, E., Bau, B., Harley, V., & Pöschl, E. (2003). SOX9 expression does not correlate with type II collagen expression in adult articular chondrocytes. Matrix Biology, 22, 363–372.
Brew, C. J., Clegg, P. D., Boot-Handford, R. P., Andrew, J., & Hardingham, T. (2010). Gene expression in human chondrocytes in late osteoarthritis is changed in both fibrillated and intact cartilage without evidence of generalised chondrocyte hypertrophy. Annals of the Rheumatic Diseases, 69, 234–240.
Chen, Q., Johnson, D. M., Haudenschild, D. R., & Goetinck, P. F. (1995). Progression and recapitulation of the chondrocyte differentiation program: cartilage matrix protein is a marker for cartilage maturation. Developmental Biology, 172, 293–306.
Acknowledgments
The authors would like to thanks Dr. Mohammadreza Gharaati for his kindly technical advisement. This study was supported by grants from Semnan University of medical science (grant No. H-4155-2014) and Royan institute (grant No. 91000211). All the authors declare that there is no conflict of interest regarding the publication of this article.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Nasrabadi, D., Rezaeiani, S., Eslaminejad, M.B. et al. Improved Protocol for Chondrogenic Differentiation of Bone Marrow Derived Mesenchymal Stem Cells -Effect of PTHrP and FGF-2 on TGFβ1/BMP2-Induced Chondrocytes Hypertrophy. Stem Cell Rev and Rep 14, 755–766 (2018). https://doi.org/10.1007/s12015-018-9816-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-018-9816-y