Abstract
The recent development of various membrane-based techniques for the purification of valuable natural products is reviewed and covers the important research that has been conducted in the last 5 years on the utilization of microfiltration, ultrafiltration and nanofiltration techniques, either carried out on their own or coupled with other separation techniques, in order to achieve concentration and purification of natural products from their biological source. There is also a special focus on the research that has been undertaken to overcome the membrane fouling encountered in their usage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The term “natural products” usually refers to chemical substances found in nature that possess distinctive pharmacological or biological activities. They are either directly recovered from their natural sources or are chemically synthesized. In recent years, natural products have been the source for the discovery of new drugs (Newman et al. 2003; Newman and Cragg 2007). As many as a quarter of today’s anticancer drugs are natural products, with another quarter being synthetic derivatives of natural products (Davidson 1995). The main categories of natural products include carbohydrates, lipids, proteins and nucleic acids. Taking into consideration that not all natural products can be obtained economically via total chemical synthesis, many of them have to be separated and isolated from higher plants and microorganisms by often tedious and time-consuming processes. Methods to concentrate and purify natural products have become a subject of dramatically increased interest in the last several decades. Advances in the recognition of the value of their various biological properties, and the expansion of food and healthcare products markets have led to the development of state-of-art strategies for the separation and isolation of natural products from plants, microorganisms and marine sources. Generally, modern strategies for the purification of natural products have been based around chromatographic techniques (Andersen and Markham 2006). An earlier review of the application of chromatographic techniques for this purpose has been conducted by Marston and Hostettmann (1991). Recent development of traditional chromatographic separations, and applications for newly developed techniques such as superficial fluid extraction, pressurized liquid extraction, microwave-assisted extraction, vacuum liquid chromatography, preparative pressure liquid chromatography, have been reviewed by Sticher (2008).
Advances in material science and membrane manufacturing technology have led to membrane-based filtration techniques becoming a mainstream technology since the early 1990s. With a number of advantages such as high efficiency, simple equipment, convenient operation and low energy consumption, membrane technology has become one of the most important industrial separation techniques and has been applied extensively to various fields, e.g. concentration of juices (Cheryan 1998), water purification and desalination, dye and sugar separation, and the recovery of valuable products (Brüschke 1995).
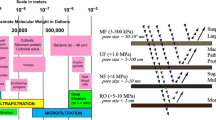
Though the term “membrane technology” refers to a number of separation processes with different characteristics, the general principle is based on the selective permeability of the membrane to allow the target substances to penetrate through the membrane, whilst unwanted substances are normally rejected by the membrane. Various methods, e.g. high pressures, concentration gradients and chemical or electrical potential differences, may be adopted as the driving force for membrane separations. Based on the size of the substances to be separated, and the resultant characteristics needed for the membrane, membrane techniques are generally classified into microfiltration, ultrafiltration, nanofiltration, reverse osmosis, dialysis, electrodialysis, pervaporation, gas permeation and membrane distillation. The latter six techniques can usually retain molecules with diameters less than 1 nm and therefore they are not suitable for natural products as these are generally larger molecules. For instance reverse osmosis is based on a solution diffusion mechanism and very high operating pressures are needed for the process. This technique is most appropriate for the molecules in the range of 0.1–1.0 nm and almost no utilization of reverse osmosis has been reported for natural products. Nanofiltration, ultrafiltration and microfiltration are based on size exclusion mechanisms and found to be suitable to separate various molecules and macromolecules with relative molecular masses ranging from several hundred to several hundred thousand, corresponding to a molecular size in the range from several nm to 1 μm. A key feature of a membrane separation process is the selection of a membrane with appropriate rejection characteristics. It is noticeable that polyvinylidene fluoride (PVDF) membranes have received by far the most attention for adoption in both food and medical fields, because of their chemical stability, durability and biocompatibility (Laroche et al. 1995).
This review is essentially a follow up to the previous reviews completed by Marston and Hostettmann (1991) and Sticher (2008) on strategies for the purification of natural products as a survey of the use of membrane techniques has mainly been neglected by previous authors. Applications of microfiltration, ultrafiltration and nanofiltration techniques in the purification of natural products are described. In addition, special emphasis has been given to the occurrence of membrane fouling, together with strategies that have been adopted to improve the deteriorating permeate flux.
Primary purification
Although purification by microfiltration and ultrafiltration used in the industrial production of fruit and vegetable juices has been extensively investigated in the past three decades (Vladisavljević et al. 2003), very few studies of the use of these techniques for the purification of natural products from plants and microorganisms have appeared in the literature. Membranes used as simple pretreatment procedures for natural products have been the most frequently reported applications. Cho et al. (2003) studied the clarification and concentration of water soluble pectin, a biopolymer with reported pharmacological properties such as cholesterol-decreasing (Kay and Truswell 1977), immuno-stimulating (Yamada et al. 1989) and anti-ulcer activities (Kiyohara et al. 1994). Pectin was isolated from an extract prepared from mature citrus peel using a 0.20 μm pore size cellulose membrane and a batch mode of operation. The use of a crossflow microfiltration step, with a 75% saving in the consumption of ethanol, resulted in the recovery yield of pectin being decreased from 10.5 to 9.9% whilst the galacturonic acid content in the pectin increased from 68 to 72.2%. Further purification was carried out by diafiltration in a fed-batch mode in order to remove the polyphenol impurities. This process was evaluated to be effective and efficient with potential for industrial application. Prodanova et al. (2008) employed ultrafiltration as a sample preparation procedure for polyphenols from almond skins before subsequent high-resolution analysis. A 30 kDa MWCO (molecular weight cut off) polyethersulfone (PES) ultrafiltration membrane has been used for the concentration and pre-purification of R-phycoerythrin from a crude extract of the macro-algae Grateloupia turuturu (Denis et al. 2009).
Membrane-based techniques have also increased in importance as techniques suited to the refining of oligosaccharides present in carbohydrates. Microfiltration and high MWCO ultrafiltration membranes have usually been used to obtain a mixture of oligosaccharides with low molecular weight impurities that have been separated from compounds of higher molecular weight (Playne and Crittenden 1996; Cheryan 1998). The use of low MWCO ultrafiltration and nanofiltration membranes provides an alternative to chromatography to concentrate and separate the target oligosaccharides from these impurities.
The earliest reports on the application of membranes for the recovery of oligosaccharides can be traced back to the 1990s (Mok et al. 1995; Matsubara et al. 1996; Urano et al. 1997; Sarney et al. 2000). More recently, Goulas et al. (2002) reported the nanofiltration on a commercial scale of a galacto–oligosaccharide mixture and investigated the significance of operating parameters, including pressure, feed concentration and filtration temperature, on the purification process. Subsequently, a study of nanofiltration for purifying fructo-oligosaccharides was reported by Li et al. (2004). Two operational modes, variable volume diafiltration and constant volume diafiltration, were applied successfully and 90% purity was achieved. In addition, the purification of xylo-oligosaccharides for use as low-calorie sweeteners and the removal of monosaccharides and other low molecular weight impurities from rice husk xylan have proved to be feasible using either a 4 kDa MWCO polymeric tubular ultrafiltration membrane or a 1 kDa MWCO ceramic monolithic nanofiltration membrane (Vegas et al. 2008). Nabarlatz et al. (2007) made successful use of commercial thin-film polymeric ultrafiltration membranes with different MWCOs to purify xylo-oligosaccharides from an autohydrolysis solution of almond shells. They were able to separate the xylo-oligosaccharides into fractions containing narrow and reproducible distributions of molar mass. Sjöman et al. (2008) reported the use of nanofiltration to recover xylose from various hemicellulose hydrolysates. The membranes used to recover this pentose sugar in their study had MWCOs between 150 and 300 Da. Hyaluronic acid is a natural glycosaminoglycan that can be used for various biomedical purposes such as treatment of arthritis, wound healing and tissue regeneration. Zhou et al. (2006) investigated the separation of hyaluronic acid from the fermentation broth of Streptococcus zooepidemicus by means of a two-stage tangential flow microfiltration and ultrafiltration process using pure water as diafiltrate. PVDF membranes with 0.45 and 0.20 μm pore size were used for microfiltration and PVDF300 (MWCO 300 kDa) and PVDF100 (MWCO 100 kDa) were used for subsequent ultrafiltration. The process was optimized to achieve an overall yield of hyaluronic acid of 77% with a purification factor of 1000. Examples of the use of membranes for primary purification purposes are summarized in Table 1.
Coupling membrane technologies with other techniques
The coupling of membrane techniques together in a series has been employed where a single membrane filtration step is not satisfactory. A classical example is an early application of a three-stage hybrid membrane separation process for the purification of active components from Echinacea plants (Johnson et al. 2002). The immunostimulatory activity of Echinacea is attributed to its high content of polysaccharides, caffeic acid derivatives and alkyl amides (Bauer and Wagner 1991). Conventional extraction of the active components from Echinacea, e.g. the use of ethanol–water extraction, usually consumes large quantities of solvents and energy. The first stage of the membrane process employed a 200 μm pore size polydimethylsiloxane (PDMS) hollow fibre membrane to remove the ethanol and some water from the crude extract in order to produce a high quality tincture by pervaporation. The second stage involved microfiltration to remove the alkyl amides, which may precipitate during tincture production, from the ethanol-free aqueous product using a 0.04 μm polysulfone (PSU) membrane. In the last stage, the microfiltration permeate was concentrated several-fold by osmotic distillation on a polypropylene (PP) membrane with a nominal pore size of 0.05 μm × 0.19 μm. This complex hybrid membrane separation process was adopted due to the fact that single osmotic distillation of the ethanol–water extract would result in severe wet through of the hydrophobic membrane. Finally, the microfiltration retentate containing the precipitated alkyl amides was added back to the osmotic distillation retentate to form a highly concentrated product that was suitable for use in capsule production. Similar work has been reported by Tessier et al. (2005) who coupled ultrafiltration (30 and 100 kDa MWCO) and nanofiltration (300 Da MWCO) techniques to purify benzylpenicillin from the fermentation broth of Penicillium chrysogenum with a recovery yield of nearly 90%. More recently, Isa et al. (2007) reported the purification of surfactin, a cyclic lipopeptide with antiviral, antibacterial and antitumor properties (Heerklotz and Seelig 2001), from the fermentation broth of Bacillus subtilis by a two-step ultrafiltration process.
There have been frequent reports of the use of membrane separations in conjunction with other purification techniques in order to isolate natural products. Ultrafiltration has been used to purify a plant peroxidase from the leaves of Ipomoea palmetto following an aqueous two-phase extraction step (Srinivas et al. 2002). Another case is the use of ultrafiltration as the first clarification step to eliminate large impurities in a lactic acid production process based on conventional and bipolar electrodialysis operations (Bouchoux et al. 2005).
Challenging the fouling of membranes
The greatest problem that has emerged in the use of membrane-based techniques is the decay of permeate flux caused by concentration polarization and fouling, which in turn increase the operating cost and shorten membrane life. During the membrane separation process, the rejected molecules arrive at the membrane surface by convective transport and thus a concentrated layer of the particles is built up at the membrane surface, forming a concentration gradient termed concentration polarization (Cheryan 1998). Fouling refers to the accumulation of unwanted material on the membrane surface leading to pore constriction and pore blockage that impedes the separation process. The decay of permeate flux caused by concentration polarization can be reversed by modifying the operating conditions, such as flow velocity, feed concentration and transmembrane pressure. However the decay of flux caused by fouling is irreversible and the membrane can usually only be cleaned using chemicals or by back-flushing with water or permeate. Fouling is an unfavourable but inevitable problem in membrane-based techniques and a key issue in the success of membrane separation is the control of fouling. Fouling of membranes is especially prevalent when they are employed in the isolation of natural products from plant sources due to the fact that more than 90% of the components present after crude extraction are non-pharmacological macromolecules with molecular weights larger than 50 kDa that can easily block the membrane pores.
Various approaches to the theoretical analysis and prediction of membrane fouling phenomena have been reported (Boerlage et al. 2002; Tung et al. 2001; Hwang et al. 2007; Kawakatsu et al. 1995; Vrouwenvelder et al. 2006). Much effort has been paid to the prevention of membrane fouling, e.g. dosing with oxidant or anti-fouling agents (Durham and Walton 1999; Sakol and Konieczny 2004). Some recent studies have developed strategies to recover the decreasing flux. Ceramic membranes have been extensively employed in the purification of active components from many Chinese herbs. Li et al. (2006) observed the effect of pH on the adsorption behaviour to zirconia particles of macromolecular impurities in an aqueous extract of Radix Rehmanniae. These authors have also studied the fouling phenomena that occur on the surface of a zirconia membrane when used in a microfiltration process with the aqueous extract. The work indicated that adsorption reached its maximum rate and extent when the pH was near the isoelectric point of the zirconia particles. This was in agreement with the pH value at which the minimum membrane flux and the highest rejection of total solids occurred with the zirconia membrane.
Modification of the properties of a membrane has also been investigated by introducing various functional groups into the existing membrane chemistry. A generally accepted hypothesis is that hydrophilic membranes are usually less susceptible to fouling than hydrophobic ones (Hester and Mayes 2002). Consequently the hydrophilicity of the membranes has been increased by introducing polymeric hydrophilic groups to the membrane. Xu et al. (2005) reported the modification of a PVDF ultrafiltration membrane with PVP (polyvinylpyrrolidone) for use in the purification of pharmacological flavonoids (Kubota et al. 2001) from extracts of Ginkgo biloba. The modified membrane contains a polyamide structure that may show a high and selective affinity for flavonoids due to hydrogen-bond effects between flavonoids and the PVP groups. With the modified PVDF-PFP membrane, the permeate flux was observed to increase linearly at low transmembrane pressures and gradually reached a steady value at high pressure; the flavonoid content in the product was increased from 21 to 35%.
For polyacrylonitrile (PAN) membranes, the simplest way to increase the hydrophilicity of the membrane is to hydrolyze the membrane by immersion into 2 M NaOH (Qiao et al. 2007). The hydrophilic PAN membranes so obtained were then used to purify danshensu and protocatechuic aldehyde from an aqueous extract from Salvia miltiorrhiza. The study suggested that increased permeate flux could be obtained by increasing the hydrophilicity of the membrane. In addition, permeates with different contents and ratios of the two desired components could be achieved by using PAN membranes with different hydrophilicities.
Another approach is the use of inorganic acids as hydrophilization agents. González Muñoz et al. (2006) immersed a nanofiltration membrane into a 1% w/v hydrofluoric acid (HF) solution for 14 days in order to improve the membrane performance. Although no investigation was carried out on the nature of the chemical modification that had occurred, improved permeate flux was demonstrated for the membrane after HF treatment. Although various synthetic polymer membranes have gained increased popularity due to the stability of their chemical structure and the availability of abundant functional groups, novel membranes containing regenerated cellulose composites may be an attractive alternative because of the occurrence of less fouling, improved mechanical strength and easy regeneration (van Reis and Zydney 1999).
A method used by You et al. (2007) to study the fouling of membranes by wastewater influent may be illuminative for similar fouling problems when membranes are used to purify natural products. They investigated the use of ozone to oxidize the fouling matter on PVDF ultrafiltration membranes. Ozonation was performed by direct continuous dosing of ozone into the wastewater influent at 8.8 mg/min and the residual concentration of ozone in the influent flow was maintained at 4 mg/l throughout the test. After 1 h, the permeate flux dropped by 40% for a membrane not subjected to ozonation, whilst the flux with the treated membrane fell by only 10%. On the basis of infra-red and energy dispersive X-ray spectroscopy analyses, the authors concluded that the ozone treatment destroyed aromatic rings within the PVDF membrane to “produce functional oxygenated groups (aldehydic, ketonic, and especially carboxylic acid), which may be coupling with calcium ions to form soluble calcium complexes that reduce the formation of calcium carbonates”.
In addition to the efforts devoted to modifying the structure and chemistry of membranes, considerable work has been focused on developing new membrane systems with reduced fouling characteristics and enhanced permeate flux. Earlier work towards this objective includes the adoption of conical-shaped rotors (Vogel and Kroner 1999), cylindrical Taylor vortex systems (Parnham and Davis 1995), rotating disk filters (Lee et al. 1995) and helical coiled Dean vortex devices (Luque et al. 1999; Gehlert et al. 1998). Recently, Auddy et al. (2004) developed a stainless steel rectangular cross-flow cell that worked as a turbulence promoter to enhance the permeate flux in a nanofiltration system. Significant transmembrane flux enhancement in the range of 40–100% was achieved and was even more pronounced at higher flow velocity. The researchers concluded that turbulence was promoted by the wires placed in the cross-flow cell and helped to reduce concentration polarization and resulted in less solute deposition on the membrane surface. Although the work was carried out in a nanofiltration process for dye solutions, the feasibility of flux enhancement in may result in the design of novel devices that work in similar ways to challenge the fouling that occurs during membrane purification processes for natural products.
Conclusions and outlook
This mini-review covers the most recent developments in the purification of natural products in which one or more membrane-based techniques are utilised as either a primary treatment or as a complete recovery process. From the work carried out so far, the general observation is that single membrane techniques, such as microfiltration, ultrafiltration and nanofiltration, have been able to perform a primary purification of natural products from plant and microorganism sources due to their inherent advantages, e.g. high efficiency, simple equipment, convenient operation and low energy consumption. In addition, these techniques can be used in conjunction with other separation processes, to achieve various demands for higher degrees of purification of natural products. Much effort has been devoted to improve the selectivity of membrane separations while maintaining their inherent high-throughput characteristics (van Reis and Zydney 2001).
The outlook for membrane-based techniques in this field will be the development of better membrane filtration system with high selectivity for target substances and better anti-fouling properties. An important trend is the improvement of the membrane structure and the emergence of functional membranes, which with appropriate function groups grafted onto their surface, are highly selective for target substances, and in addition are able to resist fouling more effectively. In conclusion, the abundant qualities of membrane-based techniques will ensure that they will remain productive tools in the purification of natural products.
References
Andersen ØM, Markham KR (2006) Flavonoids—chemistry, biochemistry and applications. CRC Press, Taylor & Francis Group
Auddy K, De S, DasGupta S (2004) Flux enhancement in nanofiltration of dye solution using turbulent promoters. Sep Purif Technol 40:31–39
Bauer R, Wagner H (1991) Echinacea species as potential immunostimulatory drugs. Econ Med Plant Res 5:253–321
Boerlage SFE, Kennedy MD, Dickson MR, Schippers JC (2002) The modified fouling index using ultrafiltration membranes (MFI-UF): characterization, filtration mechanisms and membrane selection. J Membr Sci 197:1–21
Bouchoux A, Balmann HR, Lutin F (2005) Nanofiltration of glucose and sodium lactate solutions variations of retention between single- and mixed-solute solutions. J Membr Sci 258:123–132
Brüschke H (1995) Industrial application of membrane separation processes. Pure Appl Chem 67(6):993–1002
Cheryan M (1998) Ultrafiltration and Microfiltration Handbook. Technomic Publishing Company, Lancaster
Cho CW, Lee DY, Kim CW (2003) Concentration and purification of soluble pectin from mandarin peels using crossflow microfiltration system. Carbohydr Polym 54:21–26
Davidson BS (1995) New dimensions in natural products research: culture marine microorganisms. Curr Opin Biotechnol 6(3):284–291
Denis C, Massé A, Fleurence J, Jaouen P (2009) Concentration and pre-purification with ultrafiltration of a R-phycoerythrin solution extracted from macro-algae Grateloupia turuturu: process definition and up-scaling. Sep Purif Technol 69:37–42
Durham B, Walton A (1999) Membrane pretreatment of reverse osmosis: long-term experience on difficult waters. Desalination 122:157–170
Gehlert G, Luque S, Belfort G (1998) Comparison of ultra- and microfiltration in the presence and absence of secondary flow with polysaccharides, proteins, and yeast suspensions. Biotechnol Prog 14:931–942
González Muñoz MP, Navarro R, Saucedo I, Avila M, Prádanos P, Palacio L, Martínez F, Martín A, Hernández A (2006) Hydrofluoric treatment for improved performance of a nanofiltration membrane. Desalination 191:273–278
Goulas AK, Kapasakalidis PG, Sinclair HR, Rastall RA, Grandison AS (2002) Purification of oligosaccharides by nanofiltration. J Membr Sci 209:321–335
Heerklotz H, Seelig J (2001) Detergent-like action of the antibiotic peptide surfactin on lipid membranes. Biophys J 81:1547–1554
Hester JF, Mayes AM (2002) Design and performance of foul-resistant poly(vinylidene fluoride) membrane prepared in a single-step by surface segregation. J Membr Sci 202:119–135
Hwang KJ, Liao CY, Tung KL (2007) Analysis of particle fouling during microfiltration by use of blocking models. J Membr Sci 287(2):287–293
Isa MHM, Coraglia DE, Frazier RA, Jauregi P (2007) Recovery and purification of surfactin from fermentation broth by a two-step ultrafiltration process. J Membr Sci 296:51–57
Johnson RA, Sun JC, Sun J (2002) A pervaporation–microfiltration–osmotic distillation hybrid process for the concentration of ethanol–water extracts of the Echinacea plant. J Membr Sci 209:221–232
Kawakatsu T, Nakajima M, Nakao S, Kimura S (1995) Three-dimensional simulation of random packing and pore blocking phenomena during microfiltration. Desalination 101:203–209
Kay RM, Truswell AS (1977) Effects of citrus pectin on blood lipids and fecal steroid excretion in man. Am J Clin Nutr 30(2):171–175
Kiyohara H, Hirano M, Wen XG, Matsumoto T, Sun XB, Yamada H (1994) Characterization of an anti-ulcer pectic polysaccharide from leaves of Panax ginseng C.A. Meyer. Carbohydr Res 263:89–101
Kubota Y, Naoko T, Keizo U, Hiroyuki T et al (2001) Ginkgo biloba extract-induced relaxation of rat aorta is associated with increase in endothelial intracellular calcium level. Life Sci 69:2327–2336
Laroche G, Marois Y, Guidoin R, King MW, Martin L, How T, Douville Y (1995) Polyvinylidene fluoride (PVDF) as a biomaterial: from polymeric raw material to monofilament vascular suture. J Biomed Mater Res 29:1525–1536
Lee SS, Burt A, Russotti G, Buckland B (1995) Microfiltration of recombinant yeast cells using a rotating disk dynamic filtration system. Biotechnol Bioeng 48:386–400
Li W, Li J, Chen T, Chen C (2004) Study on nanofiltration for purifying fructo-oligosaccharides I. Operation modes. J Membr Sci 245:123–129
Li W, Xing W, Jin W, Xu N (2006) Effect of pH on microfiltration of Chinese herb aqueous extract by zirconia membrane. Sep Purif Technol 50:92–96
Luque S, Mallubhotla H, Gehlert G, Kuriyel R, Dzengeleski S, Pearl S, Belfort G (1999) A new coiled hollow-fiber module design for enhanced microfiltration performance in biotechnology. Biotechnol Bioeng 63:247–257
Marston A, Hostettmann K (1991) Modern separation methods. Nat Prod Rep 8:391–413
Matsubara Y, Iwasaki K, Nakajima M, Nabetani H, Nakao S (1996) Recovery of oligosaccharides from streamed soybean waste water in tofu processing by reverse osmosis and nanofiltration membranes. Biosci Biotechnol Biochem 60:420–428
Mok CK, Ku KH, Park DJ, Kim NS, Sohn HS (1995) Ultrafiltration of soybean cooking water for the production of soyoligosaccharides. Kor J Food Sci Technol 27:181–184
Nabarlatz D, Torras C, Garcia-Valls R, Montané D (2007) Purification of xylo-oligosaccharides from almond shells by ultrafiltration. Sep Purif Technol 53:235–243
Newman DJ, Cragg GM (2007) Natural products as sources of new drugs over the last 25 years. J Nat Prod 70:46–477
Newman DJ, Cragg GM, Snader K (2003) Natural products as sources of new drugs over the period 1981–2002. J Nat Prod 66:1022–1037
Parnham CS, Davis RH (1995) Protein recovery from cell debris using rotary and tangential cross-flow microfiltration. Biotechnol Bioeng 47:155–164
Playne MJ, Crittenden R (1996) Commercially available oligosaccharide. Bull Int Dairy Fed 313:10–22
Prodanova M, Garridob I, Vacasa V, Lebrón-Aguilarc R, Dueñasd M, Gómez-Cordovésb C, Bartolomé B (2008) Ultrafiltration as alternative purification procedure for the characterization of low and high molecular-mass phenolics from almond skins. Anal Chim Acta 609:241–251
Qiao X, Zhang Z, Ping Z (2007) Hydrophilic modification of ultrafiltration membranes and their application in Salvia Miltiorrhiza decoction. Sep Purif Technol 56:265–269
Sakol D, Konieczny K (2004) Application of coagulation and conventional filtration in raw water pretreatment before microfiltration membranes. Desalination 162:61–73
Sarney DB, Hale C, Frankel G, Vulfson EN (2000) A novel approach to the recovery of biologically active oligosaccharides from milk using a combination of enzymatic treatment and nanofiltration. Biotechnol Bioeng 69:461–467
Sjöman E, Mänttäri M, Nyström M, Koivikko H, Heikkilä H (2008) Xylose recovery by nanofiltration from different hemicellulose hydrolyzate feeds. J Membr Sci 310:268–277
Srinivas ND, Barhate RS, Raghavarao KSMS (2002) Aqueous two-phase extraction in combination with ultrafiltration for downstream processing of Ipomoea peroxidise. J Food Eng 54:1–6
Sticher O (2008) Natural product isolation. Nat Prod Rep 25:517–554
Tessier L, Bouchard P, Rahni M (2005) Separation and purification of benzylpenicillin produced by fermentation using coupling ultrafiltration and nanofiltration technologies. J Biotechnol 116:79–89
Tung KL, Wang S, Lu WM, Pan CH (2001) In situ measurement of cake thickness distribution by a photointerrupt sensor. J Membr Sci 190(1):57–67
Urano H, Kawakatsu T, Nabetani H, Nakajima M (1997) Separation properties for oligosaccharides of nanofiltration membranes and its application to a purification process of Jerusalem artichoke oligosaccharides. J Food Process Preserv 44:457–462
van Reis R, Zydney AL (1999) Protein ultrafiltration. In: Flickinger MC, Drew SW (eds) Encyclopedia of bioprocess technology: fermentation, biocatalysis, bioseparation. Wiley, New York, pp 2197–2214
van Reis R, Zydney AL (2001) Membrane separations in biotechnology. Curr Opin Biotechnol 12:208–211
Vegas R, Moure A, Domínguez H, Parajó JC, Alvarez JR, Luque S (2008) Evaluation of ultra- and nanofiltration for refining soluble products from rice husk xylan. Bioresour Technol 99:5341–5351
Vladisavljević GT, Vukosavljević P, Bukvić B (2003) Permeate flux and fouling resistance in ultrafiltration of depectinized apple juice using ceramic membranes. J Food Eng 60(3):241–247
Vogel JH, Kroner KH (1999) Controlled shear filtration: a novel technique for animal cell separation. Biotechnol Bioeng 63:663–674
Vrouwenvelder JS, van Paassen JAM, Wessels LP, van Dama AF, Bakker SM (2006) The membrane fouling simulator: a practical tool for fouling prediction and control. J Membr Sci 281:316–324
Xu Z, Li L, Wu F, Tan S, Zhang Z (2005) The application of the modified PVDF ultrafiltration membranes in further purification of Ginkgo biloba extraction. J Membr Sci 255:125–131
Yamada H, Ra KS, Kiyohara H, Cyong JC, Otsuka Y (1989) Structural characterization of an anti-complementary pectic polysaccharide from the roots of Bupleurum falcatum L. Carbohydr Res 189:209–226
You SH, Tseng DH, Hsu WC (2007) Effect and mechanism of ultrafiltration membrane fouling removal by ozonation. Desalination 202:224–230
Zhou H, Ni J, Huang W, Zhang J (2006) Separation of hyaluronic acid from fermentation broth by tangential flow microfiltration and ultrafiltration. Sep Purif Technol 52:29–38
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, J., Chase, H.A. Applications of membrane techniques for purification of natural products. Biotechnol Lett 32, 601–608 (2010). https://doi.org/10.1007/s10529-009-0199-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-009-0199-7