Abstract
Excretion of 1,3-propanediol (1,3-PD) by K. pneumoniae was compared in ammonium- and phosphate-limited chemostat cultures running with an excess of glycerol. 59 and 43% catabolic flux were directed to 1,3-PD in ammonia-limited cultures and phosphate-limited cultures at dilution rate of 0.1 h−1, respectively. Ammonia-limited fed-batch cultures produced 61 g 1,3-PD l−1 and a total of 15 g l−1 organic acid in 36 h. However, phosphate-limited fed-batch cultures excreted 61 g lactate l−1 and 44 g 1,3-PD l−1.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In nature, microorganisms are often faced with a limited availability of nutrients. When the carbon source is available in a growth rate-limiting amount under aerobic conditions, it is completely oxidized to CO2. However, this is often not the case under conditions of excess carbon. Specific partially-oxidized, end-products are excreted via so-called overflow metabolism. The maintenance rates of O2 consumption in Klebsiella aerogenes (later identified as K. pneumoniae) may increase in chemostat cultures to which a sudden excess of the growth-limiting nutrient is added (Neijssel and Tempest 1976). Consequently, the maintenance of growth potential (energy spilled under conditions of carbon-substrate excess) was proposed, which was further elucidated in a kinetic model of substrate and ATP consumption rates of microbial growth under substrate sufficient conditions (Zeng and Deckwer 1995). A similar growth-yield model was also developed with a defined energy-uncoupling coefficient (Liu 1998). The coefficient could be used for quantitative interpretation of the observed growth yield in relation to the residual substrate concentration.
1,3-PD is excreted in a growth-associated manner at glycerol surplus by K. pneumoniae relying on the dha regulon (Zeng and Deckwer 1995). The other regulon glp is also involved during the glycerol dissimilation. In addition to respiratory control, these two regulons are modulated by the glycerol concentration in the growth medium (Forage and Lin 1982). While the dha is induced under anaerobic conditions, irrespective of the glycerol concentration, this regulon is induced under aerobic conditions only when glycerol is abundant. The aerobic fermentations using K. pneumoniae for 1,3-PD production has been scaled up to 50 m3 in our laboratory recently (Zheng et al. 2009). In this work, 1,3-PD excretion under ammonium or phosphate limitation was investigated in an aerobic continuous culture. We suggest a general strategy to increase the excretion of this primary metabolite.
Materials and methods
Bacterium, media and growth
Klebsiella pneumoniae CGMCC 1.6366 was stored at the China General Microbiological Culture Collection Center (CGMCC, Beijing, China). The basic culture medium contained glycerol (7–40 g), K2HPO4 (0.69 g), KH2PO4 (0.25 g), (NH4)2Cl (4 g), MgSO4·7H2O (0.2 g), yeast extract (1.5 g), and 1 ml trace element solution in 1 l H2O. The trace element solution was prepared as described previously (Zheng et al. 2009). It was grown in 5 l B. Braun Biostat B Plus (B. Braun, Germany) with a working volume of 2 l at 37°C, 320 rpm and 0.65 vvm air flow for chemostat culture. The dilution rate was set at 0.1 h−1. The pH was kept constant at 6.5 by the automatic addition of 10 M NaOH. The oxidoreduction potential (ORP) was measured with a redox meter (6308OT, RKKT, Shanghai, China) using a redox-combined electrode. The continuous culture reached steady state after 4–6 residence times. The fed-batch cultures were conducted in 5 l fermenter with a working volume of 4 l. The addition of pure glycerol into the fed-batch cultures was started when the glycerol in the broth was below 5 g l−1. The glycerol level in subsequent cultures was controlled at approximately 5–10 g l−1.
Substrate and products analysis
Dry cell mass was computed from the OD650 value. Glycerol, 1,3-PD, 2,3-butanediol (2,3-BD), lactate, succinate, acetate and ethanol were determined by HPLC system (Zheng et al. 2008a). 3-Hydroxypropionaldehyde (3-HPA) was determined using a colorimetric method (Circle et al. 1945) CO2 and O2 concentrations in the off-gas were determined using a GXH-510 infrared gas analyzer (Xibi, Beijing, China). The specific production rate of extracellular metabolite and the uptake rate of glycerol were calculated according to Weber et al. (2005).
Measurement of enzyme activities
Activities of glycerol dehydratase (GDHt), 1,3-propanediol oxidoreductase (PDOR), and glycerol dehydrogenase (GDH), were measured as previously described (Zheng et al. 2008b). One unit of enzyme is defined as the amount of enzyme required to produce 1 μmol product per min.
Results and discussion
Chemostat cultures on glycerol
To determine the influence of glycerol on the production of 1,3-propanediol, K. pneumoniae was grown in continuous cultures at various glycerol concentrations (Table 1). Glycerol was fed into the chemostat at below 30 g l−1, and ORP, residual glycerol concentration, 1,3-PD and the rate of synthesis of 2,3-BD increased almost in line with the addition of glycerol. The production of 1,3-PD displayed an optimum curve that peaked at 30 g glycerol l−1. The formation of lactate and ethanol was detected at above 15 g glycerol l−1. At higher glycerol concentrations (greater than 30 g l−1), the culture was increasingly inhibited in the presence of 3-HPA. ORP has been used as a parameter to control, optimize, scale-up fermentations and screen competent strains for 1,3-PD production (Du et al. 2007). In our studies, ORP was used to monitor the status of 1,3-PD fermentation. The sustained glycerol batch fermentations showed low ORP when the fermentation entered the exponential growth phase then leveled off. When the concentration of 3-HPA accumulated to over 10 mM, glycerol uptake and cell growth ceased, while ORP and the pH value of the fermentation broth increased (Zheng et al. 2008a).
The abnormal cessation of continuous growth at 35 and 40 g glycerol l−1 indicates that there is a threshold concentration of glycerol for sustained growth at 0.1 h−1, which is different from that of previous studies with Clostridium butyricum (Gonzalez-Pajuelo et al. 2005; Papanikolaou et al. 2000). Therefore, this high glycerol induced wash-out phenomenon should be prevented when the excess substrate and consumption rate model is employed to optimize 1,3-PD synthesis by K. pneumoniae.
Chemostat cultures under ammonia and phosphate limitation
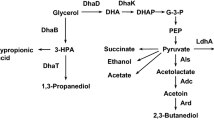
The distributions of metabolic fluxes at the central carbon pathway in this bacterium under ammonia and phosphate limitation are shown in Fig. 1. 1,3-PD was the major fermentation end-product, attaining 12.9 and 7.8 mM g−1 h−1 under ammonia and phosphate limitation, respectively. Acetate and ethanol were the main byproducts. In ammonia-limited cultures, 59% of the catabolic flux was directed to 1,3-PD, while phosphate-limited cultures showed a fermentation pattern where the 1,3-PD flux only accounted for 43% of glycerol dissimilation. The activities of GDHt, GDH, and PDOR were higher in ammonia-limited cultures, especially the activity of PDOR which was over three-fold higher than that in phosphate-limited cultures (Table 2). In addition, the activities of these enzymes indicated that the dha system expressed GDH and PDOR under aerobic conditions in glycerol surplus, confirming the proposed glycerol concentration modulation mechanism (Forage and Lin 1982). The ratio of specific acetate formation rate to specific ethanol formation rate (q acetate/q ethanol) was much higher in ammonia-limited cultures (3.55) than in phosphate-limited cultures (0.52). Unlike phosphate limitation, cell growth continues slowly, and the growth of nitrogen-starved cells was inhibited to some extent (Table 3). There were no obvious differences in biomass yield (Y glycerol) and biomass yield at 0.5 mol oxygen consumption (Y oxygen) in both conditions. However, O2 consumption per biomass per hour (\( Q_{{{\text{O}}_{2} }} \)) of 161 vs. 136 indicated that cells consumed more O2 in phosphate-limited cultures, which was consistent with the ORP value in the culture broths. 1,3-PD synthesis plays a key role in the consumption of NADH generated in the oxidation pathway to regulate the intracellular reducing equivalent balance of K. pneumoniae (Zeng et al. 1993). The metabolic flux is shifted to 1,3-PD formation under the more reduced status to maintain the internal redox state (Zheng et al. 2008b). Taken together, these findings suggest that the amount of synthesized 1,3-PD is variable, and is dependent on the nature of the limitation. The conversion of glycerol to 1,3-PD by K. pneumoniae is higher under ammonia limitation. There is a clear difference between the findings in this study and those in Klebsiella aerogenes NCTC 418, where 1,3-PD formation was maximal under phosphate limitation (Streekstra et al. 1987).
Distribution of metabolic fluxes at the central carbon pathway in K. pneumoniae CGMCC 1.6366 under ammonia limitation (top entry in the boxes) and phosphate limitation (bottom entry). The modified basic media (described in ‘Materials and methods’) contains 30 g glycerol l−1, 30.3 mM NH4Cl (ammonia limitation), or 0.35 mM K2HPO4 and 0.16 mM KH2PO4 (phosphate limitation). The other nutrients in the medium are described in ‘Materials and methods’. The average data of three individual experiments are presented
Fed-batch cultures under ammonia and phosphate limitation
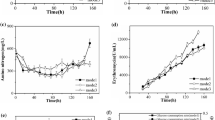
Fed-batch fermentations were conducted under ammonia and phosphate limitation in 5 l fermenters (Figs. 2, 3). A high concentration of 1,3-PD (60.8 g l−1) was obtained in 36 h (Fig. 2) in ammonia-limited cultures, leading to high volumetric productivity and a yield of 1.7 g l−1 h−1 and 0.55 mol mol−1, respectively. The final concentration and yield of 1,3-PD were only 44 g l−1 (Fig. 3) and 0.34 mol mol−1, respectively in phosphate-limited cultures. Growth was inhibited when ammonia and phosphate were limited. However, biomass in the phosphate-limited cultures showed a typical bacterial growth pattern, while cells in the ammonia-limited cultures continuously increased during the fermentation cycle. The maximum biomass was only 4.1 g l−1 in ammonia-limited fed-batch fermentation. Lactate was the major product in phosphate-limited cultures at 61 g l−1. The formation of 2,3-BD in phosphate-limited cultures was only 35% of that in ammonia-limited cultures. The total amount of organic acid was 14.8 g l−1 in ammonia-limited cultures. These results are of significant importance to the downstream processing which accounts for up to 50–70% of the total production cost of 1,3-PD (Xiu and Zeng 2008). The desalination burden in 1,3-PD purification may be reduced to some extent, by using ammonia-limited cultures.
Fermentation profile of a fed-batch culture of K. pneumoniae CGMCC 1.6366 under ammonia-limited conditions in a 5 l fermenter: acetate (filled triangle), 2,3-BD (open circle), biomass (filled circle), ethanol (inverted open triangle), glycerol (open triangle), lactate (inverted filled triangle), 1,3-PD (filled square), succinate (open square). A total 120 mM NH4Cl was added to the culture broth continuously. The average data of three individual experiments are presented
Fermentation profile of a fed-batch culture of K. pneumoniae CGMCC 1.6366 under phosphate-limited conditions in a 5 l fermenter: acetate (filled triangle), 2,3-BD (open circle), biomass (filled circle), ethanol (inverted open triangle), glycerol (open triangle), lactate (inverted filled triangle), 1,3-PD (filled square), succinate (open square). A total 1.4 mM K2HPO4 and 0.64 mM KH2PO4 were added to the culture broth continuously. The average of data of three individual experiments are presented
Conclusion
The results demonstrate that 1,3-PD excretion is enhanced by the addition of increasing glycerol concentrations (no more than 30 g l−1) to the chemostat at a dilution rate of 0.1 h−1. The conversion of glycerol to 1,3-PD by K. pneumoniae is higher under ammonia limitation than under phosphate limitation. Compared with phosphate-limited fed-batch fermentation, more carbon flux was directed to 1,3-PD formation in ammonia-limited fed-batch fermentation.
Abbreviations
- 1,3-PD:
-
1,3-Propanediol
- 2,3-BD:
-
2,3-Butanediol
- 3-HPA:
-
3-Hydroxypropionaldehyde
- GDH:
-
Glycerol dehydrogenase
- GDHt:
-
Glycerol dehydratase
- ORP:
-
Oxidoreduction potential
- PDOR:
-
1,3-Propanediol oxidoreductase
- Q :
-
Specific production/uptake rate of metabolite (mM g−1 h−1)
- \( Q_{{{\text{O}}_{2} }} \) :
-
Oxygen consumption per biomass per hour (μl mg−1 h−1)
- Y 1,3-PD :
-
Yield of 1,3-propanediol (mol mol−1)
- Y glycerol :
-
Biomass yield (g g−1)
- Y oxygen :
-
Biomass yield at 0.5 mol oxygen consumption (g (0.5 mol O2)−1)
References
Circle SJ, Stone L, Boruff CS (1945) Acrolein determination by means of tryptophan. Ind Eng Chem Anal Ed 17:259–262
Du C, Zhang Y, Li Y et al (2007) Novel redox potential-based screening strategy for rapid isolation of Klebsiella pneumoniae mutants with enhanced 1, 3-propanediol-producing capability. Appl Environ Microbiol 73:4515–4521
Forage RG, Lin ECC (1982) dha system mediating aerobic and anaerobic dissimilation of glycerol in Klebsiella pneumoniae NCIB418. J Bacteriol 151:591–599
Gonzalez-Pajuelo M, Andrade JC, Vasconcelos I (2005) Production of 1,3-propanediol by Clostridium butyricum VPI 3266 in continuous cultures with high yield and productivity. J Ind Microbiol Biotechnol 32:391–396
Liu Y (1998) Energy uncoupling in microbial growth under substrate-sufficient conditions. Appl Microbiol Biotechnol 49:500–505
Neijssel OM, Tempest DW (1976) The role of energy-spilling reactions in the growth of Klebsiella aerogenes NCTC 418 in aerobic chemostat culture. Arch Microbiol 110:305–311
Papanikolaou S, Ruiz-Sanchez P, Pariset B et al (2000) High production of 1,3-propanediol from industrial glycerol by a newly isolated Clostridium butyricum strain. J Biotechnol 77:20–191
Streekstra H, Teixeira de Mattos MJ, Neijssel OM et al (1987) Overflow metabolism during anaerobic growth of Klebsiella aerogenes NCTC 418 on glycerol and dihydroxyacetone in chemostat culture. Arch Microbiol 147:268–275
Weber J, Kayser A, Rinas U (2005) Metabolic flux analysis of Escherichia coli in glucose-limited continuous culture. II. Dynamic response to famine and feast, activation of the methylglyoxal pathway and oscillatory behaviour. Microbiology 151:707–716
Xiu ZL, Zeng AP (2008) Present state and perspective of downstream processing of biologically produced 1,3-propanediol and 2,3-butanediol. Appl Microbiol Biotechnol 78:917–926
Zeng AP, Deckwer WD (1995) A kinetic model for substrate and energy consumption of microbial growth under substrate-sufficient conditions. Biotechnol Prog 11:71–79
Zeng AP, Biebl H, Schlieker H et al (1993) Pathway analysis of glycerol fermentation by Klebsiella pneumoniae: regulation of reducing equivalent balance and product formation. Enzyme Microb Technol 15:770–779
Zheng ZM, Cheng KK, Hu QL et al (2008a) Effect of culture conditions on 3-hydroxypropionaldehyde detoxification in 1,3-propanediol fermentation by Klebsiella pneumoniae. Biochem Eng J 39:305–310
Zheng ZM, Xu YZ, Liu HJ et al (2008b) Physiologic mechanisms of sequential products synthesis in 1,3-propanediol fed-batch fermentation by Klebsiella pneumoniae. Biotechnol Bioeng 100:923–932
Zheng ZM, Guo NN, Jiao H et al (2009) Scale-up of micro-aerobic 1,3-propanediol production with Klebsiella pneumonia CGMCC 1.6366. Process Biochem 44:944–948
Acknowledgements
This study was supported by ‘863’ Hi-Tech Research and Development Program of China (2006AA020103 and 2008AA05Z302) and North China Electric Power University Fund for Teacher with Doctorate.
Author information
Authors and Affiliations
Corresponding authors
Additional information
An erratum to this article can be found at http://dx.doi.org/10.1007/s10529-010-0319-4
Rights and permissions
About this article
Cite this article
Zheng, ZM., Xu, YZ., Wang, TP. et al. Ammonium and phosphate limitation in 1,3-propanediol production by Klebsiella pneumoniae . Biotechnol Lett 32, 289–294 (2010). https://doi.org/10.1007/s10529-009-0150-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-009-0150-y