Abstract
Thaxtomin A is the main phytotoxin produced by Streptomyces scabiei, the causative agent of common scab disease of potato. Pathogenicity of S. scabiei is dependent on the production of thaxtomin A which is required for the development of disease symptoms, such as growth inhibition and cell death. We investigated whether thaxtomin A-induced cell death was similar to the hypersensitive cell death that often occurs in response to specific pathogens or phytotoxins during the so-called hypersensitive response (HR). We demonstrated that thaxtomin A induced in Arabidopsis thaliana suspension-cultured cells a genetically controlled cell death that required active gene expression and de novo protein synthesis, and which involved fragmentation of nuclear DNA, a characteristic hallmark of apoptosis. The thaxtomin A-induced form of programmed cell death (PCD) was not a typical HR, since defence responses generally preceding or associated with the HR, such as rapid medium alkalization, oxidative burst and expression of defence-related genes PR1 and PDF1.2, were not observed in plant cells following addition of thaxtomin A. Thaxtomin A has been shown to inhibit cellulose biosynthesis (Scheible et al. in Plant Cell 15:1781, 2003). We showed that isoxaben, a specific inhibitor of cellulose biosynthesis, also induced in Arabidopsis cell suspensions a PCD similar to that induced by thaxtomin A. These data suggested that rapid changes in the plant cell wall composition and organization can induce PCD in plant cells. We discuss how rapid inhibition of cellulose biosynthesis may trigger this process.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Thaxtomins, a class of 4-nitroindol-3-yl-containing 2,5 dioxopiperazines, are phytotoxins produced by the pathogenic streptomycetes Streptomyces scabiei, one of the causative agent of common scab disease in potato and other underground vegetables such as radishes, turnips and beets (King et al. 1992; Goyer and Beaulieu 1997). Pathogenicity of S. scabiei is dependent on the production of thaxtomins, thaxtomin A being the major form produced by S. scabiei (King et al. 1991, 1992; Leiner et al. 1996; Goyer et al. 1998). Application of thaxtomin A (10−5 M) alone on potato tuber tissues induced the development of scab-like lesions (Lawrence et al. 1990; King et al. 1991). Other disease symptoms, such as seedling growth inhibition, root or shoot thickening due to cell hypertrophy as well as cell death in a concentration-dependent manner have also been reported in response to thaxtomin A in a wide variety of plant species and tissues (Leiner et al. 1996; King et al. 2001; Fry and Loria 2002). The symptoms induced by thaxtomin A are very similar to those induced by specific inhibitors of cellulose biosynthesis such as dichlobenil (DCB) and isoxaben (King et al. 2001; Desprez et al. 2002; Fry and Loria 2002). Recent evidence has indicated that thaxtomin A inhibits cellulose biosynthesis in plant cells (Scheible et al. 2003; Robert et al. 2004). However, the specific mode of action of thaxtomin A is still unknown and may be wider and different from that of DCB and isoxaben.
Plants confronted to pathogens often induce an hypersensitive response (HR) characterized by a localized cell death that is generally preceded or associated with the elicitation of various defence responses. These may include production of reactive oxygen species (ROS), activation of mitogen-activated protein kinases (MAPKs), expression of defence-related genes, synthesis of phytoalexins and induction of localized hypersensitive cell death, a form of programmed cell death (PCD) which restricts pathogen infection by limiting its propagation at the site of entry (Heath 2000; Greenberg and Yao 2004). Hypersensitive-like PCD can also be activated by pathogen-derived elicitors [e.g., harpin (Desikan et al. 1998), cryptogein (Zhang et al. 1998), chitosan (Zuppini et al. 2004)] and phytotoxins [e.g., fumonisin B1 (FB1) (Asai et al. 2000), AAL-toxin (Wang et al. 1996), victorin (Navarre and Wolpert 1999)]. This cellular suicide is not specific to the HR. Different forms of PCD have also been described during normal plant development [e.g., tracheary element differentiation, aerenchyma formation, embryo morphogenesis (Pennell and Lamb 1997)], in response to various stresses [e.g., heat (Swidzinski et al. 2002), ozone (Rao and Davis 1999), salts (Huh et al. 2002), UV-C (Danon and Gallois 1998)] and are required for the removal of senescing or damaged plant cells (Lam 2004).
PCD is a genetically controlled process that occurs in all living organisms. It requires active gene expression and cell metabolism (Lam 2004). The most studied form of PCD in animal cells is apoptosis which occurs in an ordered set of events generally implicating a cascade of proteolytic enzymes, the caspases. This process leads to nuclear condensation and fragmentation, cleavage of DNA into internucleosomal fragments (DNA laddering), and dismantling of the cell into apoptotic bodies that are engulfed by neighbouring cells (Lawen 2003). Apoptotic-like features such as nuclear condensation, cleavage of DNA, activation of proteases and cytosolic release of cytochrome c from the mitochondrial intermembrane space have been described during some types of plant PCD (Lam 2004). Nonetheless, plants have evolved other specialized features and various signalling cascades to regulate the different types of PCD that take place during development or in response to pathogens or environmental stresses.
Whether S. scabiei and more particularly its major phytotoxin thaxtomin A can induce HR-like cell death and elicit defence responses in plant cells has, to our knowledge, never been examined. Other phytotoxins have been shown to induce hypersensitive-like PCD in plant cells on their own. For example, the fungal AAL-toxin induced PCD by an active process requiring de novo protein synthesis and displaying DNA fragmentation and nuclear condensation (Wang et al. 1996). Infiltration of the AAL-toxin in Arabidopsis leaves also induced the production of H2O2 and activated the expression of defence-related genes responsive to ROS, salicylic acid (SA), jasmonic acid (JA) and ethylene (ET) (Gechev et al. 2004). Similarly, the fungal toxin FB1 induced in Arabidopsis protoplasts an HR-like cell death requiring active cell metabolism and exhibiting some apoptotic features such as nuclear DNA cleavage. This PCD was also dependent on active SA, JA and ET defence-signalling pathways (Asai et al. 2000). More recently, the phytotoxin fusicoccin, which specifically activates the plasma membrane H+-ATPase, has been shown to induce PCD characterized by cell shrinkage, DNA fragmentation, nuclear condensation and cytoplasmic release of cytochrome c (Malerba et al. 2003; Malerba et al. 2004). Fusicoccin also induced defence responses, including the production of H2O2 and ET and the transcription of defence-related genes (Roberts and Bowles 1999; Malerba et al. 2003).
In the present work, we investigated whether thaxtomin A can elicit HR-like PCD in Arabidopsis suspension-cultured cells. We show that thaxtomin A induced in plant cells morphological changes and cell death that were dependent on active gene transcription and de novo protein synthesis and that displayed apoptotic-like features. However, thaxtomin A did not induce the typical defence responses normally preceding or associated with the HR. We postulated that induction of PCD by thaxtomin A would rather be caused by its inhibition of cellulose biosynthesis.
Materials and methods
Purification of thaxtomin A
Thaxtomin A was purified from oat bran broth cultures of S. scabiei as previously described (Goyer et al. 1998). In summary, cultures of S. scabiei in oat bran broth were incubated on a rotary shaker at 30°C for 7 days to 8 days. The culture supernatant was extracted twice with an equal volume of ethyl acetate. The solvent phase containing thaxtomin A was concentrated by evaporation and purified by thin layer chromatography on glass plates pre-coated with 0.25 mm Silica Gel 60. Yellow compounds with an R f of 0.27 were eluted from silica using chloroform-methanol (7:3). Thaxtomin A was quantified by HPLC using a Varian LC5500 liquid chromatograph equipped with a Water’s C18 column (10 μm particle size, 3.9×300 mm).
Plant material and treatments
Arabidopsis thaliana ecotype Landsberg erecta suspension-cultured cells were grown in Murashige and Skoog (MS) medium (pH 5.7) supplemented with B5 vitamins and 1 mg l−1 2,4-D and maintained in the dark under continuous shaking (gyratory shaker) at 120 rpm. Suspensions were sub-cultured weekly using 3:7 dilution. All treatments were carried out using log-phase cells 3 days to 4 days after sub-culture. Thaxtomin A diluted in methanol (10 mM) was added to Arabidopsis cell suspensions at the indicated final concentrations, and isoxaben (100 μM in methanol; Crescent Chemicals Co., Inc., Islandia, NY, USA) was added at a final concentration of 10 nM. The same volume of methanol was added to control cells. Actinomycin D (diluted in DMSO; Sigma) or cycloheximide (diluted in methanol; Sigma) were added at a final concentration of 20 μg ml−1 to the cells 15 min before adding thaxtomin A, isoxaben, or methanol (control).
Detection of cell death
Trypan blue staining of cell cultures was used to determine the number of dead cells. One part of cell culture was incubated for 5 min with one part of 0.4% trypan blue (Sigma) prepared in culture medium. At least three independent experiments were performed with more than 500 cells counted per condition.
TUNEL assay
Nuclear DNA cleavage was detected using the in situ cell death detection kit (Roche) according to the manufacturer’s instructions. Treated and control cells were fixed 45 min with 4% paraformaldehyde in PBS and washed twice in PBS. Cells were treated for 15 min with proteinase K (20 μg ml−1; Sigma) before 3′OH end labelling. Nuclei were stained with 5 μg ml−1 DAPI (Sigma). At least three independent experiments were performed with more than 800 nuclei counted per condition.
DNA extraction, gel electrophoresis and Southern blot analyses
Genomic DNA was prepared as described by Dean et al. (1992) and in a protocol from Janice Keller (DNAP, Oakland, CA, USA). Briefly, frozen cells were ground in liquid N2 and incubated in extraction buffer (140 mM Sorbitol, 220 mM Tris pH 8, 22 mM EDTA, 800 mM NaCl, 1% Sarkosyl, 0.8% CTAB) at 65°C. Following chloroform extraction, nucleic acids were precipitated from the aqueous phase with isopropanol. The pellet was dissolved in TE (10 mM Tris pH 8, 1 mM EDTA) and high molecular weight RNA was precipitated with 4 M lithium acetate. DNA was precipitated from the supernatant with ethanol and resuspended in TE, followed by phenol, phenol/chloroform, and chloroform extractions. DNA was precipitated from the aqueous phase with 3 M sodium acetate and ethanol. The pellet was dissolved in TE and incubated overnight with RnaseA (100 μg ml−1). After phenol/chloroform extraction, genomic DNA was precipitated and resuspended in TE. Five micrograms of genomic DNA were separated on a 2% agarose gel and DNA was visualized using SYBR Green (Sigma). For Southern blot analyses, DNA was transferred to Hybond N membranes (Amersham). Membranes were prehybridized and hybridized at 65°C with 5X SSC, 5X Denhardt’s solution, 0.5% SDS and 100 μg ml−1 herring sperm DNA, using 32P-labelled digested Arabidopsis genomic DNA as a probe. Washings were performed with 1× SSC and 0.1% SDS at 42°C. Membranes were analyzed with a Molecular Imager FX (Biorad).
Protein extraction and western blot analyses
Fractionation of cellular components for detection of cytochrome c was performed as described by Kim et al. (2003) with the following modifications. Four grams of cells treated with thaxtomin A for 2, 12, 24, 48 and 72 h were homogenized with a Polytron in extraction buffer (0.4 M Mannitol, 1 mM EGTA, 5 mM Dithiothreitol, 50 mM Tricine, 0.1% Bovine Serum Albumin, pH 7.8) for 5 min at 4°C. Extracts were centrifuged at 1,500g for 10 min at 4°C to eliminate cellular debris. Supernatant were then centrifuged at 15,000g for 5 min at 4°C to pellet mitochondria. The supernatant were further centrifuged at 16,000g for 15 min at 4°C. The resulting soluble fraction corresponded to the cytosolic fraction. Fifty micrograms of mitochondrial or cytosolic proteins were fractionated on a 12% SDS-PAGE, transferred to PVDF membranes (Amersham) and probed with the monoclonal antibody against cytochrome c (1:2000, Pharmingen) in TBS-T (TBS with 0.1% Tween 20) containing 5% non-fat dry milk. After three washes in the same buffer, membranes were probed with horseradish peroxidase-conjugated secondary antibody and detected with ECL reagent (Amersham) following the manufacturer’s instructions.
Assessment of extracellular medium alkalization
The pH of Arabidopsis cell suspensions was allowed to stabilize for 2 h before the addition of thaxtomin A. pH of the cell suspension medium was measured every 10 min for 3 h after the addition of thaxtomin A (2.0 μM).
Hydrogen peroxide quantitation
Measurement of H2O2 in extracellular medium was conducted as described in Vacca et al. (2004) every 10 min for 2 h after adding thaxtomin A (2.0 μM) or methanol (control).
RNA extraction and northern blot analyses
Total RNA was isolated from Arabidopsis suspension-cultured cells treated with thaxtomin A (2.0 μM) for 2, 12, 24, 48 and 72 h. Cells (500 mg) were ground in liquid N2 and mixed vigorously with hot extraction buffer consisting in one part of RNA buffer (100 mM Tris pH 8, 10 mM EDTA, 100 mM LiCl, 1% SDS) and one part of equilibrated phenol heated at 80°C. Samples were extracted with chloroform/isoamyl alcohol (24:1) and RNA was precipitated from the supernatant with 4 M LiCl. Pellets were resuspended in DEPC-treated water and extracted with phenol/chloroform/isoamyl alcohol (25:24:1). RNA was precipitated from the aqueous phase with 3 M sodium acetate and ethanol. RNA pellets were dissolved in DEPC-treated water. Twenty micrograms of RNA were size-separated on formaldehyde-1.5% agarose gels and transferred to Hybond N membranes (Amersham). Blots were hybridized in 7% SDS, 0.25 M Na2HPO4, 2 mM EDTA, 0.2 mg ml−1 heparin and 100μg ml−1 herring sperm DNA at 65°C with 32P-labelled probes prepared from: (1) Arabidopsis PR1 cDNA amplified by RT-PCR using the primers 5′-GTA GGT GCT CTT GTT CTT CCC-3′ and 5′-CAC ATA ATT CCC ACG AGGATC-3′; (2) Arabidopsis PDF1.2 cDNA obtained from the ABRC stock center (# 37F10) and (3) Arabidopsis PAL1 cDNA obtained from the ABRC stock center (# CD3-102). Washing conditions were 0.1× SSC, 0.1% SDS at 42°C. The same blots were also probed with a 32P-labelled probe prepared from RT-PCR amplified Arabidopsis actin cDNA using the primers: 5′-GAT TCT GGT GAT GGT GTG TCT CAC A-3′ and 5′-CCA CTG AAG AAC TGC TCT TGG CTG T-3′. Membranes were analyzed with a Molecular Imager FX (Biorad).
Results
Thaxtomin A induced morphological changes and cell death by an active process requiring gene transcription and de novo protein synthesis.
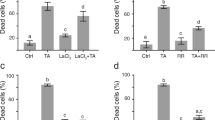
To evaluate the effects of thaxtomin A on both plant cell morphology and viability, we examined Arabidopsis suspension-cultured cells treated with different concentrations of highly purified thaxtomin A for different periods of time, in comparison with methanol-treated cells (control). As presented in Fig. 1a and b, thaxtomin A induced morphological changes in Arabidopsis cells from 24 h after addition of 2.0 μM thaxtomin A with no changes seen in the control. We observed important hypertrophy and bulging of the cells, especially for elongating cells present at the end of cell files. These enlarged cells were still alive at the time of bulging while some neighbouring cells were already dead (stained by trypan blue) and displayed a contracted cytoplasm. Changes in cell morphology were associated with an increased number of dead cells in dose and time-dependent manners (Fig. 1c). Approximately 44% of the cells were killed within 24 h of treatment with 5.0 μM thaxtomin A, and close to 54% were dead within 48 h after adding 2.0 μM thaxtomin A. Approximately 82% and 87% of the cells were dead 72 h after addition of 5.0 and 10.0 μM thaxtomin A, respectively.
a-e Thaxtomin A induces in Arabidopsis cells morphological changes and cell death that are dependent on gene expression and de novo protein synthesis. a and b Arabidopsis suspension cultures were treated with methanol (Control) (a) or thaxtomin A (2.0 μM) (b) for 24 h and stained with trypan blue. Bar = 50 μm. c Percentage of dead cells detected by trypan blue staining in Arabidopsis suspension cultures treated with the indicated concentrations of thaxtomin A for 24 h (filled square), 48 h (filled triangle) and 72 h (open circle). Percentage of dead cells in the control was less than 12% after 72 h (not shown). d and e Percentage of dead cells after 24, 48 and 72 h detected by trypan blue staining in Arabidopsis suspension cultures treated with methanol (Control), thaxtomin A (TA; 2.0μM) or pre-treated for 15 min with actinomycin D (Act D; 20 μg ml−1) (d) or cycloheximide (CHX; 20 μg ml−1) (e) before adding thaxtomin A. Data represent means (±SE) of three independent experiments including at least 500 cells each
To determine whether thaxtomin A-induced cell death was a form of hypersensitive-like PCD typically requiring active gene expression and cell metabolism, Arabidopsis suspension-cultured cells were pre-treated with actinomycin D, an inhibitor of RNA synthesis, or with cycloheximide, an inhibitor of protein synthesis, for 15 min before adding thaxtomin A. Both inhibitors could induce PCD in maize root tissues at high concentrations (Ning et al. 2001) but could be used efficiently at lower doses to inhibit transcription and translation and block the process of cell death (Solomon et al. 1999; Clarke et al. 2000; Vacca et al. 2004). Each inhibitor increased the number of dead cells when compared to control cells (by up to 8% after 48 h; Fig. 1d,e). However, the percentage of dead cells due to thaxtomin A after 48 h (46–53%) was reduced to 24% and 22% in the samples pre-treated with actinomycin D and cycloheximide, respectively. In addition, changes in cell morphology induced by thaxtomin A were markedly reduced in samples pre-treated with the transcription and translation inhibitors (data not shown). These results clearly indicated that thaxtomin A induced cell hypertrophy and cell death by an active process requiring gene transcription and de novo protein synthesis.
Thaxtomin A induced fragmentation of nuclear DNA in dying cells
Plant PCD that occurs in response to pathogens, toxins or stresses often displays apoptotic features such as nuclear DNA cleavage into a ladder of internucleosomal fragments of approximately 180 bp. We evaluated the integrity of nuclear DNA in thaxtomin A-treated Arabidopsis cells using terminal deoxynucleotidyl transferase-mediated dUTP nick end labelling (TUNEL) assay, which detects cleaved or damaged DNA (Gavrieli et al. 1992). Samples from cell suspensions treated with 2.0 μM thaxtomin A or methanol were analyzed after 2 to 72 h. Less than 1% of Arabidopsis control cells had TUNEL positive nuclei, whereas cells treated with thaxtomin A displayed an important number of positive nuclei that increased with time, from 12% after 24 h up to 39% after 72 h (Fig. 2a–c). We also performed Southern blot analyses of genomic DNA extracted from the samples mentioned above. Apoptotic-like nuclear DNA laddering, a typical hallmark of several types of plant PCDs and of apoptosis, was observed in thaxtomin A-treated cells within 12 h and the intensity of this fragmentation increased with time (Fig. 2d).
a-d Thaxtomin A induces fragmentation of nuclear DNA. a and b TUNEL assays performed on Arabidopsis suspension cultures treated with methanol (Control) (a) or thaxtomin A (TA; 2.0 μM) (b) for 72 h. DAPI staining of nuclei is shown on the left panel (represented here in red for better visibility). Each dot corresponds to a single nucleus. TUNEL positive nuclei are in green (right panel). Bar = 100 μm. c Percentage of TUNEL positive nuclei (white) or dead cells (black) detected by trypan blue staining in Arabidopsis cell suspensions treated with thaxtomin A (2.0 μM) for the indicated time. Control cells (not shown) displayed less than 1% TUNEL positive nuclei. Values represent means (±SE) of three independent experiments including at least 500 cells each. d Fragmentation of nuclear DNA as detected by Southern blot analysis using 32P-labelled digested genomic DNA as a probe. DNA was extracted from Arabidopsis cells treated with methanol for 72 h (C) or thaxtomin A (TA; 2.0 μM) from 2 h to 72 h. DNA molecular weight markers (bp) are presented on the left
Thaxtomin A induced cleavage of nuclear DNA by a genetically active process dependent on gene expression and de novo protein synthesis
Since thaxtomin A-induced cell death was dependent on active gene expression and protein synthesis, we tested whether nuclear DNA fragmentation was also regulated by a genetically active program. Actinomycin D or cycloheximide were added to Arabidopsis cell cultures 15 min before adding thaxtomin A or methanol (control), and samples were taken 48 h and 72 h after addition of thaxtomin A for TUNEL assay or DNA extraction. As shown in Fig. 3a, some positive nuclei were detected after 48 h by TUNEL assay in control samples pre-treated with actinomycin D (less than 2%) or cycloheximide (less than 8%). This correlated with an increasing number of dead cells and can be attributed to the ability of these inhibitors to induce PCD at higher concentrations (Ning et al. 2001). However, the number of positive nuclei in thaxtomin-A treated samples was significantly lower when cells were pre-treated with actinomycin D (22% compared to 10%) or cycloheximide (22% compared to 9%; Fig. 3a). Moreover, electrophoresis analysis of genomic DNA extracted after 72 h revealed reduced DNA fragmentation when actinomycin D was added to the cells before thaxtomin A (Fig. 3b). These results supported the previous data indicating that thaxtomin A-induced cell death is controlled at the genetic level.
a,b Thaxtomin A-induced nuclear DNA fragmentation is dependent on active gene transcription and de novo protein synthesis. a Percentage of TUNEL positive nuclei in Arabidopsis cell suspensions treated with thaxtomin A (TA; 2.0 μM) or methanol (Control) for 48 h, or pre-treated with actinomycin D (Act D; 20 μg ml−1) or cycloheximide (CHX; 20 μg ml−1) for 15 min before adding methanol or thaxtomin A. Control cells displayed less than 1% TUNEL positive nuclei (not shown). Values represent means (±SE) of three independent experiments including at least 800 nuclei. b Fragmentation of nuclear DNA as detected by gel electrophoresis. Genomic DNA was extracted from Arabidopsis cells treated with methanol (Control) or thaxtomin A (TA; 2.0 μM) from 72 h or pre-treated with actinomycin D (Act D; 20 μg ml−1) for 15 min before adding methanol or thaxtomin A. M: DNA molecular weight markers (bp)
Thaxtomin A did not induce cytosolic translocation of mitochondrial cytochrome c
Mitochondria have been shown to play an important role in the regulation of PCD (Yao et al. 2004). In particular, cytosolic translocation of cytochrome c during apoptosis plays a central role in the activation of caspases. Efflux of cytochrome c can also occur during some types of plant PCDs, including developmental, defence- or stress-induced PCDs (Balk and Leaver 2001; Curtis and Wolpert 2002; Pasqualini et al. 2003; Krause and Durner 2004; Malerba et al. 2004). To test if cytosolic translocation of cytochrome c was involved in the thaxtomin A-induced PCD, western blot analyses of proteins extracted from cytosolic and mitochondrial fractions from thaxtomin A- or methanol-treated Arabidopsis cells were performed using monoclonal antibodies against cytochrome c. A band of approximately 15 kD corresponding to the size of the Arabidopsis cytochrome c was detected at a constant level in mitochondrial fractions extracted from samples treated for 2 h up to 72 h (data not shown). In contrast, no cytochrome c was detected in any of the cytosolic fractions, demonstrating that thaxtomin A did not induce cytosolic translocation of cytochrome c during the induction and execution of this PCD process.
HR-like defence responses were not activated by thaxtomin A in Arabidopsis suspension cultures
HR-like cell death is generally preceded or accompanied by the activation of defence responses in the surrounding cells. One rapid response that occurs in reaction to pathogens, elicitors or various stresses is the transient change in H+ gradients across the plasma membrane (Heath 2000). This can lead to extracellular alkalization and can be conveniently detected in the medium of suspension-cultured cells (Haruta and Constabel 2003). This was examined in Arabidopsis cell suspensions treated with thaxtomin A or methanol by measuring the pH of the culture medium every 10 min for 3 h. No significant changes in pH were noted when compared to controls (data not shown).
Another characteristic response is the activation of the so-called oxidative burst (Heath 2000). Production of H2O2 in the Arabidopsis cell culture medium was measured for 2 h after adding thaxtomin A or methanol. No significant levels of H2O2 were detected in the medium in thaxtomin A-treated samples or controls, indicating that no rapid oxidative burst was induced by the addition of thaxtomin A (data not shown). Since it was reported that production of ROS in Arabidopsis during the HR would occur only after the first events of PCD (Zhang et al. 2003), H2O2 production was quantified for a longer period. It was only from 72 h after adding thaxtomin A that significant levels of H2O2 (up to 4 μM) were detected in treated samples compared to controls. Since at this time more than 65% of the cells were dead, H2O2 production was most probably a consequence of cellular decay.
Early defence responses are often followed by the expression of defence-related genes. The expression of three defence-related genes (PR1, PDF1.2 and PAL) was evaluated by northern blot analyses using total RNA extracted from thaxtomin A-treated Arabidopsis cells from 2 h to 72 h. As presented in Fig. 4, both PR1 and PDF1.2 genes were not expressed in the course of this experiment. However, expression of PAL was upregulated by thaxtomin A within 12 h, increased further at 48 h and remained at this level until the end of the experiment (Fig. 4).
a–c Expression of defence-related genes. Northern blot analyses were performed with total RNA (20 μg) extracted from Arabidopsis cell suspensions treated with methanol for 72 h (C) or with thaxtomin A (TA; 2.0 μM) for the indicated times. Blots were hybridized with the following 32P-labelled probes: a PR1 cDNA, b PDF1.2 cDNA, c PAL1 cDNA. The same blots were also hybridized with the 32P-labeled actin cDNA (lower panels) as a loading control
Activation of a genetically controlled cell death by thaxtomin A would be associated with its effect on inhibition of cellulose biosynthesis
Thaxtomin A inhibits cellulose biosynthesis (Scheible et al. 2003). Results reported above suggested that thaxtomin A induced PCD by a signalling cascade different from defence-related pathway(s) leading to the HR. We speculated that the induction of PCD was due to modifications of the cell wall composition and structure caused by thaxtomin A inhibition of cellulose synthesis. To test this hypothesis, Arabidopsis suspension-cultured cells were treated with isoxaben (10 nM), a specific inhibitor of cellulose synthesis, or pre-treated for 15 min with actinomycin D (20 μg ml−1) before adding isoxaben. As described previously (King et al. 2001; Desprez et al. 2002; Fry and Loria 2002), we observed within 24 h hypertrophy and bulging of the isoxaben-treated cells as seen in thaxtomin A-treated samples (data not shown). This result was correlated with an increased number of dead cells in a time-dependent manner, with about 32% of cell death after 24 h to reach a level close to 60% after 72 h. The percentage of dead cells after 24 h in actinomycin D pre-treated samples was significantly reduced to about 14% when compared to samples treated with isoxaben alone (data not shown). These results indicated that active gene transcription was required for the induction of cell death by isoxaben in Arabidopsis cell suspensions. We also investigated the effect of isoxaben on nuclear DNA cleavage using TUNEL assay. While less than 1% of TUNEL positive nuclei were detected in control cells after 48 h, the percentage of TUNEL positive nuclei in isoxaben-treated cells was approximately 20% (data not shown). Pre-treatment with actinomycin D reduced the percentage of TUNEL positive nuclei 48 h after adding isoxaben to about 3%. DNA cleavage was also detected by gel electrophoresis in nuclear DNA extracted from isoxaben-treated samples, but was markedly reduced in samples pre-treated with actinomycin D, indicating that this PCD feature was also dependent on active gene transcription (Fig. 5). These data suggested that inhibition of cellulose biosynthesis by thaxtomin A and isoxaben induced a genetically controlled cell death displaying some typical hallmarks of PCD.
Isoxaben-induced DNA cleavage is dependent on gene expression. Nuclear DNA cleavage was detected by gel electrophoresis. DNA was extracted from Arabidopsis cells treated with methanol (Control) or isoxaben (10 nM) for 48 h or pre-treated with actinomycin D (Act D; 20 μg ml−1) for 15 min before adding methanol or isoxaben. M: DNA molecular weight markers (bp)
Discussion
The phytotoxin thaxtomin A is essential for pathogenicity of S. scabiei and for the development of disease symptoms (Goyer et al. 1998). We investigated in Arabidopsis suspension-cultured cells whether thaxtomin A induced cell death in a fashion similar to the defence-associated HR process. First, we presented evidence that thaxtomin A induced a genetically controlled PCD in Arabidopsis suspension-cultured cells that required active gene expression and de novo protein synthesis and that was associated with apoptotic-like features, such as cytoplasm shrinkage and cleavage of nuclear DNA, as detected by DNA laddering and the prevalence of TUNEL positive nuclei in thaxtomin A-treated cells. Active gene expression and protein synthesis were also required for nuclear DNA fragmentation. These data corresponded with the widely accepted definition of PCD, which is described as a genetically controlled and ordered process that requires active metabolism, whereas necrotic cell death occurs in response to dramatic damage or trauma in an uncontrolled fashion that does not follow any specific order of events (Pennell and Lamb 1997; Lam 2004). In contrast to what is found for most apoptotic cell deaths, thaxtomin A did not induce cytosolic release of cytochrome c. These results corroborate recent data indicating that translocation of cytochrome c would not be an obligatory step in the execution of plant PCD (Yao et al. 2004). Nonetheless, there is increasing support for the importance of plant mitochondria in the control of PCD, with evidence that mitochondrial permeability transition precedes plant PCD (Yu et al. 2002; Yao et al. 2004). It is thus possible that the thaxtomin A-induced cell death process also involves mitochondria.
Pathogenic signals, including phytotoxins, can trigger in plant cells an HR that is normally accompanied by characteristic defence responses (Heath 2000). We found that PCD induced by thaxtomin A was not associated with typical defence responses. Addition of thaxtomin A to Arabidopsis cells did not induce medium alkalization nor production of ROS such as H2O2. This is consistent with results obtained by Fry and Loria (2002) in thaxtomin A-treated tobacco cells, indicating that no rapid modification of the plasma membrane H+-ATPase activity occurred after addition of thaxtomin A. While ROS have often been described as important molecules for the execution of HR cell death, there are some controversial reports on their actual involvement in the PCD process (Dorey et al. 1999; Vacca et al. 2004). For example, direct addition of H2O2 to Arabidopsis cell suspensions could elicit an HR-like PCD (Desikan et al. 1998) while ROS were not required nor sufficient for induction of cell death by elicitin in tobacco cell suspension cultures (Dorey et al. 1999; Sasabe et al. 2000). Similarly, when specific ROS scavengers were added to tobacco cell suspensions treated with cryptogein, an HR-elicitor, the cell death process was not significantly affected by the absence of ROS in the extracellular medium (Hirasawa et al. 2005). While production of H2O2 is not required for thaxtomin A-induced PCD, we cannot exclude the possibility that other forms of ROS (superoxide anion or hydroxyl radical) were produced extracellularly after the addition of thaxtomin A, or that ROS produced inside the cells participated in the cell death process. Finally, we showed that expression of defence-related genes PR1 and PDF1.2 was not induced by thaxtomin A in Arabidopsis cells, indicating that the resulting PCD did not involve the classical SA, JA or ET-defence signalling pathways. However, expression of the PAL gene was upregulated 12 h after adding thaxtomin A. While PAL expression is often associated with defence responses, it can also be induced by a wide range of stresses and is modulated by developmental cues. Recent data using molecular phenotyping of T-DNA insertion mutants in the Arabidopsis PAL1 and PAL2 genes have also shown that PAL activity can influence various metabolic processes (Rohde et al. 2004). Whether the expression of PAL in thaxtomin A-treated cells is a true defence response is uncertain. It is possible that PAL is specifically involved in the induction of defence and PCD by modulating the synthesis of defence related-compounds, such as phytoalexins. It was also reported that cellulose-deficient mutants can exhibit defence responses as well as ectopic deposition of lignins, which are derived from the phenylpropanoid pathway involving PAL (Caño-Delgado et al. 2003). Since thaxtomin A inhibits cellulose biosynthesis, expression of PAL may be induced in response to low levels of cellulose in the plant cell wall. While cellulose-deficient mutants can adjust their development to compensate for low cellulose levels, diminution of cellulose synthesis in thaxtomin A-treated suspension-cultured cells is much more sudden. Whether this rapid change in cell wall composition could lead to PAL expression remains to be shown.
As reported in other plant species (Leiner et al. 1996; Fry and Loria 2002), thaxtomin A also induced changes in cell morphology in Arabidopsis. Interestingly, we found that these changes in cell morphology were reduced when inhibitors of transcription or translation were added before thaxtomin A. This suggested that these morphological changes could be involved in inducing the cell death process. Rapid inhibition of cellulose synthesis can modify the plant cell wall composition and organization. We postulated that these alterations were somehow perceived by the cell which in turn initiated a cell death program. This was supported by the fact that another inhibitor of cellulose biosynthesis, isoxaben, also induced changes of cell morphology as well as a genetically controlled PCD in Arabidopsis suspension-cultured cells, demonstrating that rapid inhibition of cellulose synthesis was a key factor in the induction of PCD. It was also reported that tobacco protoplasts were resistant to thaxtomin A (Fry and Loria 2002), thus corroborating the importance of the cell wall in initiating PCD.
How changes in cell wall composition, structure or mechanical strength can activate PCD has never been examined as such. The strong but flexible primary plant cell walls control the rate and direction of cell expansion and can regulate cell volume and cell shape (Fry 2004). They are also required for the build-up of the turgor pressure and for the protection of plant cells from bursting when placed into hypotonic environments. Generally, the cell wall composition and structure gradually adjust to the growing conditions. For example, progressive reduction of cellulose levels in cell or callus cultures treated with cellulose inhibitors such as DCB and isoxaben can be gradually compensated by the accumulation of other cell wall components such as pectins (Encina et al. 2001; Manfield et al. 2004). However, sudden changes in the properties of the cell wall can trigger pathways essential for metabolic adjustments or can even induce alarm responses eventually leading to cell death (Kacperska 2004; Passardi et al. 2004). We propose here some hypotheses to explain how sudden inhibition of cellulose synthesis may induce PCD.
Recent evidence has indicated that cellulose synthesis needs to be maintained at a certain rate and at sufficient levels for adequate cellulose microfibril orientation (Sugimoto et al. 2003; Wasteneys 2004). It is quite possible that the rapid reduction in cellulose content of plant cell walls caused by thaxtomin A and isoxaben led to microfibril reorganization and subsequent relaxation of the cell wall structure. This would in turn decrease the turgor pressure and allow water to come into the cell to maintain an adequate turgor, thus mimicking the effect of placing cells into hypotonic surroundings. We observed that inhibition of cellulose biosynthesis induced important swelling of expanding cells, suggesting that water was taken up by the cells. Perturbations of the osmotic and ionic equilibrium in cells have been shown in some cases to activate PCD (Kültz and Burg 1998; Huh et al. 2002; Kacperska 2004). However, the first cells to die after the addition of thaxtomin A or isoxaben were not the bulging cells, suggesting that initiation of PCD was not a consequence of hypoosmotic stress. Pollen tubes grown in the presence of isoxaben also exhibited swelling at the elongating tip (Lazzaro et al. 2003). The swollen morphology could not be attributed to simple changes in turgor pressure, but were clearly associated with disorganization of the microtubules array at the extending end, indicating that rapid inhibition of cellulose biosynthesis altered the organization of microtubules. This correlates with the most recent model on cellulose synthesis which proposes that synthesis and integrity of long cellulose microfibrils require the participation of microtubules (Wasteneys 2004).
Modifications in the cell wall structure can profoundly modify the dynamic interactions that exist between the plant cytoskeleton, the membrane and components that connect the cell wall to the membrane. How these changes are perceived by the cells and what signals are involved to induce the cell response remain uncertain. One possibility resides at the level of microtubules, which are bound by a wide variety of proteins. Transient or massive reorganization of the cytoskeleton, as induced by rapid inhibition of cellulose synthesis, could release these proteins into the cytoplasm to activate specific signalling pathways (Wasteneys 2004). Specific molecules were proposed to act as linkers between the plant cell walls and plasma membranes, such as cell wall-associated kinases (WAKS), arabinogalactan proteins (AGPS), pectins, cellulose synthases (CESA) and phospholipase D (PLD) (Baluška et al. 2003; Dhonukshe et al. 2003; Kacperska 2004). There is increasing evidence that some of these molecules would be closely linked to the activation of signalling cascades initiated at the plasma membrane level. In particular, AGPs, which are located at the plasma membrane and cell wall, are important signalling molecules in various activities of plant cells, including the regulation of PCD (Gao and Showalter 1999; Baluška et al. 2003). Other possible linker molecules are the transmembrane CESA required for the synthesis of cellulose microfibrils, which have been proposed to somehow regulate the microtubule polymer status (Wasteneys 2004). PLD is another possible linker that can associate with microtubules and the plasma membrane (Baluška et al. 2003). Activation of PLD can trigger various signalling pathways and can also perturb microtubule organization (Dhonukshe et al. 2003). It is quite possible that reorganization of microtubules in response to inhibition of cellulose synthesis could also activate PLD which in turn would initiate a program leading to cell death.
While rapid inhibition of cellulose biosynthesis clearly triggers a genetic program of cell death in Arabidopsis suspension-cultured cells, it remains to be determined whether important reduction in cellulose levels during the normal plant life cycle are associated with the induction of some developmental or stress-related PCD. At least one report has indicated that changes in the cell wall composition occur as an early event in the PCD process leading to the formation of the aerenchyma (Gunawardena et al. 2001). Nevertheless, our results obtained with cell suspensions demonstrate the importance of the mechanical constraints and structure of the cell wall in regulating cell viability. Additional work investigating the regulation and execution of PCD in response to thaxtomin A will certainly provide important clues on how perturbations in the cell wall composition can be sensed and transduced by the cells into the activation or repression of PCD.
Abbreviations
- AGPs:
-
Arabinogalactan proteins
- CESA:
-
Cellulose synthase
- DCB:
-
Dichlobenil
- ET:
-
Ethylene
- FB1:
-
Fumonisin B1
- HR:
-
Hypersensitive response
- JA:
-
Jasmonic acid
- MAPK:
-
Mitogen-activated protein kinase
- PAL:
-
Phenylalanine ammonia-lyase
- PCD:
-
Programmed cell death
- PLD:
-
Phospholipase D
- ROS:
-
Reactive oxygen species
- SA:
-
Salicylic acid
- TUNEL:
-
Terminal deoxynucleotidyl transferase-mediated dUTP nick end labelling
- WAKS:
-
Cell wall-associated kinases
References
Asai T, Stone JM, Heard JE, Kovtun Y, Yorgey P, Sheen J, Ausubel FM (2000) Fumonisin B1-induced cell death in Arabidopsis protoplasts requires jasmonate-, ethylene-, and salicylate-dependent signaling pathways. Plant Cell 12:1823–1835
Balk J, Leaver CJ (2001) The PET1-CMS mitochondrial mutation in sunflower is associated with premature programmed cell death and cytochrome c release. Plant Cell 13:1803–1818
Baluška F, Šcaron amaj J, Wojtaszek P, Volkmann D, Menzel D (2003) Cytoskeleton-plasma membrane-cell wall continuum in plants. Emerging links revisited. Plant Physiol 133:482–491
Caño-Delgado A, Penfield S, Smith C, Catley M, Bevan M (2003) Reduced cellulose synthesis invokes lignification and defense responses in Arabidopsis thaliana. Plant J 34:351–362
Clarke A, Desikan R, Hurst RD, Hancock JT, Neill SJ (2000) NO way back: nitric oxide and programmed cell death in Arabidopsis thaliana suspension cultures. Plant J 24:667–677
Curtis MJ, Wolpert TJ (2002) The oat mitochondrial permeability transition and its implication in victorin binding and induced cell death. Plant J 29:295–312
Danon A, Gallois P (1998) UV-C radiation induces apoptotic-like changes in Arabidopsis thaliana. FEBS Lett 437:131–136
Dean C, Sjodin C, Page T, Jones J, Lister C (1992) Behavior of the maize transposable element Ac in Arabidopsis thaliana. Plant J 2:69–81
Desikan R, Reynolds A, Hancock JT, Neill SJ (1998) Harpin and hydrogen peroxide both initiate programmed cell death but have differential effects on defence gene expression in Arabidopsis suspension cultures. Biochem J 330:115–120
Desprez T, Vernhettes S, Fagard M, Refrégier G, Desnos T, Aletti E, Py N, Pelletier S, Höfte H (2002) Resistance against herbicide isoxaben and cellulose deficiency caused by distinct mutations in same cellulose synthase isoform CESA6. Plant Physiol 128:482–490
Dhonukshe P, Laxalt AM, Goedhart J, Gadella TWJ, Munnik T (2003) Phospholipase D activation correlates with microtubule reorganization in living plant cells. Plant Cell 15:2666–2679
Dorey S, Kopp M, Geoffroy P, Fritig B, Kauffmann S (1999) Hydrogen peroxide from the oxidative burst is neither necessary nor sufficient for hypersensitive cell death induction, phenylalanine ammonia lyase stimulation, salicylic acid accumulation, or scopoletin consumption in cultured tobacco cells treated with elicitin. Plant Physiol 121:163–171
Encina AE, Moral RM, Acebes JL, Álvarez JM (2001) Characterization of cell walls in bean (Phaseolus vulgaris L.) callus cultures tolerant to dichlobenil. Plant Sci 160:331–339
Fry SC (2004) Primary cell wall metabolism: tracking the careers of wall polymers in living plant cells. New Phytol 161:641–675
Fry BA, Loria R (2002) Thaxtomin A: evidence for a plant cell wall target. Physiol Mol Plant Pathol 60:1–8
Gao M, Showalter AM (1999) Yariv reagent treatment induces programmed cell death in Arabidopsis cell cultures and implicates arabinogalactan protein involvement. Plant J 19:321–331
Gavrieli Y, Sherman Y, Ben-Sasson SA (1992) Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol 119:493–501
Gechev TS, Gadjev IZ, Hille J (2004) An extensive microarray analysis of AAL-toxin-induced cell death in Arabidopsis thaliana brings new insights into the complexity of programmed cell death in plants. Cell Mol Life Sci 61:1185–1197
Goyer C, Beaulieu C (1997) Host range of streptomycete strains causing common scab. Plant Dis 81:901–904
Goyer C, Vachon J, Beaulieu C (1998) Pathogenicity of Streptomyces scabies mutants altered in thaxtomin A production. Phytopathology 88:442–445
Greenberg JT, Yao N (2004) The role and regulation of programmed cell death in plant-pathogen interactions. Cell Microbiol 6:201–211
Gunawardena AHLAN, Pearce DME, Jackson MB, Hawes CR, Evans DE (2001) Rapid changes in cell wall pectic polysaccharides are closely associated with early stages of aerenchyma formation, a spatially localized form of programmed cell death in roots of maize (Zea mays L.) promoted by ethylene. Plant Cell Environ 24:1369–1375
Haruta M, Constabel CP (2003) Rapid alkalinization factors in poplar cell cultures. Peptide isolation, cDNA cloning, and differential expression in leaves and methyl jasmonate-treated cells. Plant Physiol 131:814–823
Heath MC (2000) Hypersensitive response-related death. Plant Mol Biol 44:321–334
Hirasawa K, Amano T, Shioi Y (2005) Effects of scavengers for active oxygen species on cell death by cryptogein. Phytochemistry 66:463–468
Huh GH, Damsz B, Matsumoto TK, Reddy MP, Rus AM, Ibeas JI, Narasimhan ML, Bressan RA, Hasegawa PM (2002) Salt causes ion disequilibrium-induced programmed cell death in yeast and plants. Plant J 29:649–659
Kacperska A (2004) Sensor types in signal transduction pathways in plant cells responding to abiotic stressors: do they depend on stress intensity? Physiol Plant 122:159–168
Kim M, Ahn JW, Jin UH, Choi D, Paek KH, Pai HS (2003) Activation of the programmed cell death pathway by inhibition of proteasome function in plants. J Biol Chem 278:19406–19415
King RR, Lawrence CH, Clark MC (1991) Correlation of phytotoxin production with pathogenicity of Streptomyces scabies isolates from scab infected potato tubers. Am Potato J 68:675–680
King RR, Lawrence CH, Calhoun LA (1992) Chemistry of phytotoxins associated with Streptomyces scabies, the causal organism of potato common scab. J Agric Food Chem 40:834–837
King RR, Lawrence CH, Gray JA (2001) Herbicidal properties of the thaxtomin group of phytotoxins. J Agric Food Chem 49:2298–2301
Krause M, Durner J (2004) Harpin inactivates mitochondria in Arabidopsis suspension cells. Mol Plant Microbe Interact 17:131–139
Kültz D, Burg M (1998) Evolution of osmotic stress signaling via MAP kinase cascades. J Exp Biol 201:3015–3021
Lam E (2004) Controlled cell death, plant survival and development. Nat Rev Mol Cell Biol 5:305–315
Lawen A (2003) Apoptosis-an introduction. BioEssays 25:888–896
Lawrence CH, Clark MC, King RR (1990) Induction of common scab symptoms in aseptically cultured potato tubers by the vivotoxin, thaxtomin. Phytopathology 80:606–608
Lazzaro MD, Donohue JM, Soodavar FM (2003) Disruption of cellulose synthesis by isoxaben causes tip swelling and disorganizes cortical microtubules in elongating conifer pollen tubes. Protoplasma 220:201–207
Leiner RH, Fry BA, Carling DE, Loria R (1996) Probable involvement of thaxtomin A in pathogenicity of Streptomyces scabies on seedlings. Phytopathology 86:709–713
Malerba M, Cerana R, Crosti P (2003) Fusicoccin induces in plant cells a programmed cell death showing apoptotic features. Protoplasma 222:113–116
Malerba M, Crosti P, Cerana R, Bianchetti R (2004) Fusicoccin affects cytochrome c leakage and cytosolic 14–3-3 accumulation independent of H+-ATPase activation. Physiol Plant 120:386–394
Manfield IW, Orfila C, McCartney L, Harholt J, Bernal AJ, Scheller HV, Gilmartin PM, Mikkelsen JD, Knox JP, Willats WGT (2004) Novel cell wall architecture of isoxaben-habituated Arabidopsis suspension-cultured cells: global transcript profiling and cellular analysis. Plant J 40:260–275
Navarre DA, Wolpert TJ (1999) Victorin induction of an apoptotic/senescence-like response in oats. Plant Cell 11:237–249
Ning SB, Wang L, Li ZY, Jin WW, Song YC (2001) Apoptotic cell death and cellular surface negative charge increase in maize roots exposed to cytotoxic stresses. Ann Botany 87:575–583
Pasqualini S, Piccioni C, Reale L, Ederli L, Della Torre G, Ferranti F (2003) Ozone-induced cell death in tobacco cultivar Bel W3 plants. The role of programmed cell death in lesion formation. Plant Physiol 133:1122–1134
Passardi F, Penel C, Dunand C (2004) Performing the paradoxical: how plant peroxidases modify the cell wall. Trends Plant Sci 9:534–540
Pennell RI, Lamb C (1997) Programmed cell death in plants. Plant Cell 9:1157–1168
Rao MV, Davis KR (1999) Ozone-induced cell death occurs via two distinct mechanisms in Arabidopsis: the role of salicylic acid. Plant J 17:603–614
Robert S, Mouille G, Höfte H (2004) The mechanism and regulation of cellulose synthesis in primary walls: lessons from cellulose-deficient Arabidopsis mutants. Cellulose 11:351–364
Roberts MR, Bowles DJ (1999) Fusicoccin, 14–3-3 proteins, and defense responses in tomato plants. Plant Physiol 119:1243–1250
Rohde A, Morreel K, Ralph J, Goeminne G, Hostyn V, De Rycke R, Kushnir S, Van Doorsselaere J, Joseleau JP, Vuylsteke M, Van Driessche G, Van Beeumen J, Messens E, Boerjan W (2004) Molecular phenotyping of the pal1 and pal2 mutants of Arabidopsis thaliana reveals far-reaching consequences on phenylpropanoid, amino acid, and carbohydrate metabolism. Plant Cell 16:2749–2771
Sasabe M, Takeuchi K, Kamoun S, Ichinose Y, Govers F, Toyoda K, Shiraishi T, Yamada T (2000) Independent pathways leading to apoptotic cell death, oxidative burst and defense gene expression in response to elicitin in tobacco cell suspension culture. Eur J Biochem 267:5005–5013
Scheible WR, Fry B, Kochevenko A, Schindelasch D, Zimmerli L, Somerville S, Loria R, Somerville CR (2003) An Arabidopsis mutant resistant to thaxtomin A, a cellulose synthesis inhibitor from Streptomyces species. Plant Cell 15:1781–1794
Solomon M, Belenghi B, Delledonne M, Menachem E, Levine A (1999) The involvement of cysteine proteases and protease inhibitor genes in the regulation of programmed cell death in plants. Plant Cell 11:431–443
Sugimoto K, Himmelspach R, Williamson RE, Wasteneys GO (2003) Mutation or drug-dependent microtubule disruption causes radial swelling without altering parallel cellulose microfibril deposition in Arabidopsis root cells. Plant Cell 15:1414–1429
Swidzinski JA, Sweetlove LJ, Leaver CJ (2002) A custom microarray analysis of gene expression during programmed cell death in Arabidopsis thaliana. Plant J 30:431–446
Vacca RA, de Pinto MC, Valenti D, Passarella S, Marra E, De Gara L (2004) Production of reactive oxygen species, alteration of cytosolic ascorbate peroxidase, and impairment of mitochondrial metabolism are early events in heat shock-induced programmed cell death in tobacco Bright-Yellow 2 cells. Plant Physiol 134:1100–1112
Wang H, Li J, Bostock RM, Gilchrist DG (1996) Apoptosis: a functional paradigm for programmed plant cell death induced by a host-selective phytotoxin and invoked during development. Plant Cell 8:375–391
Wasteneys GO (2004) Progress in understanding the role of microtubules in plant cells. Curr Opin Plant Biol 7:651–660
Yao N, Eisfelder BJ, Marvin J, Greenberg JT (2004) The mitochondrion—an organelle commonly involved in programmed cell death in Arabidopsis thaliana. Plant J 40:596–610
Yu XH, Perdue TD, Heimer YM, Jones AM (2002) Mitochondrial involvement in tracheary element programmed cell death. Cell Death Differ 9:189–198
Zhang S, Du H, Klessig DF (1998) Activation of the tobacco SIP kinase by both a cell wall-derived carbohydrate elicitor and purified proteinaceous elicitins from Phytophthora spp. Plant Cell 10:435–449
Zhang C, Czymmek KJ, Shapiro AD (2003) Nitric oxide does not trigger early programmed cell death events but may contribute to cell-to-cell signaling governing progression of the Arabidopsis hypersensitive response. Mol Plant Microbe Interact 16:962–972
Zuppini A, Baldan B, Millioni R, Favaron F, Navazio L, Mariani P (2004) Chitosan induces Ca2+-mediated programmed cell death in soybean cells. New Phytol 161:557–568
Acknowledgements
Financial support was provided by NSERC as individual grants to N.B. and C.B., and by FQRNT and Université de Sherbrooke to N.B. We also wish to thank K. Bouarab for the Arabidopsis suspension cultures, O. Domingue for initial characterization of the effects of thaxtomin A, A. Djoumad for help with DNA gel electrophoresis and northern blot analyses, and G. Grondin for help with microscopy work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Duval, I., Brochu, V., Simard, M. et al. Thaxtomin A induces programmed cell death in Arabidopsis thaliana suspension-cultured cells. Planta 222, 820–831 (2005). https://doi.org/10.1007/s00425-005-0016-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-005-0016-z