Abstract
Protein disulfide isomerase (PDI) is a multifunctional polypeptide presents in the endoplasmic reticulum of the cell. Silkworm (Bombyx mori) pupae were used as hosts to produce recombinant PDI (rPDI). The concentration-dependent chaperone activity of rPDI was evidenced by the inhibition of the aggregation of rhodanese. Approximately 297 μg rPDI was purified from a single silkworm pupa. Results of rPDI treated with endoglycosidase H and N-glycanase, PNGase F, indicate that non-N-glycosylated rPDI (occupying 90%) and N-glycosylated rPDI are expressed in the silkworm expression system. The difference in glycosylation between silkworm pupae and yeast is discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Protein disulfide isomerase (PDI), which is present in the endoplasmic reticulum of yeast, cattle, rats, humans, etc (Mizunaga et al. 1990), catalyzes the formation, reduction, and isomerization of disulfide bonds in vitro (Hillson et al. 1984) and facilitates the folding of disulfide-bonded proteins in vivo (Shusta et al. 1998). PDI, which promotes in vitro folding, can be used to improve the expression of foreign genes. In fact, in a coexpression system, PDI exhibits chaperone activity wherein it suppresses the aggregation and increases the yield of heterologous proteins (Gilbert 1990; Hayano et al. 1995). Furthermore, as an ideal fusion partner in the expression system of Escherichia coli and Bacillus brevis (Kajino et al. 2000; Liu et al. 2005), PDI facilitates the folding process of nascent peptides to prevent the unintended intermolecular association of hydrophobic domains because of its role as a molecular chaperone.
In this study, we report the cloning and expression of the yPDI gene in the silkworm expression system. The molecular size of yPDI was estimated by SDS-PAGE to be 70 kDa, which changed to 60 kDa via endoglycosidase H (Endo H) treatment. This indicates that yPDI is N-glycosylated and that the sugar moiety accounts for about 10 kDa for the yPDI subunit (Mizunaga et al. 1990). yPDI exhibits twice as much isomerase activity as human PDI (hPDI) and has 4-fold the chaperone activity of hPDI (Kimura et al. 2004).
Lepidopteran insect cells are widely used as hosts for the expression of heterologous protein by infection with recombinant baculoviruses. A large variety of mammalian proteins have been expressed in this system, such as porcine lactoferrin (Wang et al. 2005), bovine interferon-τ (Nagaya et al. 2004), and human IL-17B (Li et al. 2000). However, the expression of heterologous protein derived from microorganisms, such as yeast has not yet been reported. In the silkworm expression system, the expressed polypeptide can be expected to undergo appropriate post-translational modifications such as acetylation, phosphorylation, and glycosylation. Therefore, compared with the prokaryotic expression system, use of the silkworm expression system can produce proteins with high biological activity (Yu et al. 2006). Furthermore, the B. mori nuclear polyhedrosis virus (BmNPV) does not transmit to humans (Yu et al. 2006). These features make the silkworm expression system an ideal expression system and delivery package for producing oral medicinal proteins.
Materials and methods
Construction of the recombinant transfer vector and recombinant baculovirus
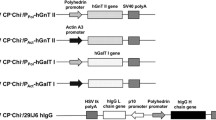
To clone the PDI gene, we used plasmid pMTE6 derived from pMTY 17 (Tachikawa et al. 1991) and provided by Professor T. Mizunaga, as a template DNA. The primers for PCR amplification were designed as follows: forward primer 5′-TATCCCGTTATGAAGTTTTCTGCT-3′ and reverse primer 5′-CTCTTTCCCTAAGGCGTTCG-3′ The reaction was run for 30 cycles (denaturing at 94°C for 45 s, annealing at 55°C for 45 s, and extension at 72°C for 2 min) with Pfu DNA polymerase (Fermentas UAB, Vilnius, Lithuania). The PCR product was inserted in the SmaI (Takara Bio, Tokyo, Japan) site of pBluescript II KS vector (Toyobo, Osaka, Japan). The recombinant plasmid DNA obtained was named pBS-PDI. Subsequently, we treated pBS-PDI and pET16b (Takara Bio) with XhoI and BamHI (Takara Bio), respectively, and subcloned the PDI DNA located downstream of the His-tag coding region of pET16b. The recombinant plasmid DNA obtained was named pET16b-PDI. The His-tagged PDI DNA was cut from pET16b-PDI by digestion with XbaI (Takara Bio) and BamHI (Takara Bio) and then treated with T4 polymerase (Takara Bio). Next, the His-tagged PDI fragment was inserted into the SmaI (Takara Bio) site of the baculovirus transfer vector pBM31 provided by Professor S. Matsumoto (Riken, Saitama, Japan). The recombinant vector obtained was named pBM31-PDI (Fig. 1a).
(a) Construction of baculovirus transfer vector. The diagram shows the genetic features of the recombinant pBM31-PDI DNA encoding the His-tagged PDI gene under the control of the high-level expression polyhedron promoter BmNPV. (b) Inoculation of silkworm pupae with recombinant virus. (c) Eighty-four hours after infection, silkworm pupae inoculated with transformation virus BAMM-PDI showed a significant transgenic characteristic; they changed to black and became weak
To generate the recombinant baculovirus, plasmid pBM31-PDI treated with Bsu36I (Promega) was cotransfected with baculovirus BAMM, provided by Professor S. Matsumoto, by using lipofectin reagent (Invitrogen). The recombinants were subsequently purified through plaque assay. Finally, high titer virus stocks were prepared and named BAMM-PDI.
Expression of rPDI in BmN cells and silkworm pupae
BmN cells (Riken) (4 × 106 cells/60 mm flask) were infected with the recombinant virus at a medium dose (MOI = 5) in TC-100 medium (Funakoshi, Tokyo) supplied with 10% (v/w) fetal bovine serum (Irvine, Santa Ana, CA, USA) using wild-type virus infection as control. The infected cells were cultured at 27°C and collected 72 h after infection. Subsequently, the cells were resuspended in sodium phosphate buffer (50 mM NaPO4, 50 mM NaCl, and 20 mM EDTA; pH 7.5), and then lysed by repeated freezing and triturating with a Multi-beads shocker (Yasui Kikai, Osaka). The samples were stored at −80°C for further analysis after trituration and centrifugation.
After confirming the expression in cultured cells, the silkworm pupae (just after pupation) were used as hosts for rPDI production. The recombinant viral solution was needle inoculated into the body cavity of the pupae (Fig. 1b) and incubated for 72 to 96 h at 27°C; wild virus infection was used as control. The infected pupae were collected between 72 and 96 h after infection, frozen by liquid nitrogen, and triturated using a Multi-beads shocker (Yasui Kikai). The samples were stored at −80°C until further analysis.
Purification of the expressed rPDI
Eighty-four hours after infection, the pupae were ground and suspended in sodium phosphate buffer (50 mM NaPO4, 50 mM NaCl, and 20 mM EDTA; pH 7.5) supplied with protease inhibitor cocktail (Complete Mini; Roche, Mannheim, Germany). The desalting the crude protein extraction was performed with an Econo-Pac 10DG column (Bio-Rad). Because the rPDI carried 10 × His-tag at the N-terminal, they could be easily purified using a TALON metal affinity resins column (Clontech Takara Bio, Kyoto) under native conditions. The desalted PDI fractions were applied to the TALON metal affinity resins column, which were equilibrated with an equilibration/wash buffer (50 mM NaPO4 and 300 mM NaCl; pH 7.5) for binding. This purification procedure was performed twice. To further purify the expressed rPDI, we used a Bio-gel P column (Bio-Rad) to eliminate non-specific protein. The recombinant protein was finally eluted by native elution buffer (50 mM NaPO4, 300 mM NaCl, and 150 mM imidazole; pH 7.0).
SDS-PAGE and Western blot analysis
SDS-PAGE followed by Western blot analysis was performed to detect the rPDI. The cell-lysed supernatant fluid or pupae protein samples were electrophoresed in a 7.5% slab gel. For Western blot analysis, proteins were transferred onto a PVDF membrane. After blocking in TTBS buffer (20 mM Tris-buffered saline, pH 7.4; 0.5 M NaCl and 10% Tween 20), the membrane was incubated in a 1:1000 yPDI antibody solution (provided by Professor T. Mizunaga) for 2 h at room temperature. The membrane was washed and then incubated in 1:3000 GAR-AP (Bio-Rad) for 1 h at room temperature. The bound antibody was detected with an AP Conjugate substrate kit (Bio-Rad).
Treatment of rPDI with Endo H and PNGase F
For release of asparagine N-acetylglucosamine-linked carbohydrate moieties, rPDI was denatured by boiling in a 10 μl assay buffer (250 mM NaHPO4; pH 5.5) and a 2 μl denaturation buffer (2% SDS, 1 M β-mercaptoethanol) for 3 min. Subsequently, 2 μl Endo H (Calbiochem, San Diego, CA) was added to the reaction, and the mixture stood overnight at 37°C. rPDI was also treated with PNGase F (New England Biolabs, Beverly, MA, USA) using the reagents supplied with this enzyme. The digests were subjected to electrophoresis as described above.
Assay of rPDI chaperone activity
The chaperone activity of preventing denatured rhodanese aggregation was performed according to the method of Martin et al. (1991). The aggregation of denatured rhodanese was investigated by monitoring the increase in absorbance at 320 nm. In this experiment, bovine PDI was used as positive control.
Results and discussion
Construction of recombinant baculovirus BAMM-PDI and expression in BmN cells and silkworm pupae
Recombinant pBM31-PDI (Fig. 1a) was identified by PCR and DNA sequencing using an ABI Prism 3100-Avant sequencer (Applied Biosystems, Tokyo), indicating that PDI fusion gene was inserted into the transfer vector pBM31 correctly under the control of a powerful polyhedron promoter derived from polyhedron genes of BmNPV. Recombinant baculovirus BAMM-PDI was purified by plaque assay and identified by PCR. To confirm the expression of rPDI, BmN cells were infected with BAMM-PDI. Seventy-two hours after infection, the cells were collected by centrifugation. Results of SDS-PAGE and Western blot suggested that rPDI was expressed by BmN cells. After infection with this recombinant virus, the silkworm pupae presented typical symptoms of BmNPV infection (Fig. 1c). Time-course analysis was conducted to assess the expression of PDI in the infected pupae, and maximum expression was noticed approximately 84 h after infection. rPDI was purified twice using the TALON metal affinity resins column (Fig. 2; lanes 3 and 4) and the Bio-gel P-100 column (Fig. 2; lane 5). A clear band of approximately 60 kDa was observed, which suggested that rPDI was well expressed and purified. 297 μg rPDI was purified from a single silkworm pupa. However, the duration from the construction of recombinant virus to the production of rPDI from silkworm pupae was only 1 month. This method, established in our study, will pave the way for efficient industrial production of rPDI on a large scale in a short period of time.
Expression, purification, and identification of rPDI. The post-infected pupae’s protein was dissolved by SDS-PAGE on 7.5% gel and subsequently visualized by staining with Coomassie blue. Lane 1, molecular marker; lane 2, crude extraction after desalting; lanes 3 and 4, first and second purification of the crude rPDI extraction by the Talon metal affinity resins column; and lane 5, purified rPDI
Comparison of protein glycosylation in silkworm pupae and yeast
As shown in Fig. 3, the molecular size of rPDI was estimated by Western blot to be 70 and 60 kDa (lane 2). This indicates that two types of rPDI were expressed from silkworm pupae; 10% of the expressed rPDI changed to 60 kDa via endoglycosidase H (Endo H) treatment. It suggests that the 10% of rPDI carried oligomannosidic-type or hybrid-type N-linked glycans (Fig. 3; lane 2, a faint band indicated by a black arrow). This result is similarly to that seen with yPDI. yPDI is known as a high mannosidic-type glycoprotein, and the glycans of yPDI are released after treatment with Endo H (Mizunaga et al. 1991) (Fig. 3; lanes 5 and 6). The fact that more than 90% of the expressed rPDI (Fig. 3; lane 2, a sharp band indicated by a white arrow) did not change after treatment with Endo H and PNGase F (Fig. 3; lanes 3 and 4) suggested that 90% of the expressed rPDI did not contain N-linked glycans. Most mammalian recombinant glycoproteins produced by the baculovirus-mediated silkworm expression system are produced with mannose-terminated oligosaccharides or otherwise less-complete compared with the native protein produced in the original tissue (Luckow. 1995; März et al. 1995). Interestingly, complex-type glycans have thus far been reported to occur only on recombinant human plasminogen produced in Lepidopteran insect cell lines (Davidson et al. 1990, 1991, 1991a, 1991b) and are secreted in alkaline phosphatase (SEAP) produced in insect larvae (Davis and Wood. 1995). However, the results of our study indicate that heterologous proteins, derived from microorganisms such as yeast, expressed in the silkworm expression system mostly did not undergo N-terminal glycosylation and were produced as non-N-glycosylated proteins. We suggest that the difference in molecular properties of proteins between mammalian and microorganism systems are responsible for the differences in glycosylation when expressed in the silkworm expression system.
Chaperone activity of rPDI
Generally, protein aggregation is a non-productive and off-pathway reaction, and chaperones such as GroEL-GroES interact with folding intermediates and prevent or minimize aggregation, which increases the refolding yield (Winter et al. 2002). Many reports have stated that PDI influences the refolding of denatured and reduced proteins with multiple disulfide bonds as an isomerase and a chaperone (Winter et al. 2002). In our study, we used rhodanese as a substrate, which contains no disulfide bonds, to examine the chaperone activity of rPDI, and bovine PDI was used as positive control. Inhibition of the aggregation of rhodanese is shown in Fig. 4 (triangle and square), which indicates that rPDI has a concentration-dependent chaperone activity. Furthermore, we confirmed that the chaperone activity of rPDI is approximately 2.5-fold higher than that of bovine PDI (Fig. 4; triangle and circle). These results indicate that the lack of N-glycosylation does not influence the chaperone activity of rPDI.
References
Davidson DJ, Castellino FJ (1991a) Asparagine-linked oligosaccharide processing in lepidopteran insect cells temporal dependence of the nature of the oligosaccharides assembled on asparagine-289 of recombinant human plasminogen produced in baculovirus vector infected Spodoptera frugiperda (IPLB-SF-21AE) cells. Biochemistry 30:6167–6174
Davidson DJ, Castellino FJ (1991b) Structures of the asparagine-289-linked oligosaccharides assembled on recombinant human plasminogen expressed in a Mamestra brassicae cell line (IZD-MB0503). Biochemistry 30:6689–6696
Davidson DJ, Fraser MJ, Castellino FJ (1990) Oligosaccharide processing in the expression of human plasminogen cDNA by lepidopteran insect (Spodoptera frugiperda) cells. Biochemistry 29:5584–5590
Davidson DJ, Bretthauer RK, Castellino FJ (1991) [alpha]-Mannosidase-catalyzed trimming of high-mannose glycans in noninfected and baculovirus-infected Spodoptera frugiperda cells (IPLB-SF-21AE). A possible contributing regulatory mechanism for assembly of complex-type oligosaccharides in infected cells. Biochemistry 30:9811–9815
Davis TR, Wood HA (1995) Intrinsic glycosylation potentials of insect cell cultures and insect larvae. In Vitro Cell Dev Biol 31:659–663
Gilbert HF (1990) Protein disulfide isomerase and assisted protein folding. J Biol Chem 272: 505–511
Hayano T, Hirose M, Kikuchi M (1995) Protein disulfide isomerase mutant lacking isomerase activity accelerates protein folding in the cell. FEBS Lett 377:505–511
Hillson DA, Lambert N, Freedman RB (1984) Formation and isomerization of disulfide bonds in proteins: protein disulfide-isomerase. Methods Enzymol 48:487–492
Kajino T, Ohto C, Muramatsu M, Obata S, Udaka S, Yamada Y, Takahashi H (2000) A protein disulfide isomerase gene fusion expression system that increase the extracellular productivity of Bacillus brevis. Appl Environ Microbiol Feb. 638–642
Kimura T, Hosoda Y, Kitamura Y, Nakamura H, Horibe T, Kikuchi M (2004) Functional difference between human and yeast protein disulfide isomerase family protein. Biochem Biophys Res Commun 320:359–365
Li H, Chen J, Huang A, Stinson J, Heldens S, Foster J, Dowd P, Gurney LA, Wood IW (2000) Cloning and characterization of IL-17B and IL-17C, two new members of the IL-17 cytokine family. Pnas 97:773–778
Liu Y, Zhao T, Yan Y, Zhou H (2005) Increase of soluble expression in Escherichia coli cytoplasm by a protein disulfide isomerase gene fusion system. Protein Expr Purif 44:155–161
Luckow VA (1995) Protein production and processing from baculovirus expression vectors. In: Shuler ML, Wood HA, Granados RR, Hammer DA (eds) Baculovirus expression systems and biopesticides. John Wiley & Sons, New York, pp 51–90
Martin J, Langer T, Boteva R, Schramel A, Horwich AL, Hartl F-U (1991) Chaperon-mediated protein folding at the surface of groEL through a ‘molten globule’-like intermediate. Nature 352(6330):36–42
März L, Altmann F, Staudacher E, Kubelka V (1995) Protein glycosylation in insects. In: Montreuil J, Vliegenthart JFG, Schachter H (eds) Glycoproteins. Elsevier, Amsterdam, pp 543–563
Mizunaga T, Katakura Y, Miura T, Maruyama Y (1990) Purification and characterization of yeast protein disulfide isomerase. J Biochem 108:846–851
Nagaya H, Kanaya T, Kaki H, Tobiya Y, Takahashi M, Takahashi H, Yokomizo Y, Inumaru S (2004) Establishment of a large-scale purification procedure for purified recombinant bovine interferon-τ produced by a silkworm-baculovirus gene expression system. J Vet Med Sci 66(11):1395–1401
Shusta EV, Rains RT, Pluckthum A, Wittrup KD (1998) Increasing the secretory capacity of Saccharomyces cerevisiae for the production of single-chain antibody fragment. Nat Biotechnol 42:358–363
Tachikawa H, Miura T, Katakura Y, Mizunaga T (1991) Molecular structure of a yeast gene, PDI1, encoding protein disulfide isomerase that is essential for cell growth. J Biochem 110:306–313
Wang Y, Wu X, Liu G, Cao C, Huang H, Xu Z, Liu J (2005) Expression of porcine lactorerrin by using recombinant baculovirus in silkworm, Bombyx mori L., and its purification and characterization. Appl Microbiol Biotechnol 69(4):385–389
Winter J, Klappa P, Freedman RB, Lilie H, Rudolph R (2002) Catalytic activity and chaperone function of human protein-disulfide isomerase are required for the efficient refolding of proinsulin. J Biol Chem 277:310–317
Yu W, Chen J, Zhao X, Lv Z, Nie Z, Zhang Y (2006) Expression of polyhedron-hEGF fusion protein in cultured cells and larvae of Bombyx mori. Afr J Biotechnol 5(11):1034–1040
Acknowledgments
We would like to thank the members of Molecular Entomology Laboratory, Discovery Research Institute, Riken for their valuable support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, L., Shimizu, Y., Mizunaga, T. et al. Expression, purification and characterization of yeast protein disulfide isomerase produced by a recombinant baculovirus-mediated silkworm, Bombyx mori, pupae expression system. Biotechnol Lett 30, 625–630 (2008). https://doi.org/10.1007/s10529-007-9582-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-007-9582-4