Abstract
Saccharomyces cerevisiae grows very poorly in dilute acid lignocellulosic hydrolyzate during the anaerobic fermentation for fuel ethanol production. However, yeast cells grown aerobically on the hydrolyzate have increased tolerance for the hydrolyzate. Cultivation of yeast on part of the hydrolyzate has therefore the potential of enabling increased ethanol productivity in the fermentation of the hydrolyzate. To evaluate the ability of the yeast to grow in the hydrolyzate, fed-batch cultivations were run using the ethanol concentration as input variable to control the feed-rate. The yeast then grew in an undetoxified hydrolyzate with a specific growth rate of 0.19 h−1 by controlling the ethanol concentration at a low level during the cultivation. However, the biomass yield was lower for the cultivation on hydrolyzate compared to synthetic media: with an ethanol set-point of 0.25 g/l the yield was 0.46 g/g on the hydrolyzate, compared to 0.52 g/g for synthetic media. The main reason for the difference was not the ethanol production per se, but a significant production of glycerol at a high specific growth rate. The glycerol production may be attributed to an insufficient respiratory capacity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The yeast Saccharomyces cerevisiae gives a high ethanol yield and high specific ethanol productivity from sugars such as glucose and mannose, and is among the most tolerant microorganisms to various inhibitors found in hydrolyzates of lignocellulosic materials (Olsson and Hahn-Hagerdal 1993). S. cerevisiae is therefore a preferred organism for fuel ethanol production based on lignocellulose. Even though it is one of the most tolerant microorganisms, it grows very poorly while fermenting lignocellulosic hydrolyzates (Nilsson et al. 2001). It has, however, been shown that propagation of yeast on hydrolyzate confers increased resistance to the inhibitors present in the hydrolyzate (Alkasrawi et al. 2006). It will therefore be of considerable advantage to produce yeast from lignocellulose hydrolyzates, a carbon source which will be locally available at the fuel ethanol plant. Cultivation of yeast should preferably be performed with low ethanol formation in order to get a high biomass yield. Even though ethanol is the desired product in the fermentation of lignocellulose hydrolyzate formation of it should be limited during cultivation since it will not be economically feasible to extract the ethanol from the cultivation broth. The cells, on the other hand, can be allowed to settled and thereby easily separated from the broth and used for the fermentation.
In the sugar cane-based ethanol industry of today, the yeast used in the fermentation is normally reused—either by a simple batch-wise reuse of settled yeast, or in continuous processes with cell recycle (Wheals et al. 1999). The need for fresh supply of yeast is therefore limited. The lignocellulosic material may however also contain fibers, which makes cell recycling difficult or even impossible. The latter case applies to Simultaneous Saccharification and Fermentation (SSF) processes, in which the enzymatic hydrolysis of the cellulose takes place simultaneous with the fermentation (Wright 1988). After finished fermentation solids, in terms of both lignin and yeast, are present in the liquid, making re-use of the yeast very difficult. In SSF, yeast will thus have to be freshly supplied to each batch fermentation.
Aerobic fed-batch processes have traditionally been used for industrial production of baker’s yeast (Rose 1979). The well-known reason for this is that the Crabtree effect exhibited by S. cerevisiae can be avoided if the feed-rate is controlled in a proper way. By controlling the feed rate of the carbon source, such that a critical specific uptake rate of sugar is not exceeded, carbon flux diversion into ethanol formation can be avoided and thereby a high biomass yield can be ensured (Sonnleitner and Kappeli 1986). A second problem is O2 transfer limitation, which is likely to occur at a late stage of the fed-batch process. The maximum allowable feed rate will at this stage be determined by the O2 transfer capacity of the reactor.
One principle which has been used for aerobic fed-batch control of S. cerevisiae with high yield is that of maintaining a low and constant ethanol concentration in the broth (Axelsson et al. 1988; Noronha et al. 1999; Cannizzaro et al. 2004). If the flow rate is lower than the maximum respiratory sugar uptake, ethanol will be consumed, signalling that the flow rate can be increased. On the other hand, if the concentration of the fermentable sugar is too high or if O2 is lacking, ethanol would start to accumulate in the reactor. Maintaining a constant ethanol concentration will therefore ensure an optimal feed-rate near the critical feed-rate. The principle has been shown for yeast cultivation on molasses (Axelsson et al. 1988, 1984), where a simplified model of the dynamics from the feed rate to the ethanol concentration was derived by mass balances and used as a basis for the developed of the PID regulator. The regulator was subsequently used in cultivation of S. cerevisiae from molasses with a set-point concentration of 0.4 g ethanol/l
A different kind of problem arises in cultivations where lignocellulosic hydrolyzate is the carbon source compared to a untoxic carbon source. In the case of cultivation on hydrolyzate not only overflow metabolism, but also inhibition effects, may cause ethanol formation and possible also stopped growth by a different mechanism than glucose repression. Effects of the inhibitors present in hydrolyzates on S. cerevisiae have mainly been characterized under anaerobic conditions. However, some results suggest that the inhibition effects on S. cerevisiae are larger during aerobic conditions than during anaerobic conditions (Horvath et al. 2003). In the same study, a high furfural conversion rate resulted in respiro-fermentative metabolism well below the critical dilution rate and a consequent decrease in biomass yield. It thus appears plausible that a changed metabolism from purely respiratory to respiro-fermentative is an early indication of inhibition effects in furan containing media, such as hydrolyzates.
Provided that a switch from purely respiratory to respiro-fermentative metabolism is indeed a first indication of inhibition, the same control principle as described above may in fact be used to find the maximum allowable substrate addition rate for aerobic cultivation directly on hydrolyzate without any detoxification of the medium. With an ethanol sensor, either in the liquid or in the effluent gas stream, the feed rate of substrate may be controlled using a suitably designed controller. The objective of the present study was to investigate the effectiveness of fed-batch cultivation of Saccharomyces cerevisiae on a lignocellulose hydrolyzate controlled at a constant ethanol concentration. A robust strain of S. cerevisiae was grown on a dilute-acid hydrolyzate at different ethanol concentration set-points and the biomass yield as well as specific growth rate was compared to corresponding cultivations using a fully synthetic glucose medium.
Material and methods
Cultivation procedures
Wood material and hydrolysis
The hydrolyzate used was produced from forest residues originating mainly from spruce. A two-stage dilute-acid hydrolysis process was used to hydrolyze the material. Hydrolysis of the material was carried out as described elsewhere (Nilsson et al. 2001), and the composition of the hydrolyzate is given in Table 1. The hydrolyzate was stored at 8°C until used.
Yeast strain and medium
Saccharomyces cerevisiae TMB 3000 (ATCC 96581), obtained from Department of Applied Microbiology (Lund Institute of Technology, Lund, Sweden) (Linden et al. 1992) was used in the experiments. The strain was maintained on agar plates made from yeast extract 10 g/l, soy peptone 20 g/l and agar 20 g/l with d-glucose 20 g/l as additional carbon source. Inoculum cultures were grown in 300 ml cotton-plugged-conical flask on a rotary shaker at 30°C for 24 h. The liquid volume was 100 ml and the shaker speed 150 rpm. The growth medium was a defined medium according to (Taherzadeh et al. 1996) with the exception of the glucose concentration, which here was 15 g/l.
Cultivation conditions
Cultivations were made in a 3.3 l BioFlo III bioreactor (New Brunswick Scientific, Edison, NJ, USA) at 30°C and stirring at of 700 rpm. The pH value in the medium was controlled at 5.0 by addition of 2 M NaOH. The reactor was initially filled with 1 l of defined medium according to (Taherzadeh et al. 1996), containing 10 g glucose. However, the concentrations of mineral salts and vitamins were four times as high as in the reference medium to compensate for dilution and a different sugar concentration in the current experiments. At the start of each experiment, 20 ml inoculum culture was added to the reactor. The flow rate of air was constant throughout the experiments, and was set a rate corresponding to 1 vvm for the initial volume. After almost complete depletion of the ethanol (<0.1 g/l) during the diauxic batch growth the feeding—of either synthetic media containing 35 g glucose/l or hydrolyzate—was started. The feed was turned off when 1500 ml had been added.
On-line measurements and control
The software used for data acquisition and feed-rate control was developed locally in Visual Basic. Analog signals from on-line measurements were translated to digital signals using a 12-bit A/D board. Feed rate control, according to a developed model presented below, was obtained by manipulating the speed of a peristaltic pump (Watson-Marlow Alitea AB, Sweden). The medium bottle was placed on a balance connected to the computer to allow measurement of the amount of added hydrolyzate or synthetic media.
Cultivations were performed at two different ethanol set-points. For an ethanol set-point of 0.5 g/l, 75 ml of synthetic medium (or hydrolyzate) was added at the start of the fed-batch phase in order to obtain a concentration of ethanol close to the desired set-point. For the cultivations at a set-point of 0.25 g/l, 37.5 ml of synthetic medium (or hydrolyzate) was added. The feed-rate was controlled around a feed-profile corresponding to a specific growth rate of 0.2 h−1. Open-loop fed-batch cultivations at a feed-rate corresponding to a specific growth rate of 0.1 h−1 were also performed, both with synthetic media containing 35 g/l glucose and with hydrolyzate.
Analytical methods
Biomass concentration
Cell dry weight was determined from duplicate 10-ml samples taken five times for each cultivation. The samples were centrifuged, washed with distilled water and dried for 24 h at 105°C.
Gas analysis
CO2, O2 and ethanol content of the outlet gas were continuously measured with an acoustic gas monitor (model 1311, Innova, Denmark) described in (Christensen et al. 1995). Gas measurement signals were averaged for 30 s. The gas analyzer was equipped with three channels, one for oxygen, one for CO2 and a third channel for analysis of hydrocarbons (at a wave length of 3.4 μm). The third channel was in the present work used for on-line estimation of ethanol concentration in the medium. Calibration for O2 and carbon dioxide was done using a gas containing 20% of O2 and 5% CO2 The ethanol signal was calibrated against known ethanol broth concentrations as determined by HPLC.
Metabolite analysis
Samples for HPLC-analysis were withdrawn from the reactor, centrifuged and filtered through 0.2 μm filters. The concentrations of glucose, xylose, galactose, mannose and arabinose were determined using a polymer column (Aminex HPX-87P, Bio-Rad, USA) at 85°C. The concentrations of ethanol, glycerol, acetic acid, furfural, 5-hydroxymethyl furfural (HMF) were determined on an Aminex HPX-87H column (Bio-Rad, USA) at 65°C. All compounds were quantified using a refractive index (RI) detector, except for furfural and HMF which were quantified using a UV detector at 210 nm.
Results
Parameters for the PI-controller
The PI-controller can be described by the following equation:
where F is the feed rate and e is the differences between the set-point value and the online measured value of the ethanol concentration. The parameters for the controller were initially set at levels estimated to be reasonable and thereafter tuned to further improve the control. The parameter values of K (gain constant) and T i (integral time constant) finally used were 3 × 10−5 m6 g−1 h−1 and 1.2 h, respectively.
Performance of the PI-controller
The cultivations were started by the addition of 75 or 37.5 ml synthetic medium (or hydrolyzate) for ethanol set-points of 0.25 g/l and 0.5 g/l, respectively. This initial addition resulted in some ethanol formation. After the ethanol concentration had reached a plateau value, the feeding was started using the tuned PI-controller that controlled the feed-rate around a base profile corresponding to a specific growth rate of 0.2 h−1. The initial feed-rate at the start of the fed-batch phase corresponded to an estimated consumption rate of hexose using the following equation:
The values used were: μ: 0.2 h−1 (specific growth rate), X: 3.33 g/l (biomass concentration), V : 1 l (volume), Y SX: 0.5 g/g (yield of biomass from hexoses), and S F: 35 g/l (hexose concentration in the feed).
The performance of the controller with respect to maintaining the ethanol concentration around the chosen set-point was found satisfactory (Fig. 1). The small off-set between the ethanol concentrations as measured by off-line HPLC analysis and the on-line measured value in the off-gas may be due to inaccuracies in the gas analyser caused by interferences with of other compounds present in the off-gas, e.g. acetaldehyde.
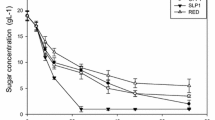
Measured amounts of products formed in cultivations with PI-control at a set-point of 0.5 g ethanol/l. The fed-batch phase was started after the ethanol concentration had decreased to less 0.1 g/l after the initial batch-phase (time = 0 h). Left: Fed-batch with synthetic media as feed. Right: Fed-batch with lignocellulose as feed. Upper row: feed-rate (right hand scale), ethanol concentration (♦ off-line, — on-line). Lower row: biomass (♦) and glycerol (▲) formed during the cultivations
The feed-rate reached a higher value for the cultivation with synthetic medium. The reason for this was both that the sugar concentration was slightly lower in the synthetic media (35 g/l) compared to the concentration of fermentable sugars (glucose, mannose and galactose) in the hydrolyzate (36.6 g/l), but also because the specific growth rate was higher in synthetic media compared to in hydrolyzate (Table 2). The level of dissolved O2 in the broth did not decrease below 10% of saturation at any time. The concentrations of the furan compounds furfural or HMF were below detection level at all times, indicating a complete conversion of the compounds.
Biomass productivity and yield
With the applied PI-controller, growth was observed during the entire feeding phase (Fig. 1), indicating that severe inhibition was avoided. The specific growth rates were lower for the hydrolyzate compared to synthetic media, but it was indeed possible to grow cells even on the undetoxified hydrolyzate at specific growth not far from the growth rate of 0.2 h−1 which was used as the feed profile (Table 2). Also the biomass yields were somewhat lower for cultivations on lignocellulose hydrolyzate than for the cultivations on synthetic medium (Table 2). The yield calculations on lignocellulose hydrolyzate are based on the added amounts of the fermentable sugars glucose, mannose and galactose. The cells were able to consume most of the fermentable sugars and no glucose and mannose were detected by the end of the feeding phase, but 10–15% of the added galactose was not consumed at that stage. It was furthermore concluded that the biomass yields decreased with increased set-point of ethanol (Table 2). Some ethanol will be formed even in a in a fed-batch culture with a constant ethanol concentration and this will cause carbon loss. The reasons for this are both the necessary compensation for evaporation of ethanol, and the compensation for the dilution caused by feeding. The evaporation effect will increase with a higher ethanol set-point. However, the decrease in biomass yield was not only due to this effect, but also to formation of glycerol, since unexpectedly high levels of glycerol were found in the PI-controlled cultivations (Fig. 1 and Table 2). Furthermore, the levels were higher when hydrolyzate was used as the growth media compared to synthetic for both set-points.
Discussion
This study demonstrates that it is indeed possible to achieve high biomass productivity on undetoxified lignocellulosic hydrolyzate in a closed-loop fed-batch process using ethanol as control variable. By maintaining a constant ethanol concentration throughout the fed-batch phase, growth near the critical specific growth rate could be obtained. Furthermore, it was possible to maintain constant ethanol concentrations throughout the cultivations despite the fact that the PI control parameters where maintained constant. The biomass yields obtained in the PI-controlled cultivations, however, were somewhat lower than to be hoped for, especially when hydrolyzate was used as growth medium.
The basis for the PI-controller used in this work has previously been demonstrated in cultivations of molasses (Axelsson et al. 1988). Unfortunately, the biomass yield was not reported and could not be calculated from the data given. Thus, the yield cannot be compared to the yields obtained in this study. A similar control strategy for production of baker’s yeast from synthetic media was applied in (Dairaku et al. 1981), in which biomass yields obtained were around 0.5 g biomass/g sugar. In that study, however, the ethanol set-points examined were in the range 150–200 ppm, i.e. lower than in the present study.
The biomass yield on hydrolyzate was, as to be expected, lower than on synthetic media, indicating that the inhibitors present in the hydrolyzate affected the cellular metabolism. A tentative reason for the lower biomass yields for growth on hydrolyzate compared to synthetic medium could be an increased maintenance requirements caused by inhibition compounds present in the hydrolyzate. Furfural, for example, at 2.25 g/l caused the non-specific ATP consumption to increase almost threefold, which resulted in a decrease of the biomass yield from 0.51 g/g (in the absence of furfural) to 0.43 g/g for the strain CBS8066 (Horvath et al. 2003). No difference in glycerol formation was found in this case. The furfural concentration in the hydrolyzate used in the present study, was only about 0.4 g/l, but most likely other similar compounds, such as HMF or other aldehydes, might give rise to increased maintenance requirements.
However, the prime explanation for the lower biomass yield found in the current study was a significantly higher glycerol production in hydrolyzate compared to in a synthetic medium. A high glycerol yield is normally to be expected during anaerobic conditions when glycerol is produced in order to generate NAD+ needed in anabolic reactions (Nordstrom 1966). In the presence of O2, NAD+ is generated in the respiratory chain and the need for glycerol formation is not required for redox reasons, but may instead be needed as an osmolyte (Siderius and Mager 2003). The enzymes enabling glycerol formation are also induced during other stress conditions, although this does not necessary leads to any net production of this compound (Hohmann 2002). It has previously been concluded that the oxidation of furfural to furoic acid during purely respirative growth of S. cerevisiae most likely is NAD+-coupled (Horvath et al. 2003), but the cofactor preference for HMF oxidation has to our knowledge not been reported. NADH is formed in anabolic reactions, and might also be formed in connection to the oxidation of furfural and HMF. We therefore hypothesize that glycerol formed during the PI-controlled cultivations on hydrolyzate is due to excess NADH formed both in the anabolic reactions and as a result of furan oxidation. This could explain why glycerol formation is lower for a synthetic medium, in which case NADH is only generated in the anabolic reactions, compared to growth on hydrolyzate where more NADH is formed due to furfural and (possibly also) HMF oxidation. At lower feed-rates no glycerol is formed in either a synthetic medium or hydrolyzate since the respiratory chain has sufficient capacity for NAD + regeneration.
In conclusion, we have shown that the yeast S. cerevisiae can indeed be grown on undetoxified hydrolyzate using a closed-loop control strategy of maintaining a constant ethanol concentration in a fed-batch cultivation. A high specific growth rate was obtained. However, this was accompanied by an a priori unexpected glycerol formation. The glycerol formation could be explained by an insufficient respiratory capacity in combination with oxidation of compounds present in the hydrolyzate.
References
Alkasrawi M, Rudolf A, Liden G, Zacchi G (2006) Influence of strain and cultivation procedure on the performance of simultaneous saccharification and fermentation of steam pretreated spruce. Enzyme Microbial Technol 38:279–286
Axelsson JP, Hagander P, Holst O, Mandenius CF, Mattiasson B (1984) Control of baker’s yeast production based on ethanol measurement. Third European Congress of Biotechnology, Munich vol 2, pp 629–634
Axelsson JP, Mandenius CF, Holst O, Hagander P, Mattiasson B (1988) Experience in using an ethanol sensor to control molasses feed-rates in bakers-yeast production. Bioprocess Eng 3:1–9
Cannizzaro C, Valentinotti S, von Stockar U (2004) Control of yeast fed-batch process through regulation of extracellular ethanol concentration. Bioprocess Biosyst Eng 26:377–383
Christensen LH, Schulze U, Nielsen J, Villadsen J (1995) Acoustic off-gas analyser for bioreactors: precision, aacuracy and dynamic detection. Chem Eng Sci 16:2601–2610
Dairaku K, Yamasaki Y, Kuki K, Shioya S, Takamatsu T (1981) Maximum production in a bakers-yeast fed-batch culture by a tubing method. Biotechnol Bioeng 23:2069–2081
Hohmann S. (2002) Osmotic stress signaling and osmoadaptation in Yeasts. Microbiol Mol Biol Rev 66:300
Horvath IS, Franzen CJ, Taherzadeh MJ, Niklasson C, Liden G (2003) Effects of furfural on the respiratory metabolism of Saccharomyces cerevisiae in glucose-limited chemostats. Appl Environ Microbiol 69:4076–4086
Linden T, Peetre J, Hahn-Hagerdal B (1992) Isolation and characterization of acetic acid-tolerant galactose-fermenting strains of Saccharomyces-Cerevisiae from a spent sulfite liquor fermentation plant. Appl Environ Microbiol 58:1661–1669
Nilsson A, Taherzadeh MJ, Liden G (2001) Use of dynamic step response for control of fed-batch conversion of lignocellulosic hydrolyzates to ethanol. J Biotechnol 89:41–53
Nordstrom K (1966) Yeast growth and glycerol formation. Acta Chem Scand 20:1016
Noronha SB, Wagner LW, Matheson NH, Shiloach J (1999) Use of an ethanol sensor for feedback control of growth and expression of TBV25H in Saccharomyces cerevisiae. Biotechnol Bioeng 63:285–289
Olsson L, Hahn-Hagerdal B (1993) Fermentative performance of bacteria and yeasts in lignocellulose hydrolysates. Process Biochem 28:249–257
Rose AH (1979) History and scientific basis of large-scale production of microbial biomass. Econ Microbiol 4:1–29
Siderius M, Mager WH (2003) Conditional response to stress in yeast. Monatshefte fur Chemie 134:1433–1444
Sonnleitner B, Kappeli O (1986) Growth of Saccharomyces cerevisiae is controlled by its limited respiratory capacity—formulation and verification of a hypothesis. Biotechnol Bioeng 28:927–937
Taherzadeh MJ, Liden G, Gustafsson L, Niklasson C (1996) The effects of pantothenate deficiency and acetate addition on anaerobic batch fermentation of glucose by Saccharomyces cerevisiae. Appl Microbiol Biotechnol 46:176–182
Wheals AE, Basso LC, Alves DMG, Amorim HV (1999) Fuel ethanol after 25 years. Trends Biotechnol 17:482–487
Wright JD (1988) Ethanol from biomass by enzymatic-hydrolysis. Chem Eng Progress 84:62–74
Acknowledgements
This work was financially supported by the Swedish National Energy Administration. Bernt Nilsson is gratefully acknowledged for valuable discussions during the course of this work. The authors are thankful for technical assistance by Yoseph Goldberg.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Petersson, A., Lidén, G. Fed-batch cultivation of Saccharomyces cerevisiae on lignocellulosic hydrolyzate. Biotechnol Lett 29, 219–225 (2007). https://doi.org/10.1007/s10529-006-9227-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-006-9227-z