Abstract
At high growth rates, the biomass yield of baker’s yeast (Saccharomyces cerevisiae) decreases due to the production of ethanol. For this reason, it is standard industrial practice to use a fed-batch process whereby the specific growth rate, μ, is fixed at a level below the point of ethanol production, i.e., μcrit. Optimally, growth should be maintained at μcrit, but in practice, this is difficult because μcrit is dependent upon strain and culture conditions. In this work, growth was maintained at a point just above μcrit by regulating ethanol concentration in the bioreactor. The models used for control design are shown, as are the experimental results obtained when this strategy was implemented. This technique should be applicable to all microorganisms that exhibit an “overflow” type metabolism.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In order to maximize biomass yield, Saccharomyces cerevisiae and Escherichia coli are often cultivated in a fed-batch manner such that overflow metabolites (ethanol and acetate, respectively) do not accumulate [1–3]. Overflow metabolite organisms are limited in their capacity to oxidize a given substrate. Below a certain critical specific growth rate, μcrit, the substrate will be completely oxidized at a high biomass to substrate yield, Yoxsx (Fig. 1). However, from μcrit to the maximum growth rate, μmax, the excess substrate will be converted reductively to an overflow metabolite at a low biomass to substrate yield, Yredsx.
Idealized case of biomass yield, Ysx, vs. specific growth rate μ for an overflow metabolite organism. The biomass yield is equal to the oxidative yield, Yoxsx until a critical specific growth rate μcrit is reached. Above this point, Ysx is a function of Yoxsx and Yredsx, the reductive yield. The critical growth rate is represented as an arrow within a grayed area since it is not a fixed parameter, but may vary with culture conditions. In the proposed control methodology, the desired biomass yield is specified just below Yoxsx (setpoint) such that a small quantity of metabolite is always formed through reductive metabolism. Overflow metabolite production is thought to occur due to a limitation or “bottleneck” in the metabolism. For case 1, there is excess capacity and no metabolites are formed (Reaction 1a). Case 2 represents growth at the critical point where any excess substrate will “overflow” to a metabolite. In case 3, the excess growth above the critical point is represented by an added metabolite flux (Reaction 1b)
If the objective is to maximize the biomass concentration starting from a sufficiently concentrated inoculum, it is necessary to grow the cells at a rate as close as possible to the critical one [4]. Yet, the critical growth rate may vary from batch to batch, and even during the experiment [2]. In order to avoid the risk of decreasing the yield, a conservative approach is commonly used, whereby the cells are grown at μconservative. An alternative is to use the overflow metabolite as an indicator of how close or far the actual growth rate is from μcrit. Thus, if ethanol production is maintained constant, it is possible to fix the growth rate at a value slightly above the critical growth rate.
In this work, a recently developed control methodology [4] was implemented to accomplish exactly this task by modeling the system as two independent reactions. The first one describes the conversion of substrate into overflow metabolite. It is assumed that this reaction is always present. However, this reaction must be limited by an appropriate control strategy. The second reaction describes the cell growth that can be considered as a disturbance for the first process, since it consumes the substrate that is otherwise used to produce the overflow metabolite. The optimum control strategy is to run the process with a very small, but constant, concentration of overflow metabolite such that a high percentage of substrate is converted to biomass at the fastest possible rate. The developed control algorithm utilizes the measured and desired metabolite concentrations to obtain the necessary feed rate to maintain exponential growth and constant metabolite concentration. This approach requires the knowledge of only two system parameters: the yield coefficient, Ysp, and the instantaneous concentration of the overflow metabolite. The yield determines how much overflow metabolite, P, is produced per unit of substrate fed, S, while the metabolite concentration is the controlled variable.
In order to implement the proposed control strategy, the overflow metabolite must be measured on-line. Fortunately, due to modern developments in this field, a wide selection of methods are available [9, 10], such as flow injection analysis [11], near infrared spectroscopy [12, 13], mid-infrared spectroscopy [14–16], HPLC [6], inexpensive gas sensors [8], biosensors [17, 7, 18, 19] and mass spectrometry [20].
Although not considered explicitly in this work, alternative methods to ascertain the metabolic state of a culture could be used in place of measuring the overflow metabolite concentration. For example, one common fed-batch control approach is to maintain the respiratory quotient, RQ, at a value indicative of saturated oxidative capacity [21, 22]. The control strategy can also be based upon a calorimetric quotient by substituting the oxygen uptake rate with the heat production rate [23–27]. Alternatively, when the bioreactor is sufficiently instrumented, on-line mass balances can be constructed, from which a control strategy can be formulated [28, 29]. However, note that for all of these approaches at least two measurements are required, versus only one when the overflow metabolite concentration is used.
2 Reaction system
2.1 Yeast as a model system
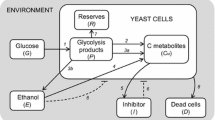
In order to be able to understand the processes that take place in a bioreactor, a macroscopic description of the interactions between the cells and the environment is needed. This basic description can be written in the same way as chemical reactions. Let S be the substrate fed into the reactor. Based on the overflow metabolite model for yeast proposed by Sonnleitner and Käppeli [11], in the presence of oxygen, this substrate is oxidized via the following reaction:
Reaction components are microorganisms, X, oxygen, O2, and carbon dioxide, CO2. If a limitation in the oxygen uptake rate exists, the substrate that cannot be oxidized is reduced into product P, which is the case of baker’s yeast, is ethanol:
Finally, if enough oxidative capacity is available, possibly due to a decrease in the concentration of S, the product P will also be metabolized:
The constants a1, a3, b1, b2, b3, c1, c2, c3, and d2 are the stoichiometric coefficients, i.e., the yields of the three reactions and r1, r2, and r3 are the rates at which the reactions take place. Substrate and/or product can either be oxidized (Reactions 1a and 1c) or reduced (Reaction 1b) [11]. The values for the constants are given in Table 1.
2.2 Macroscopic modeling
From a macroscopic point of view, the relationship between the concentrations of the different reaction components in Eq. 1 is given by the following mass balances:
The specific biomass growth rate, μ, can be obtained by adding the biomass production for each reaction [12]:
3 Modeling for control
3.1 Exponential cell growth
Assumption 1: biomass production in Reaction 1 is much higher than in Reaction 2
With this assumption, and the fact that Reaction 1 is saturated, biomass growth rate μ can be considered as constant and equal to \( \ifmmode\expandafter\bar\else\expandafter\=\fi{\mu }. \) Thus, it is possible to integrate Eq. 2a to give:
In this way, biomass will be considered as a growing exponential evolving at a constant \( \ifmmode\expandafter\bar\else\expandafter\=\fi{\mu }. \) This simple observation allows decoupling the biomass growth rate from the substrate feed rate.
3.2 Ethanol production
Assumption 2: ethanol is constantly being produced, i.e., r3=0
This assumption implies Reaction 1a is saturated with respect to oxidative capacity. Thus, the reaction rate is maximized: r1(S)=r1,max.
Assumption 3: dynamics of the substrate concentration S are fast, i.e., S is in quasi-steady state
This assumption can be made since a small variation in the substrate feed rate, F, will result in an almost instantaneous change in the substrate concentration, S. In this way, dS/dt≈0, and from Eq. 2b, the following expression is obtained:
From Eq. 2c, the ethanol evolution in the reactor can then be expressed by:
This last equation shows that the rate at which glucose is transformed into ethanol is obtained from the difference between the rate at which substrate is fed into the reactor, F(Sin−S), and the rate at which the cells oxidize it, r1,maxVX. A controller based on Eq. 6 would need to have the value of the volume at each time instant. However, this measurement may sometimes be difficult to obtain in practice. For example, consider its evolution for the presented experiment (Fig. 2).
Even if the starting and ending reactor volumes, Vo and V f , respectively, are precisely known, all volume added and removed must be accurately accounted for in order to have continuous reactor volume data. In this particular case, it was not possible to place the reactor on a balance. Additionally, even with knowledge of the mass in the reactor, the total volume must be further corrected for density, which changes significantly in high cell density fed-batch experiments. Even with proper accounting of flows, volume correction remains a major source of error. Thus, a further simplification needs to be made. Applying the chain rule of derivation to Eq. 6 gives:
where \( \ifmmode\expandafter\bar\else\expandafter\=\fi{V} = {{\left( {V_{{\text{o}}} + V_{{\text{f}}} } \right)}} \mathord{\left/ {\vphantom {{{\left( {V_{{\text{o}}} + V_{{\text{f}}} } \right)}} 2}} \right. \kern-\nulldelimiterspace} 2 \) is an averaged volume. Since P will be maintained at a low value, P<<d2(Sin−S) and S<<Sin, the dilution effect on P and S can be neglected, i.e., (F/V)S≈0 and (F/V)P≈0. Notice how in Eq. 7 the feed rate, F, and the biomass growth term, VX, have been decoupled. The fermentation goal is now to maintain a constant ethanol concentration. If this is achieved, biomass will grow exponentially at a growth rate close to μcrit. Thus, the control strategy can be stated as: feed enough substrate so that the cells can grow at a constant \( \ifmmode\expandafter\bar\else\expandafter\=\fi{\mu } \), while at the same time, maintain a constant product concentration in the reactor. In this case, the controller design can be divided in two. One part of the controller should ensure that the cells would always grow exponentially; a second part should guarantee that the desired metabolite concentration will be maintained. The controller derivation has been published elsewhere [4] and the reader is referred to this reference for further information.
4 Material and methods
4.1 Microorganism and medium
The yeast S. bayanus CBS426 was used, which, until recently, belonged to the S. cerevisiae species. Cells were stored frozen at −80°C in 1.8 ml aliquots. A single aliquot added to a 500ml Erlenmeyer flask containing 150 ml of medium (0.67 g/l Yeast Nitrogen Base, Difco; 10 g/l glucose) and grown for 24 h at 30°C provided the bioreactor inoculum. The defined media for the batch and fed-batch experiments were adapted from [13]. Unless otherwise noted, all chemicals were from Fluka (Buchs, Switzerland). The batch medium was sterilized by filtration and contained per liter: 20 g glucose, 5 g (NH4)2SO4, 3 g KH2PO4, 0.5 g MgSO4·7H2O. The medium also contained vitamins and trace elements: 0.01 g CaCl2·2H20, 2.67 mg H3BO3, 0.8 mg CuSO4·5H2O, 0.27 mg KI, 2.67 mg MnCl2, 1.07 mg Na2MoO4·2H2O, 12 mg ZnSO4·7H2O, 40 mg EDTA, 0.8 mg CoCl2, 8 mg FeSO4·7H2O, 2.67 Ca pentothenate, 0.13 mg biotin, 66.67 mg m-inositol, 2.67 mg nicotinic acid, 0.53 mg para-amino benzoic acid (PABA), 2.67 mg pyridoxine hydrochloride, 2.67 mg thiamine hydrochloride. The feed medium was sterilized by filtration and contained per liter: 300 g glucose, 0 g (NH4)2SO4, 12 g KH2PO4, 1.5 g MgSO4·7H2O. The medium contained the same vitamins and trace elements as in the batch medium, though concentrated 15-fold.
4.2 Cultivation conditions
Cultivations were performed in a 2-l stirred tank bioreactor (BioRC1 calorimeter) with a working volume of between 0.5 and 1.8 l. The temperature and agitation were controlled at 30°C and 1,000 rpm, respectively. The culture sparging rate was 1.5 l/min. Dissolved oxygen tension was monitored but not controlled with a pO2 probe (Mettler Toledo, Greifensee, Switzerland). During batch and nitrogen limited phases of cultivation, 2 M NaOH was used to control pH at 5.0. During fed-batch phase, 14% NH4OH was used in order to provide a source of nitrogen. No acid control was necessary to maintain pH. A silicone-based antifoam (Antifoam A, Sigma, St. Louis, MO, USA) was added at regular intervals to eliminate foaming.
4.3 Analytical methods
Cell mass concentration was quantified gravimetrically. A volume of 10 ml of culture broth was added to pre-weighed glass tubes, followed by immediate centrifugation for 10 min at 3,000 rpm. The supernatant was removed and the tubes dried for 36 h at 100°C. Medium components and extracellular metabolites were quantified by HPLC (1,100 system, Agilent, Palo Alto, CA, USA) with a refractive index (RI) detector and a diode array detector (DAD). Samples were filtered, placed into sealed vials, and frozen at −20°C until analysis. Analyses were performed with an ion exchange chromatography column (300 mm Supelco H, Supelco, Bellefonte, PA, USA) and a guard column (Supelguard C610H, Supelco) at 60°C. The mobile phase consisted of 0.02 M H2SO4 solution made with MilliQ water (Millipore, Bedford, MA, USA). The flow rate of mobile phase was 0.8 ml/min.
Off-gas composition was monitored with an infrared analyzer for CO2 and ethanol (model PSA-401 Servomex, Crowborough, UK), and a paramagnetic (model 1100-A Servomex) analyzer for oxygen. The infrared analyzers had an iridium light source and a LiTaO3 detector. The CO2 analyzer measured the difference in absorbance at 2,335 cm−1 (CO2) and 2,531 cm−1 (reference). The ethanol analyzer measured the difference in absorbance at 2,940 cm−1 (ethanol) and 2,531 cm−1 (reference). Measured signals were corrected for a water vapor content of 3.5% [14]. The ethanol concentration in the liquid phase was related to the gas phase concentration with an empirical partition coefficient.
The biomass concentration was estimated on-line, based upon the rate of base consumption [3]. Since the medium was defined and there was no significant production of organic acids, the ammonia uptake rate was directly proportional to the rate of base addition. The biomass was then estimated by assuming constant nitrogen content. The biomass composition was taken to be: XC=1.00, XN=0.19, XH=1.78, XO=0.613, ash = 9.6%, MW=26.9 g/C-mol [15]. The on-line estimate of biomass was in agreement with off-line dry cell weight data.
5 Results and discussion
Several fed-batch experiments were conducted in a 2-l bioreactor to validate the control strategy. The ethanol was measured in the gas phase, and then related to liquid phase concentration through an empirically determined partition coefficient Ke. As shown in Fig. 3, the control strategy was able to maintain the ethanol concentration in bioreactor around the desired setpoint of 0.5 g/l. At the cessation of feeding, the residual ethanol was rapidly consumed.
On-line ethanol measurement in gas phase and HPLC validation points. Overshoot occurs at 15.2 h where oxygen became limiting. At 18.8 h, an unknown disturbance caused a spike in ethanol concentration, while at 21.2 h, an increase in the oxygen transfer rate caused ethanol concentration to decrease below setpoint
As expected for a constant overflow metabolite concentration, the biomass growth rate was also constant, and gave rise to an exponential increase in the total biomass, XV. This is clearly seen in Fig. 4, where XV increased exponentially until ~15 h, at which point the mass transfer of oxygen into liquid phase was the limiting factor, not the oxidative capacity of cells.
The controller automatically adapted the feed rate of glucose to compensate for this disturbance. As shown in Fig. 5, the molar rates q i V increased exponentially until ~15 h, and linearly thereafter. The molar rates started increase more rapidly at ~21 h because the reactor volume had reached a sufficient levele that the second Rushton turbine in the bioreactor could now contribute to oxygenation of medium, and hence exponential growth could resume. This is interesting because it demonstrates two points: (1) that oxygen was really limiting growth, not some other factor (e.g., nutrient deficiency), and (2) that the controller was able to adapt once again to a new growth regime.
Constant production of ethanol implies a constant specific cell growth rate above μcrit. This can be verified by looking at the respiratory quotient (RQ), which is defined as the ratio between the carbon dioxide production rate rc and the oxygen uptake rate ro. If only glucose oxidation takes place, i.e., μ≤μcrit, then RQ≅1. The RQ evolution for this experiment is shown in Fig. 6.
Initial oscillations in the RQ signal, resulting from the oscillations in the substrate feed rate, converged to a constant value of 1.10, indicating that the cells were consuming glucose via Reactions 1a and 1b. Notice how this growth metabolism is maintained even when dissolved oxygen concentration is limiting in the latter part of experiment. Thus, even if the critical growth rate diminishes, the actual growth rate is always maintained slightly above it.
The specific carbon dioxide and oxygen rates (rc and ro) were approximately constant during exponential growth phase (Fig. 7). The maximum oxygen uptake rate r MAXo was between 5.3 and 5.5 mmol/g-h, which is in the same range found by Duboc et al. [16] for the same strain of S. cerevisiae. Duboc et al. also found that r MAXo was not fixed, but rather, varied between 3.3 and 5.6 mmol/g-h, depending upon culture history. In this work, growth was maintained at r MAXo without a priori knowledge of its value. From r MAXo , the critical growth rate was calculated from the stoichiometry according to:
where MWo is the molecular weight of oxygen (g/mol), Yso is the oxygen to substrate yield (g/g), and Ysx the biomass to substrate yield (g/g). A μcrit of 0.26 h−1 is consistent with the value estimated from the XV regression line seen in Fig. 4.
Ethanol is the principal overflow metabolite in S. cerevisiae. However, there was a risk that, by constraining ethanol flux to a low value, the excess glycolytic flux would then overflow to another metabolite. Concentrations of acetate, acetaldehyde, glycerol, succinate, fumarate, pyruvate, and 2,3-butanediol all remained below 0.25 g/l, even with over 70 g/l biomass in the bioreactor.
6 Conclusion
In this article, a recently developed control strategy was successfully applied to a fed-batch experiment with S. bayanus. The controller ensured keeping the ethanol concentration at a very small non-zero level, thereby, forcing the culture to grow both very close to the critical growth rate and with a near-optimal biomass yield. Even though the controller was based upon a very simple “bottleneck” model, exponential growth was maintained near the critical point until oxygen was limiting, and, thereafter, the feed profile was adapted to maintain growth in the low oxygen environment. Furthermore, production of by-products other than ethanol was minimal. Unlike many other strategies, the only input to the controller was the ethanol concentration measured in the gas phase. The method also provided a quick means to characterize strains. Both the critical growth and the maximum oxygen uptake rates were determined on-line from fed-batch experiments, as opposed to the more time consuming continuous cultures. Finally, the method was very straightforward to implement, and should be widely applicable to many industrially important processes. In the case of baker’s yeast fermentation, ethanol can be measured in the gas phase very inexpensively, whereas for non-volatile metabolites (e.g., pyruvate or lactate) measurements could be made directly in the liquid phase with spectroscopic or enzymatic techniques.
References
Ejiofor AO, Solomon BO, Posten C, Deckwer WD (1994) Analysis of the respiro-fermentative growth of Saccharomyces cerevisiae on glucose in a fed-batch fermentation strategy for accurate parameter estimation. Appl Microbiol Biotechnol 41:664–669
van Hoek P, van Dijken JP, Pronk JT (1998) Effect of specific growth rate on fermentative capacity of Baker’s yeast. Appl Environ Microbiol 64:4226–4233
Xu B, Jahic M, Enfors SO (1999) Modeling of overflow metabolism in batch and fed-batch cultures of Escherichia coli. Biotechnol Prog 15:81–90
Valentinotti S, Holmberg U, Srinivasan B, Cannizzaro C, Rhiel M, von Stockar U, Bonvin D (2002) Optimal operation of fed-batch fermentations via adaptive control of overflow metabolite. Control Eng Pract 11(6):665–674
Axelsson JP (1988) Experimental techniques and data treatment for studying the dynamics of ehanol production/consumption in baker’s yeast. Anal Chim Acta 213:151–163
Turner C, Gregory ME, Thornhill NF (1994) Closed-loop control of fed-batch cultures of recombinant Escherichia coli using on-line HPLC. Biotechnol Bioeng 44:819–829
Rank M, Gram J, Nielsen KS, Danielsson B (1995) Online monitoring of ethanol, acetaldehyde and glycerol during industrial fermantations with Saccharomyces cerevisiae. Appl Micorbiol Biotechnol 42:813–817
Noronha SB, Wagner LW, Matheson NH, Shiloach J (1999) Use of an ethanol sensor for feedback control of growth and expression of Tbv25H in Saccharomyces cerevisiae. Biotechnol Bioeng 63285–289
Locher G, Sonnleitner B (1992) On-line measurement in biotechnology: techniques. J Biotechnol 25:23–53
Polakovic M, Mandenius CF (1994) Evaluation of sensors for the control of a contiuous ethanol fermentation. Bioprocess Eng 10:217–223
Kellerhals MB, Kessler B, Witholt B (1999) Closed-loop control of bacterial high-cell-density fed-batch cultures—production of mcl-PHAs by Pseudomonas putida KT2442 under single-substrate and cofeeding conditions. Biotechnol Bioeng 65:306–315
Hall JW, McNeil B, Rollins MJ, Draper I, Thompson BG, Macaloney G (1996) Near-infrared spectroscopic determination of acetate, ammonium, biomass, and glycerol in an industrial Escherichia coli fermentation. Appl Spectrosc 50:102–108
Yano T, Aimi T, Nakano Y, Tamai M (1997) Prediction of the concentrations of ethanol and acetic acid in the culture broth of a rice vinegar fementation using near-infrared spectroscopy. J Ferment Bioeng 84:461–465
Faxolle P, Picque D, Corrieu G (2000) On-line monitoring of fermentation processes by a new remote disperisve middle-infrared spectrometer. Food Control 11:291–296
Rhiel M, Cohen MB, Murhammer DW, Arnold MA (2002) Nondestructive near-infrared spectroscopic measurement of multiple analytes in undiluted samples of serum-based cell culture medium. Biotechnol Bioeng 77:73–82
Kornmann H, Rhiel M, Cannizzaro C, Marison I, von Stockar U (2003) Methodology for real-time multi-analyte monitoring of fermentations using an in situ mid-infrared sensor. Biotechnol Bioeng 82:702–709
Phelps MR, Hobbs JB, Kilburn DG, Turner RFB (1995) an autoclavable glucose biosensor for microbial fermentation monitoring and control. Biotechnol Bioeng 46:514–524
Buttler T, Liden H, Jonsson JA, Gorton L, Markovarga G, Jeppsson H (1996) Evaluation of detection and sample cleanup techniques for on-line and off-line fermentation monitoring systems. Anal Chim Acta 324:103–113
Wild R, Citterio D, Spichiger J, Spichiger UE (1996) Continuous monitoring of ethanol for bioprocess control by a chemical sensor. J Biotechnol 50:37–46
Srinivasan N, Kasthurikrishnan N, Cooks RG, Krishnan MS, Tsao GT (1995) On-line monitoring with feedback control of bioreactors using a high ethanol tolerance yeast by membrane introduction mass spectrometry. Anal Chim Acta 316:269–276
Meyer C, Beyeler W (1984) Control strategies for continous bioprocesses based on biological activities. Biotechnol Bioeng 26:916–925
Zigova J, Mahle M, Paschold H, Malissard M, Berger EG, Weusterbotz D (1999) Fed-batch production of a soluble beta-1,4-galactosyltransferase with Saccharomyces cerevisiae. Enz Microb Technol 25:201–207
Randolph TW, Marison IW, Martens DE, von Stockar U (1990) Calorimetric control of fed-batch fermentations. Biotechnol Bioeng 36:678–684
Larsson C, Lidén G, Niklasson C, Gustafsson L (1991) Calorimetric control of fed-batch cultures of Saccharomyces cerevisiae. Bioprocess Eng 7:151–155
Larsson C, Lidén G, Blomberg A, Niklasson C, Gustafsson L (1993) Monitoring and control of batch and fed-batch cultures of Saccharomyces cerevisiae by calorimetry. Pure Appl Chem 65:1933–1937
Duboc P, Cascao Pereira LG, von Stockar U (1998) Identification and control of oxidative metabolism in Saccharomyces cerevisiae during transient growth using calorimetric measurements. Biotechnol Bioeng 57:610–619
Voisard D, Pugeaud P, Kumar AR, Jenny K, Jayaraman K, Marison IW, von Stockar U (2002) Development of a large scale biocalorimeter to monitor and control bioprocesses. Biotechnol Bioeng 80:125–138
Ringbom K, Rothberg A, Saxen B (1996) Model-based automation of baker’s yeast production. J Biotechnol 51:73–82
Jobe A, Herwig C, Surzyn M, Walker B, Marison I, von Stockar U (2003) A generally applicable fed-batch culture concept based on the detection of metabolic state by on-line balancing. Biotechnol Bioeng (in press)
Sonnleitner B, Kappeli O (1986) Growth of Saccharomyces cerevisiae is controlled by its limited respiratory capacity: formulation and verification of a hypothesis. Biotechnol Bioeng 28:927–937
Valentinotti S (2001) Adaptive rejection of unstable disturbances: Application to fed-batch fermantation process. Thèse N° 2405. Swiss Federal Institute of Technology
Verduyn C, Postma E, Sheffers WA, van Dijken JP (1992) Effect of benzoic acid on metabolic fluxes in yeasts: a continuous culture study on the regulation of respiration and alcoholic fermentation. Yeast 8:501–517
Vicente A, Castrillo JI, Teixeira JA, Ugalde U (1998) Online estimation of biomass through pH control analysis in aerobic yeast fermentation systems. Biotechnol Bioeng 58:445–450
Duboc P, Marison I, Von Stockar U (1996) Physiology of Saccharomyces cerevisiae during cell cycle oscillations. J Biotechnol 51:57–72
Duboc P, von Stockar U (1998) Systematic errors in data evaluation due to ethanol stripping and water vaporization. Biotechnol Bioeng 58:428–439
Acknowledgements
Funding by the Swiss National Science Foundation is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cannizzaro, C., Valentinotti, S. & von Stockar, U. Control of yeast fed-batch process through regulation of extracellular ethanol concentration. Bioprocess Biosyst Eng 26, 377–383 (2004). https://doi.org/10.1007/s00449-004-0384-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-004-0384-y