Abstract
Pretreatment of biomass with dilute H2SO4 results in residual acid which is neutralized with alkalis such as Ca(OH)2, NaOH and NH4OH. The salt produced after neutralization has an effect on the fermentation of Pichia stipitis. Synthetic media of xylose (60 g total sugar/l) was fermented to ethanol in the presence and absence of the salts using P. stipitis CBS 6054. CaSO4 enhanced growth and xylitol production, but produced the lowest ethanol concentration and yield after 140 h. Na2SO4 inhibited xylitol production, slightly enhanced growth towards the end of fermentation but had no significant effect on xylose consumption and ethanol concentration. (NH4)2SO4 inhibited growth, had no effect on xylitol production, and enhanced xylose consumption and ethanol production.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lignocelluloses of plant cell walls are composed of cellulose, hemicellulose, pectin and lignin. The major sugars produced after hydrolysis of lignocellulosic biomass are glucose, galactose, mannose, xylose, and arabinose. Efficient conversion of biomass to ethanol requires microorganisms with the ability to ferment the sugars generated after hydrolysis. The yeast, Pichia stipitis, produces ethanol from glucose, galactose, mannose, xylose, and cellobiose with high ethanol yields and low amounts of xylitol (Dellweg et al. 1984; du Preez et al. 1986).
One promising technology for converting lignocellulosic biomass to ethanol is the enzyme-based process, where enzymes are used to hydrolyze the fibers after pretreatment. Dilute sulphuric acid pretreatment at high temperatures extensively hydrolyzes the hemicellulose to soluble sugars (Schell et al. 2003; Fenske et al. 1998). The residual acid after pretreatment is neutralized with alkalis such as Ca(OH)2 (van Zyl et al. 1988; Eken-Saracoglu and Arslan 2000), NH4OH (Alriksson et al. 2005; Persson et al. 2002) and NaOH (Nilvebrant et al. 2005). In some cases, these alkalis are added in excess to reduce inhibitor concentrations in dilute acid pretreated biomass (Tran and Chambers 1986; Nigam 2001a, b).
Although the inhibitors generated after dilute acid pretreatment can decrease ethanol production (Delgenes et al. 1996; Tran and Chambers 1986), Persson et al. (2002) have shown that the improved fermentability after alkali treatment are difficult to explain by the removal of inhibitors only. Therefore, the salts produced after neutralizing the excess H2SO4 with alkalis could play a role in the fermentation. The purpose of this study was to determine how CaSO4, Na2SO4, and (NH4)2SO4 salts affect the cell growth, xylose consumption, ethanol production, ethanol yield, and xylitol production in Pichia stipitis.
Materials and methods
Microorganism
Pichia stipitis CBS 6054 was generously supplied by Dr. Thomas Jeffries of the Forest Products Laboratory, USDA. The cells were grown overnight in a filter-sterilized fermentation medium containing per liter: 1.7 g yeast nitrogen base (without amino acid or ammonium sulfate), 2.27 g urea, 6.56 g peptone, and 20 g xylose. The cells were centrifuged at 3,000g for 5 min and resuspended in 5 ml sterile water to serve as inoculum.
Media and fermentation
Three different salt media were prepared by adding 3.3 ml H2SO4 (7.6 M) to 25 ml Ca(OH)2 (1 M), 50 ml NH4OH (1 M) or 50 ml NaOH (1 M). The salts were diluted with distilled water to a total liquid volume of 200 ml and a final pH of 6.0. The control was 200 ml distilled water at the initial pH of 6.1. Xylose, 12 g, was dissolved in each solution to give 60 g/l. Each sugar solution was filter-sterilized using a 0.2 μm filter. Nutrient solution (50× the concentration used) was prepared by dissolving 1.7 g yeast nitrogen base, 2.27 g urea and 6.56 g peptone in 20 ml water. Fermentations were performed in sterile 125 ml Erlenmeyer flasks (with 0.2 μm vent cap) in at 30°C and shaken at 100 rev/min. Each Erlenmeyer flask contained 50 ml sugar media, 1 ml nutrient solution, and 2 ml inoculum. All these experiments were performed in triplicate at the same initial cell concentration of 1.5 g/l.
Analytical methods
Samples, 1 ml, were periodically removed for analyses. The concentrations of xylose, xylitol, and ethanol were determined using an Agilent HPLC System with an analytical Bio-rad Aminex HPX–87H column and a Bio-rad Cation H refill guard column. The cell concentrations were determined as OD600 values; an OD of 1 = 0.23 g of dry cells/l.
Results and discussion
Effect of the salts on cell growth
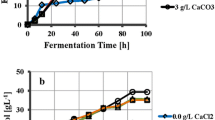
The growth of Pichia stipitis on different salt media is shown in Fig. 1 and the pH during fermentation is shown in Fig. 2. Xylose consumption is shown in Fig. 3 and ethanol production in Fig. 4. A synopsis of the key fermentation data is given in Table 1. Pichia stipitis reached its final cell concentration after 90 h (Fig. 1). An initial cell concentration of 1.5 g/l grew to different final cell concentrations on the different salt media after 118 h of fermentation (Fig. 1). Inhibition by (NH4)2SO4 on the growth of Pichia stipitis is in agreement with observations by Guebel et al. (1992). The medium containing (NH4)2SO4 had the lowest cell concentration. Cell concentration on Na2SO4 medium was lower than the control initially (t < 40 h), and became higher towards the end (Fig. 1). The initial pH for all the salts and control was 5.5–6.0 and the final pH was 4.0–4.5. Studies on Saccharomyces cerevisiae suggest that the initial response of cells to saline conditions is the efflux of water, which leads to cell shrinkage (Blomberg 2000). Pichia stipitis might also respond similarly to Saccharomyces cerevisiae on Na2SO4 medium, since the cells did not grow well initially (compare to control). The highest cell growth was on CaSO4 medium. Guebel and Nudel (1994) obtained maximum cell growth rates at low Ca2+ (0.34 mM) concentrations and low growth at high Ca2+ (1 mM) concentrations. The high cell growth of Pichia stipitis in our study might be because of low Ca2+ in solution due to the poor solubility of CaSO4.
The effect of salts on cell growth of Pichia stipitis. The control was fermentation with no salts added. CaSO4, Na2SO4, and (NH4)2SO4 were produced by adding 3.3 ml H2SO4 (7.6 M) to 25 ml Ca(OH)2 (1 M), 50 ml NH4OH (1 M), and 50 ml NaOH (1 M) respectively, and adding distilled water to a final liquid volume of 200 ml. Pichia stipitis fermentations in 60 g xylose/l at an initial cell concentration of 1.5 g/l in a shake flask incubator at 30°C. Cell concentrations were determined from OD600 values using Cary 3C UV-Visible spectrophotometer *Error bars are ± 1 std
Effect of the salts on xylose consumption and ethanol production
The xylose consumption was initially (t < 60 h) faster on the CaSO4 medium but towards the end, xylose consumption was faster in the (NH4)2SO4 medium (Fig. 3). The fast rate of xylose consumption initially in CaSO4 medium can be attributed the high cell growth in Fig. 1. However, towards the end of the fermentation (t > 90 h), xylose consumption slowed down in all the treatments apart from the (NH4)2SO4 medium (Fig. 3). This makes the xylose consumption of the entire fermentation period (140 h) higher in the (NH4)2SO4 medium compared to the other treatments.
The highest ethanol concentration was produced in the (NH4)2SO4 medium and the lowest ethanol concentration was in the CaSO4 medium (Fig. 4). The low ethanol concentration in CaSO4 medium is due to high cell biomass production, because the xylose was used to produce cell mass instead of ethanol. Although (NH4)2SO4 medium produced the highest ethanol concentration, the cell biomass produced was the lowest. The NH3 produced from the dissociation of NH +4 stimulate ethanol production in Pichia stipitis (Guebel et al. 1992). Xylose consumption and ethanol production in the control and Na2SO4 medium were similar (Figs. 3 and 4).
The highest ethanol yield was in the control 0.39 g/g, which is not significantly different from 0.38 g/g for (NH4)2SO4 medium, whereas the ethanol yield was 0.35 g/g and 0.36 g/g in CaSO4 and Na2SO4 media respectively (Table 1). Ethanol yield per g of cell was higher on (NH4)2SO4 compared to the other treatments because of stimulatory effect of ammonia on ethanol production in Pichia stipitis and the low cell concentration in (NH4)2SO4 medium. Xylitol yield in CaSO4 medium, 0.02 g/g was higher than all the other treatments. The control and (NH4)2SO4 media had a xylitol yield of 0.01 g/g whereas there was no xylitol in Na2SO4 medium. CaSO4 stimulated xylitol production, (NH4)2SO4 had no effect on xylitol production and Na2SO4 inhibited xylitol production in Pichia stipitis.
Conclusion
Xylose consumed after 140 h of fermentation was highest in the (NH4)2SO4 medium compared to the other treatments. The maximum ethanol concentration after 140 h of fermentation was 18.9 g/l in the (NH4)2SO4 medium whilst the lowest ethanol concentration was 15.7 g/l in the CaSO4 medium. The production of ammonia from (NH4)2SO4 enhanced ethanol production by Pichia stipitis, and therefore ethanol yield per g of cells was 3.3 g/g. The salts produced after neutralizing the excess H2SO4 with alkalis such as Ca(OH)2, NaOH and NH4OH have an effect on the cell growth, xylose consumption, ethanol production, ethanol yield, and xylitol production in Pichia stipitis.
References
Alriksson B, Horvath IS, Sjode A, Nilvebrant N-O, Jonsson LJ (2005) Ammonium hydroxide detoxification of spruce lignin hydrolysates. Appl Biochem Biotechnol 121–124:911–922
Blomberg A (2000) Metabolic surprises in Saccharomyces cerevisiae during adaptation to saline conditions: questions, some answers and a model. FEMS Microbiol Lett 182:1–8
Delgenes JP, Moletta R, Navarro JM (1996) Effects of lignocellulose degradation products on ethanol fermentations of glucose and xylose by Saccharomyces cerevisiae, Zymomonas mobilis, Pichia stipitis, and Candida shehatae. Enzyme Microb Technol 19:220–225
Dellweg H, Rizzi M, Methner H, Debus D (1984) Xylose fermentation by yeasts 3. Comparison of Pachysolen tannophilus and Pichia stipitis. Biotechnol Lett 6:395–400
du Preez JC, Bosch M, Prior BA (1986) The fermentation of hexose and pentose sugars by Candida shehatae and Pichia stipitis. App Microbiol Biotechnol 23:228–233
Eken-Saracoglu N, Arslan Y (2000) Comparison of different pretreatments in ethanol fermentation using corn cob hemicellulosic hydrolysate with Pichia stipitis and Candida shehatae. Biotechnol Lett 22:855–858
Fenske JJ, Hashimoto A, Penner MH (1998) Relative fermentability of lignocellulosic dilute-acid prehydrolysates. Application of a Pichia stipitis-based toxicity assay. Appl Biochem Biotechnol 73:145–157
Guebel DV, Nudel C (1994) Antagonism between growth and flocculation in Pichia stipitis NRRL Y-7124: Influence of Ca+2 and Mg+2 ions. Biotechnol Lett 16:143–148
Guebel DV, Cordenons A, Cascone O, Giulietti AM, Nudel C (1992) Influence of the nitrogen source on growth and ethanol production by Pichia stipitis NRRL Y-7124. Biotechnol Lett 14:1193–1198
Nigam JN (2001a) Ethanol production from wheat straw hemicellulose hydrolysate by Pichia stipitis. J Biotechnol 87:17–27
Nigam JN (2001b) Development of xylose-fermenting yeast Pichia stipitis for ethanol production through adaptation on hardwood hemicellulose acid prehydrolysate. J Appl Microbiol 90:208–215
Nilvebrant N-O, Persson P, Reimann A, De Sousa F, Gorton L, Jonsson LJ (2003) Limits of alkaline detoxification of dilute-acid lignocellulose hydrolysates. J Appl Microbiol 90:208–215
Persson P, Andersson J, Gorton L, Larsson S, Nilvebrant N-O, Jonsson LJ (2002) Effect of different forms of alkali treatment on specific fermentation inhibitors and on the fermentability of lignocellulose hydrolysates for production of fuel ethanol. J Agric Food Chem 50:5318–5325
Schell DJ, Farmer J, Newman M, McMillan JD (2003) Dilute-sulphuric acid pretreatment of corn stover in pilot-scale reactor. Appl Biochem Biotechnol 105–108:69–85
Tran AV, Chambers RP (1986) Ethanol fermentation of red oak acid prehydrolysate by the yeast Pichia stipitis CBS 5776. Enzyme Microb Technol 8:439–444
Van Zyl C, Prior BA, du Preez JC (1988) Production of ethanol from sugarcane bagasse hemicellulose hydrolysate by Pichia stipitis. Appl Biochem Biotechnol 17:357–369
Acknowledgement
This investigation was supported by the Abengoa-DOE project. We like to thank Dr. Thomas Jeffries for generously providing the yeast strain, David Milam and James Palmer for analytical support on HPLC. We also like to thank Dr. Guillermo Coward-Kelly, Dr. Mads Torry-Smith and Dr. Frank D. Haagensen for useful discussions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Agbogbo, F.K., Wenger, K.S. Effect of pretreatment chemicals on xylose fermentation by Pichia stipitis . Biotechnol Lett 28, 2065–2069 (2006). https://doi.org/10.1007/s10529-006-9192-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-006-9192-6