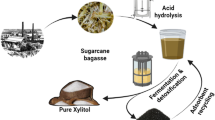
Abstract
The valorization of C5 sugars (xylose) from hemicellulose of agro-industrial residues to xylitol, as one of the multi-products biorefinery approach, mandates the pretreatment of biomass which releases fermentable sugars along with the generation of biological inhibitors affecting xylitol fermentation. This study was therefore evaluated to understand the inhibitory kinetics of furfural, 5-hydroxymethylfurfural and acetic acid on xylitol fermentation. Xylitol fermentation was established using Pichia stipitis NCIM 3497 with xylose as a pure substrate optimized for xylitol yield and productivity of 0.48 g/g of xylose and 0.13 g/L/h, respectively. The functional relationship of yeast specific growth rate and limiting substrate (xylose) was expressed by Monod-type kinetics. The inhibition kinetics results indicated that the effect of inhibitors on xylitol fermentation was furfural > acetic acid > HMF. Furfural (500 mg/L) and acetic acid (1000 mg/L) reduced xylitol yield by 59% and 44%, respectively, with least reduction of 9.89% exhibited by HMF. The synergistic effect of 500 mg/L furfural, 500 mg/L HMF and 1000 mg/L acetic acid showed the highest reduction in xylitol yield of 67.6% as compared to the control. Kinetic studies predicted that the maximum concentration of furfural, HMF and acetic acid which inhibited P. stipitis growth was 884 mg/L, 3258 mg/L and 2922 mg/L, respectively, whereas xylitol production was completely inhibited at 1069 mg/L furfural, 3498 mg/L HMF and 3714 mg/L acetic acid. Furfural and acetic acid were found to be a competitive inhibitor, while uncompetitive inhibition was observed with HMF indicating negligible effect on xylitol fermentation.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Reducing the burden of food and energy availability, with concomitant increase in revenue, can be addressed by deriving the value-added products from industrial and food waste. This approach involving process integration, optimization and final product development requires a biorefinery platform for scaling up to industrial level [1]. The utilization of agro-residues; rich in hemicellulose component for the production of value-added product such as xylitol which is applicable in food and pharmaceutical industries, along with the production of second-generation biofuels, is a promising approach towards sustainability [2, 3]. Xylitol, a low-calorie sweetener, is considered as a sugar substitute to sucrose with similar sweetness, along with health beneficial characteristics such as low glycaemic index, high cooling power (negative heat of dissolution) [4], insulin-independent metabolic pathway [2], anticariogenic and non-interfering with food nutritional value [5]. As large-scale production of xylitol is still carried out through chemical route by dehydrogenation of xylose under high pressure and temperature, the biotechnological route which involves milder process conditions is the potential substitute for chemical route which can utilize both industrial and agricultural wastes, thereby reducing the overall production cost [6]. Pretreatment of agro-residues enhances the accessibility of fermentable sugars by disintegration of lignin and hemicellulosic linkage. The sugars released from the pretreated lignocellulosic biomass are considered as a low-cost source of xylose for xylitol production by microbial fermentation process [7]. Among the various pretreatments, acid and alkali have been widely used as an efficient process with respect to higher sugar yield and lignin breakage [8]. But, considering the harsher treatment associated with acid/alkali such as acid concentrations and pretreatment time, it may lead to inhibitors generation which interferes with enzymatic/fermentation process.
Among the inhibitory compounds generated during pretreatment, carboxylic acids mainly acetic acid released from the hemicellulosic breakage; furan derivatives such as furfural and 5-hydroxymethylfurfural (HMF) from degradation of pentoses and hexoses, respectively; and phenolic compounds from lignin degradation are produced to an extent intolerant to microbial biocatalyst [9, 10]. Cortez et al. [11] studied that presence of high levels of inhibitory compounds, such as acetic acid (8.4 g/L) and furans (2.5 g/L), affected the metabolism of P. stipitis by preventing the efficient conversion of sugars to ethanol. The concentration of inhibitors generated depends on the severity of pretreatment and pretreatment conditions such as temperature, pH, residence time, presence of acid/alkali and type of biomass [12]. Also, the ratio of total sugars to inhibitors generated (∑C/∑I) was used as an indicator for evaluating the fermentable ability of xylose (20 g/L) in the presence of inhibitors such as furfural, HMF and acetic acid. The ratio of sugars/inhibitors released can be considered together to assess the severity of pretreatment conditions. [13]. Hence, the choice of milder pretreatment avoiding the use of harsh chemicals and expensive enzymes for higher sugar yield with minimum inhibitor generation can be a sustainable option [14].
As most of the fermentation process employs yeast such as Candida and Pichia sp., as an efficient biocatalyst, which utilizes the cellulosic and hemicellulosic-derived monomeric sugars for bioconversion to bioethanol, xylitol, sorbitol and other value-added products. The widely used yeast, Saccharomyces cerevisiae, is capable of utilizing only glucose but not pentose sugar such as xylose [15]. However, Pichia stipitis has the highest native capacity of xylose fermentation, which helps in improving the process economics with respect to utilization of both glucose and xylose and, hence, can ferment xylose to xylitol under micro-aerobic conditions [16].
Earlier reported studies are mostly related to the effect of inhibitors on ethanol fermentation using various species such as Candida, Saccharomyces and Pichia. In the case of ethanol fermentation, various studies have been reported on the mechanism involved in the inhibitory effect of various inhibitors on growth of the yeast, but very few studies have been reported on inhibitory effect of major inhibitors such as furfural, HMF and acetic acid on xylitol fermentation representing the maximum threshold concentration of inhibitors negatively affecting xylitol fermentation. It has been found that furfural and HMF reduces enzymatic and biological activities of yeast by breaking down DNA and inhibiting protein and RNA synthesis [17, 18]. The inhibitory effect of furfural was observed in S. cerevisiae as it inhibited glycolytic enzymes and aldehyde dehydrogenase activity, which resulted in accumulation of acetaldehyde, responsible for the lag phase [19]. The mitochondria were found damaged in the presence of furfural and induced accumulation of reactive oxygen species in S. cerevisiae [20]. Another inhibitor which is generated from the glucose degradation is 5-hydroxymethyl furfural which has been shown to negatively impact on the fermentation performance of S. cerevisiae, which was used as biocatalyst for fermentation of mixed sugar of glucose and xylose [18]. Although HMF is considered as an inhibitor generated during pretreatment, it does not exhibit higher effect on enzymatic hydrolysis and fermentation as it is further degraded to 2,5-bis-hydroxymethylfuran [19]. The effect of HMF was minimal as compared to furfural and acetic acid; however, the presence of 0.5 g/L HMF inhibited xylose consumption in fermentation media. The results showed that the fermentation profiles were similar between control and media containing HMF exhibiting least effect on fermentation [21]. Almarsdottir et al. [22] investigated that the toxicity of acetate in yeasts was increased by the presence of furfural. Acetic acid, the primary inhibitor released from the acetyl hemicellulose linkage during pretreatment, affected the cell growth by entering the cell through diffusion and then dissociated due to the neutral cytosolic pH. The dissociation of the acid leads to a decrease in the intracellular pH, which may lead to cell death. And also, the regeneration of ATP in mitochondria is inhibited in the presence of acetic acid at the plasma membrane. Acetic acid can have a positive or negative effect on microbes during fermentation. It was reported that acetic acid as a substrate for Meyerozyma guilliermondii was co-consumed with sugars (xylose, arabinose and glucose) even when furfural was present. With the increase of acetic acid concentration from 4.5 to 19.5 g/L, there was no decrease in specific growth rate. M. guilliermondii metabolized acetic acid in the presence of both pentoses (xylose and arabinose) and glucose and did not interfere with the sugar uptake. Furthermore, a decrease of 20–30% in cell concentrations of immobilized S. cerevisiae was observed when the acetic acid concentration increased from 2.5 to 20 g/L which might be due to the stress by acetic acid leading to cell death [23].
Considering the unfavourable effect of furfural, HMF and acetic acid on yeast growth and subsequent xylose fermentation, it is necessary to reduce or eliminate these inhibitors during the upstream process, with minimum use of acids/alkali during the pretreatment and detoxification methods. Several studies have been carried out on the detoxification methods of the hydrolysate, which includes physical methods, where the volatile compounds such as acetic acid, furfural and vanillin can be eliminated with vacuum-assisted evaporation. Physico-chemical methods using activated carbon with the adsorption mechanism and ion-exchange resins and biological methods by microbial biocatalysts are some of the followed detoxification process [24]. Physico-chemical methods are fast but have significant sugar loss, are time-consuming and require several filtration steps and accessories which increases the operation cost at larger scale. Enzymatic detoxification, modified fermentation strategies and microbial pretreatment of lignocellulose are slow and time-consuming. Hence, the choice of detoxification methods should be considered by evaluating their effectiveness and extent of substrate weight loss [25]. Also, the development of more tolerant yeast strains that can detoxify the inhibitors in situ to produce bioethanol in a sustainable and cost-effective industrial process has been studied [26]. The adaptation of Candida tropicalis for xylitol production which improved cell growth and xylose uptake rate in successive batch cultivations with xylose as sole carbon source was reported [27]. Similar work was carried out containing 2% of xylose as sole carbon source for xylitol production by Kluyveromyces marxianus NIRE-K1, which showed better performance for xylose consumption (more than 80%) and xylitol production (xylitol yield was 1.65 higher compared to the non-adapted yeast [28]. However, the development of engineered yeast strains and detoxification processes of the hydrolysate using chemicals increase the cost of the downstream processing and also the waste discharge, which reinforces to find an alternative method for the use of hydrolysate with minimum concentration of the inhibitors directly for the fermentation process. Therefore, it is necessary to identify the concentration range of furfural, acetic acid and HMF downregulating the xylitol fermentation and, hence, develop the kinetic model of individual and synergistic effect of inhibitors on xylitol fermentation with respect to yeast growth rate and xylitol fermentation rate. Wannawilai et al. [27] demonstrated that furfural competitively inhibits the xylitol fermentation using Candida magnoliae TISTR 5663 and the kinetic models have been developed for yeast growth, substrate consumption and xylitol production. Also, the models developed for inhibitory effects can be useful for the prediction of fermentation behaviour at various levels of inhibitors in the pretreated hydrolysate. The kinetic model developed predicted the maximum inhibitor concentrations which affected biomass growth and xylitol production. The effect of major inhibitors such as furfural, HMF and acetic acid can be modelled for its inhibitory effects to predict the fermentation with respect to yield reduction in biomass growth and xylitol production for various levels of inhibitor concentration in the substrate. The yeast growth profiles, substrate consumption and xylitol production profiles can be evaluated in the presence of major inhibitors such as furfural, HMF and acetic acid. Furfural concentration of 40 mg/L inhibited growth of M. guilliermondii and ethanol production, which was verified by slower sugar uptake and lower ethanol titres [23]. Earlier reported studies are mostly related to the effect of inhibitors on ethanol fermentation using various species such as Candida, Saccharomyces and Pichia. The yeast Pichia stipitis was reported to be inhibited during ethanol fermentation [21], where fermentation process was completely inhibited at acetic acid concentration of 3.5 g/L. Furfural (0.5–2 g/L) caused delay on sugar consumption rates, and ethanol productivity decreased to half the value of the control after 24 h, but productivity was almost similar to control at the end of fermentation. HMF was studied in the concentration range of 0.1–0.5 g/L and did not have a significant effect at the concentration range studied.
The present study was, therefore, carried out to understand the insights of the inhibitory effect of furfural, acetic acid and HMF on xylose consumption and xylitol production by the yeast, P. stipitis. The novelty of the work was to evaluate the factors such as yeast growth rate, xylose consumption and xylitol production in the presence of inhibitors. The kinetic model (Luong’s model) well represented the primary data obtained in this study and the prediction of toxicity of furfural, HMF and acetic acid on yeast growth and xylitol production which can be adapted for the design of pretreatment conditions for various lignocellulosic feedstocks.
2 Materials and methods
2.1 Substrates and inhibitory compounds
Pure D-xylose procured from Sigma-Aldrich, Inc., was used as synthetic media for xylitol production using P. stipitis. The inhibitors used in the present study, furfural, HMF and acetic acid, were procured from Sigma-Aldrich, Inc. All the standards used for analytical experiments were procured from Sigma Aldrich, India, and were of ≥ 99% purity. The representative concentration range of inhibitors studied was selected based on the inhibitors generated during the alkaline-assisted pretreatment of wheat straw, with acetic acid of 2.3% per 100 g biomass, whereas the furans varied with respect to pretreatment conditions and duration [29]. Furfural and HMF concentrations were in the range of 100–500 mg/L, whereas acetic acid concentration was in the range of 100–1000 mg/L as acetic acid is found in higher concentration in the pretreated liquid hydrolysate due to the hemicellulose solubilization. However, furfural and HMF concentrations can be balanced based on the mild pretreatment conditions with higher sugar recovery and reduced generation of HMF and furfural. Stock solutions of the inhibitors were prepared in deionized water in the individual concentrations of 100, 250 and 500 mg/L of furfural and HMF and 100, 500 and 1000 mg/L of acetic acid.
2.2 Yeast culture and growth conditions
Yeast strain, P. stipitis (NCIM 3497), was procured from the National Collection of Industrial Microorganisms, Council of Scientific and Industrial Research - National Chemical Laboratory, Pune, India. Therefore, this study reports the bioconversion of xylose to xylitol using the biocatalyst, P. stipitis, generally regarded as safe (GRAS) organism for food applications [30]. Also, it does not require the addition of vitamins to the fermentation of xylose and is able to use a wide range of sugars as substrate [16, 21]. The chemicals used for maintenance of yeast culture and fermentation were procured from HiMedia, India. Growth media comprising of 10 g/L D-glucose, 3 g/L malt extract, 3 g/L yeast extract and 5 g/L peptone was used to maintain the yeast culture at 4 °C and pH 5.5. Yeast suspension culture (YPX media) was prepared using 10 g/L yeast extract, 20 g/L peptone and 30 g/L xylose and was maintained at 30 °C with agitation of 200 rpm and pH 5.5.
2.3 Batch fermentation of xylitol
Xylitol fermentation from pure D-xylose was carried out in 500-ml conical flask containing 250 ml of fermentation media: 20 g/L xylose, 2 g/L peptone, 3 g/L yeast extract, 1 g/L ammonium sulphate, 2 g/L potassium dihydrogen phosphate and 1 g/L magnesium sulphate. The fermentation media was inoculated with 24 h culture of yeast with initial cell concentration of 1.5 g/L of cell dry weight which was measured using UV-visible spectrophotometer (UV-1800, Shimadzu, UV Spectrophotometer) [31]. Xylitol production was optimized for xylose concentration in the range of 10–80 g/L.
The flasks were placed in incubator shaker under agitation at 200 rpm maintained at 30 ± 0.5 °C, pH 5.5 for 72 h of fermentation. The samples were collected at time interval of 6 h for the analysis of biomass concentration, xylose consumption and xylitol production. Inhibitory studies for xylitol fermentation were also carried out in the similar method with addition of furfural, HMF and acetic acid in the concentration range as mentioned in Table 1. Xylitol produced with inhibitors was compared and analysed for its effect on yeast growth rate and xylitol fermentation rate to that of control (xylitol fermentation without inhibitors from pure xylose).
2.4 Quantitative analysis by high-performance liquid chromatography
Samples collected at different time intervals during the fermentation period was centrifuged at 5000 rpm for 15 min. The supernatant was extracted with equal volume of ethyl acetate, and the solvent layer containing xylitol was further concentrated at 50 °C with vacuum pressure of 153 mBar using rotary evaporator (Rotavac vario pumping unit, Heidolph Instruments). Xylitol was quantified by high-performance liquid chromatography (HPLC Alliance waters, e2695) as described by Banerjee et al. [32]. The standards of xylose and xylitol and the extracted samples were filtered through 0.2μ syringe filter. Xylose and xylitol were quantified using Bio Rad (Hercules, CA) Aminex HPX-87H column at 55 °C with acetonitrile: water (80:20) as eluant, at a flow rate of 1 ml/min and an injection volume of 20 μl using refractive index detector (Hitachi High-Technologies Corporation model L-2490, Japan).
2.5 Kinetic model for yeast growth and xylitol production
The growth of the yeast P. stipitis using xylose as sole source of carbon for xylitol production was assumed to follow the Monod type kinetics [33], in which the specific growth rate (μ) depended on xylose concentration expressed by Eq. (1)
where μmax is the maximum specific growth rate (h-1), S is the substrate concentration (g/L) and KS is the rate-limiting substrate concentration (g/L) at which the specific growth rate is half of its maximum value, generally referred to as the saturation constant.
2.6 Inhibitory kinetics of furfural, HMF and acetic acid on xylitol fermentation
The inhibitory effect of furfural, HMF and acetic acid on xylitol fermentation was analysed using pure xylose with initial concentration of 20 g/L. The effects of furfural, HMF and acetic acid on yeast growth, xylose consumption and xylitol production study were evaluated using Luong’s model [34]. The proposed kinetic model predicted the growth rate of P. stipitis and xylitol production in low, moderate and high concentrations of selected inhibitors in comparison with control. Luong’s model is a well-known model for representation of inhibitory effect, which determines the toxicity level exhibited by the inhibitors on yeast growth and xylitol production. The primary data such as the percentage of decrease in yeast growth rate was calculated by decrease in yeast concentration in presence of specific inhibitor with respect to the concentration of yeast estimated without inhibitors (control). The experimental data were used to estimate the kinetic parameters such as μo, μI, Pm, Pm’, vo and vI, for xylitol fermentation with and without inhibitors. The inhibition on yeast growth and xylitol production was evaluated by Eqs. (2) and (3), respectively.
P is the concentrations of inhibitors (mg/L)
μ O and μI are the maximum specific growth rate of P. stipitis without and with inhibitors, respectively
P m and \({P}_m^{' }\) are the inhibitor concentration (mg/L) above which yeast cells do not grow and do not produce xylitol, respectively
vO and vI are the specific rate of xylitol production without and with inhibitors
To determine the type of inhibition (competitive inhibition, uncompetitive inhibition and non-competitive inhibition) by furfural, HMF and acetic acid on xylitol fermentation, the plot of 1/V versus 1/S for various concentrations of xylose in presence of low, moderate and high concentrations of inhibitors is represented in Fig. 8. The models for competitive, uncompetitive and non-competitive inhibition [35] are analysed from the following equations:
3 Results and discussion
3.1 Batch fermentation of xylitol
Xylitol fermentation by P. stipitis NCIM 3497 was optimized with varying initial xylose concentrations from 10 to 80 g/L, and the xylitol concentration was found to be in the range of 0.89–9.561 g/L (data not shown). The optimum xylitol yield was observed at xylose concentration of 20 g/L. Further, decrease in xylitol yield was observed because of substrate inhibition. Xylose consumption by P. stipitis during fermentation was estimated by measuring the xylose concentration at respective time interval of 6 h. It was observed that almost 75% of the xylose was utilized during 72 h of fermentation. P. stipitis has been studied widely in fermenting pentoses and hexoses to ethanol from pretreated lignocellulosic biomass [36,37,38] for its efficient xylose utilization as compared to other yeasts [16]. The initial xylose concentration of 20 g/L was reduced to 6.174 g/L after 72 h of fermentation. It was estimated that xylitol produced by P. stipitis was quantified to be 9.561 g/L for fermentation of 72 h and is represented in Fig. 1a with productivity of 0.13 g/L/h, which was almost similar as reported in literature of 0.18 g/L/h [31].
Furthermore, to evaluate the effect of varying xylose concentrations as a limiting substrate on yeast growth, the plot of μ versus S (Fig. 1b) which followed the Monod-type kinetics. The maximum specific growth rate (μmax) was found to be 0.186 h-1. The saturation constant, KS, is the value of [S] when μ/μmax = 0.5 was estimated to be 14 g/L. Similar results were reported by Wannawilai (2017) where the yeast, Candida magnoliae TISTR 5663, depended on substrate concentration such as glucose, xylose or combination of both which followed the Monod-type kinetics [27]. Both μmax and KS differ depending on the species of microorganism and fermentation conditions such as temperature, pH and composition of the culture medium. If S < KS, then the reaction follows first-order kinetics, where the substrate utilization is directly proportional to substrate concentration [34].
3.2 Substrate inhibition of xylitol fermentation
The higher concentration of substrate (xylose) could reduce the yeast cell viability by the hypertonic environment affecting xylitol fermentation. If the substrate concentration is higher than an optimum value of 20 g/L, the product (xylitol) and yeast concentrations will not increase with additional substrate which will eventually waste resources and energy. In the presence of higher xylose concentration, the yeast growth declined as the additional energy and xylose was consumed for the survival of yeast in the hypertonic environment rather than increase in the yeast growth. Also, the long-term exposure of yeast cell to a higher sugar concentration can lead to decrease in cell membrane fluidity affecting the transport of biomolecules through transmembrane layer of the yeast cell [38]. Therefore, higher xylose in the fermentation media was consumed by the yeast to maintain the activity of the transport system instead of bioconversion of xylose to xylitol. As shown in Fig. 2a, YX/S, yield of biomass on substrate (yeast cell growth per unit mass of xylose consumed, (gyeast/gxylose)), decreased with an increase in the initial xylose concentration, C0, which is represented in Eq. (7):
Equation 7 represents the substrate inhibition of xylose on xylitol fermentation induced by a high xylose concentration analysed in the range of 10 to 80 g/L. YX/S decreased with an increase in the substrate concentration due to excessive xylose concentration in the fermentation media affecting the yeast growth.
3.3 Effect of furfural, HMF and acetic acid on yeast growth
The effect of furfural, HMF and acetic acid generated during pretreatment of agro-residues which inhibits yeast growth and xylitol fermentation impacts the scale up and process economics of xylitol production. Therefore, it is necessary to determine the optimum concentration of inhibitors that can be tolerated by P. stipitis for obtaining higher xylitol yield and productivity. Effect on inhibitors on the yield of biomass (YX/C) was represented as shown in Fig. 2b using the plot of YX/C (gyeast /gxylose) versus inhibitor concentrations (CI). The higher concentration of furfural (500 mg/L) showed a biomass yield reduction of 79.3% followed by acetic acid (1000 mg/L) with 58.95%, and HMF (500 mg/L) reduced the biomass yield by 15.07%. From Fig. 2b, it can be inferred that the biomass yield reduction to 0.25 g/g in the presence of 350 mg/L of furfural was equivalent to the biomass yield at initial xylose concentration of 40 g/L. Similar reduction in biomass yield to 0.45 g/g was observed with acetic acid of 200 mg/L and initial xylose concentration of 30 g/L, indicating similar inhibitory effect by substrate concentration and inhibitor. This observation inferred that substrate concentration may equally affect yeast growth as inhibitors, as represented in Fig. 2a and b.
Figure 3a shows the effect of low, moderate and higher concentrations of furfural on the growth of yeast, P. stipitis, during the fermentation period of 72 h. Furfural resulting from the degradation of C5 sugars is inhibitory to yeast growth, and its inhibitory effect depends on concentration. In the present study, the furfural concentration in the range of 100–500 mg/L was evaluated for its effect on growth rate of P. stipitis. The yeast concentration in the presence of lower concentration of furfural (100 mg/L) was almost similar to that of yeast growth without inhibitors of 11.97 g/L. With further increase in the concentrations of furfural to 250 mg/L and 500 mg/L, the growth rate of P. stipitis substantially decreased 45.76% and 79.30%, respectively, as compared to the control. This might be due to the shock stress, which results in the change in physiological environment of yeast and the substrate utilization for the tolerance of yeast to various concentrations of inhibitors. It also affects glycolytic activity and the tricarboxylic acid cycle, causing oxidative stress and this reduces the enzyme activity [39, 40]. On the contrary, the higher concentration of HMF of 500 mg/L did not affect the yeast growth which was estimated to be 12.034 g/L at 54 h of fermentation that declined to 10.165 g/L at 72 h. HMF concentrations of 100 mg/L and 250 mg/L did not have noticeable effect on yeast growth (Fig. 3b). Similar results were observed in inhibitory tolerance by Rhodotorula mucilaginosa strain PTD3 which was capable of tolerating HMF at concentration of 15 g/L [41].
Another known major inhibitor from breakdown of hemicellulose structures is acetic acid, its generation cannot be avoided during pretreatment, and hence, it is present in higher concentrations compared to furfural and HMF. Kashid and Ghosalkar [42] proposed a model to understand the inhibition kinetics of xylose to ethanol fermentation by P. stipitis in the presence of acetic acid. The kinetic model consisting of linear differential equation was developed that described cell mass, ethanol production, xylose consumption and oxygen concentration with time which predicted that xylose fermentation in the presence of acetic acid depicted low value of maximum specific growth rate of 0.15 h-1 as compared to 0.23 h-1 in the absence of acetic acid. The lower cell mass yield can be attributed due to the presence of acetic acid in undissociated form in the fermentation broth at pH 5.5. Undissociated form of acetic acid diffuses through yeast cell membrane and penetrates to the cytoplasm leading to acidic conditions inside the cell. Therefore, ATP is utilized to maintain the intracellular pH by removing the H+ ions outside, which decreases the ATP necessary for yeast growth. Hence, to understand the effect of acetic acid on yeast growth rate, the present study was conducted in the concentration range of 100–1000 mg/L as represented in Fig. 3c. The growth of P. stipitis was not found to be affected in the presence of 100 mg/L of acetic acid, and the maximum concentration was estimated to be 13.093 g/L almost similar to control of 13.26 g/L, whereas further increase in concentration to 500 and 1000 mg/L was found to decrease the maximum yeast growth to 8.376 and 5.987 g/L, respectively.
It was also observed that the specific growth rate (μo) which was defined by Monod equation was 1.131 h-1 without the inhibitors was reduced in the presence of furfural and acetic acid but not in the presence of HMF. It was estimated that the specific growth rate in the presence of inhibitors (μI) such as furfural and acetic acid at concentration of 500 mg/L and 1000 mg/L reduced to 0.862 h-1 and 0.755 h-1, respectively. Similar observations were made by Wannawilai et al. [27] that even the lower concentrations of furfural of 0.164 g/L negatively impacted both specific growth rate and the final biomass concentration. The action of furfural is dose-dependent as it affects glycolytic activity and the tricarboxylic acid cycle, which causes oxidative stress and, therefore, decrease in enzyme activity of dehydrogenases [27].
3.4 Inhibitory effect of furfural, HMF and acetic acid on xylitol fermentation
The pretreatment of lignocellulosic biomass (LCB) necessary for hydrolysis releases the constituent sugars which are the carbon sources for yeast growth and production of value-added products. Along with C5 and C6 sugars, compounds such as furans, aliphatic acids and phenolic compounds that can inhibit microorganisms are generated during pretreatment of LCB. Inhibitors present or formed in pretreated hydrolysates can limit the consumption of the carbon source and may even inhibit the fermentation process [43]. Therefore, it is necessary to understand the inhibitory effect of furfural, HMF and acetic acid individually and in combination on xylitol fermentation.
In the present study, the xylitol yield was estimated with and without inhibitors like furfural, HMF and acetic acid in the fermentation medium. The yield of xylitol from pure xylose without the inhibitors (control) for 6, 24, 48 and 72 h of fermentation were 29, 215, 345 and 478 mg of xylitol/g of xylose (Table 1). It is evident that xylitol production increased by 186 and 130 mg of xylitol/g of xylose from 6 to 24 h and 24 to 48 h, respectively. Also, the fermentation period from 24 to 48 h increased the yield of xylitol by 60%, signifying the major conversion of xylose to xylitol occurred within 24 to 48 h.
Initially, the presence of furfural in the fermentation media resulted in lower yield of xylitol. Xylitol fermentation containing 100 mg/L and 250 mg/L of furfural in the fermentation media produced 24.21 and 23.1 mg of xylitol/g of xylose in 6 h of fermentation, and the yield at 72 h of fermentation with 250 mg/L of furfural was 390 mg of xylitol/g of xylose approached to that of control. Furfural concentration of 500 mg/L showed highest inhibition of xylitol fermentation compared to 100 and 250 mg/L with 285.03 mg of xylitol/g of xylose at 72 h of fermentation (Table 1). It was observed that furfural inhibited the growth of yeast by reducing the specific growth rate due to the prolonged lag phase which decreased the xylitol yield to 8.03 mg xylitol/g xylose as compared to 29 mg xylitol/g of xylose (control). Also, the length of lag phase increased nonlinearly after a threshold concentration of furfural was present which affected the xylitol fermentation [27]. The growth of yeast was not completely inhibited at higher concentration of furfural (500 mg/L) as yeasts are capable of reducing the inhibition impact of furfural on fermentation by metabolizing it to lesser toxic products such as furoic acid or furfuryl alcohol [39].
However, the extent of inhibition on xylitol fermentation in the presence of HMF was low as compared to furfural and acetic acid. Similar study was carried out and concluded from the inhibition experiments that furfural is more inhibitory than 5-HMF. The inhibitory effect of HMF was studied in the concentration range of 100 to 500 mg/L, in which 425 mg of xylitol/g of xylose was produced in the presence of 500 mg/L of HMF which was almost similar to control (478 mg of xylitol/g of xylose) (Table 1). Acetic acid, major inhibitor generated during the pretreatment by deacetylation of hemicellulosic linkages, showed remarkable inhibitory effect on xylitol fermentation. Acetic acid in the concentration range of 100 to 1000 mg/L was analysed for its inhibitory effect on xylitol fermentation, and a higher concentration of 1000 mg/L acetic acid during xylitol fermentation yielded 406.34 mg of xylitol/g of xylose. Overall, based on the effect of individual inhibitors on growth rate of P. stipitis and xylitol production, it could be concluded that furfural had higher inhibitory effect followed by acetic acid and HMF. Similar results were observed by Noronha et al. [44] that furfural was more toxic for xylose fermentation than acetic acid as it affected the assimilation pathways for the pentose-fermenting yeast. Acetic acid directly enters the KREBS cycle via acetyl-CoA, where some amount of acetic acid may continue to be directed toward the Krebs cycle and the remaining may be utilized by another energy-consuming metabolic pathway such as the glyoxylate cycle. This might result in the lack of energy for the maintenance of the overall metabolism of the yeast leading to reduced cell growth [45]. The threshold concentration of inhibitors provides the insights about the severity of pretreatment which affects the yeast growth and xylitol fermentation. The severity of pretreatment can be evaluated by ratio of total sugars to inhibitors ratio [13] and was also assessed in the present study. Depending on the constant xylose concentration of 20 g/L and inhibitor concentration range of furfural and HMF, ratio (∑S/∑I) was between 4 and 20, whereas in the presence of acetic acid, the ratio was between 2 and 20 (Table 2). Higher value of ∑S/∑I can be considered as better pretreatment, with minimum inhibitory effect which will be further suitable for enzymatic hydrolysis/fermentation. Also, the type of lignocellulosic biomass and pretreatment conditions affects the ∑S/∑I. Yeast growth and xylitol production were affected when ∑S/∑I was decreased to 4 for furfural and HMF, with the least value of 2 in the presence of acetic acid.
3.5 Synergistic inhibition of furfural, HMF and acetic acid on xylitol fermentation
The inhibitory effect of compounds generated during pretreatment on yeast growth can be enhanced by the presence of other compounds. The effect of inhibitors can be additive or synergistic on fermentation if the inhibition increases significantly more than individual compounds, respectively. Inhibitory compounds usually work synergistically to reduce metabolic output; however, presence of weak acids such as acetic acid in lower concentration actually improved tolerance to hydroxymethyl furfural (HMF) and furfural in S. cerevisiae as reported by Greetham et al. [46]. Another study reported that ethanol fermentations by P. stipitis from steam exploded wheat straw slurry were completely inhibited by a synergistic effect due to the presence of 1.5 g/L acetic acid, 0.15 g/L furfural and 0.05 g/L HMF in the pretreated liquid hydrolysate [30]. Several studies have reported that the combination of acetic acid, aromatic aldehydes and alcohols, 2-furfural and furfuryl alcohol showed to increase the inhibitory potential, resulting in synergistic inhibition of growth and ethanol yield in Escherichia coli [47, 48]. Also, the presence of furfural with acetic acid showed the negative effect in the case of S. cerevisiae [20]. Therefore, to compare the synergistic effect with individual effect on inhibitors, ∑S/∑I was also estimated for additive effect of furfural, HMF and acetic acid at low, moderate and high concentrations. The ratio of xylose to total inhibitors was in the range of 6.67 to 1 (Table 2), indicating the increase in inhibitory effect due to the presence of three major inhibitors in the fermentation media. At a higher value of ∑S/∑I of 1, xylitol yield was reduced by 67.57%. Similar effect was observed by Bellido et al. [21], where the ternary mixture of acetic acid, furfural and HMF affected sugar consumption, with complete inhibition of xylose consumption and lower glucose consumption during ethanol fermentation.
Therefore, to understand the synergistic effect on xylose consumption and xylitol fermentation, the present work was carried out in the presence of combinations of furfural, HMF and acetic acid and analysed for xylitol yield. Xylose consumption and xylitol production were analysed in combination of low, medium and high concentrations of furfural, HMF and acetic acid as represented in Fig. 4a and b, respectively. In the presence of lower and medium concentrations of inhibitors, the yield was found to be 423 and 239 mg of xylitol/g of xylose, respectively. It was estimated that there were 11.5% and 50% reduction found in the xylitol yield in the presence of lower and moderate concentration of inhibitors, respectively, as compared to control. In the presence of higher concentration of furfural, HMF and acetic acid, there were 66.67% and 67.57% reduction found in the yield at 48 and 72 h of fermentation, respectively, indicating that the highest reduction in xylitol yield occurred between 24 and 72 h of fermentation (Table 1). Similar studies were also reported by Vajzovic et al. [41] which was evaluated in a synthetic medium (containing glucose or xylose, 30 g/L) for xylitol production in the presence of inhibitors (furfural, HMF and acetic acid). This showed that high concentrations of inhibitors (above 3 g/L) negatively affected xylitol production.
(a) Xylose consumption and (b) xylitol production in the presence of low, moderate and high concentrations of inhibitors; low conc. of inhibitors (mg/L), furfural (100) + HMF (100) + acetic acid (100); moderate conc. of inhibitors (mg/L), furfural (250) + HMF (250) +acetic acid (500); and high conc. of inhibitors (mg/L), furfural (500) + HMF (500) +acetic acid (1000)
Figure 5a and b illustrates the difference of inhibitory effect of furfural, HMF and acetic acid on yeast growth and xylitol fermentation rate. As shown in Fig. 5a, when the concentration of inhibitors was lowest at 100 mg/L, it showed no obvious difference on yeast growth inhibition. However, when the concentration of furfural and HMF was increased to 250 mg/L and acetic acid to 500 mg/L, respectively, the yeast specific growth rate was found to decrease in the presence of furfural as compared to HMF and acetic acid. But there was sudden reduction in the yeast growth rate observed when the furfural concentration was 500 mg/L, during which the yeast growth rate reduced to 0.862 h−1 from 1.131 h−1 (without inhibitor). In case of HMF, there was not much reduction in yeast specific growth rate which affected the fermentation. However, there was noticeable fall observed in the specific growth rate of P. stipitis when the acetic acid concentration exceeded 500 mg/L, which reduced to 0.755 h−1 at 1000 mg/L of acetic acid.
The effect of inhibitors on xylitol fermentation rate is depicted in Fig. 5b, during which furfural exhibited a greater inhibitory effect on the fermentation rate than the same amount of HMF. There was linear decrease in the xylitol fermentation rate to 3.95 × 10-3 gxylitol/(gxylose.h) observed from 6.9 × 10-3 gxylitol/(gxylose.h) (control) with increase in concentration of furfural, whereas the presence of acetic acid and HMF did not considerably affect the rate of xylitol fermentation as HMF might be further degraded to 2,5-bis-hydroxymethylfuran. Also, the ability of the yeast to dissociate acetic acid to acetate which did not hamper the internal pH of yeast cell.
3.6 Kinetic studies of inhibitory potential of furfural, HMF and acetic acid on xylitol fermentation
In the present work, the kinetic studies of xylitol fermentation with and without the presence of inhibitors were evaluated, and the yeast growth and xylitol production were estimated for fermentation time of 72 h. It is known that xylitol fermentation depends on substrate concentration, specificity of the microorganisms and the process parameters which affects metabolic regulations. Therefore, monitoring of the substrate and end product concentration which affects the growth of yeast cells and fermentation defines the relationship between specific growth rate (μ) and the rate-limiting substrate concentration (xylose). The growth was assumed to follow Monod-type kinetics in which specific growth rate depended on the concentration of xylose. The effect of varying xylose concentrations on yeast growth was modelled by Monod-type kinetics which showed μmax of 0.186 h-1, which declined when the initial xylose concentration was higher than 20 g/L. Several studies reported on mathematical modelling of xylitol production from xylose [49, 50], and kinetic modelling of xylose fermentation by P. stipitis in pure synthetic xylose [31, 51].
The kinetics of inhibitory effects which was evaluated as per Luong’s model as described showed that at low concentration of inhibitors (P→ 0), μI approaches μo and vI approaches vo. The value of μI or vI approaches zero when P approaches Pm or \({P}_m^{' }\), respectively. The magnitude of constant α is the toxic severity coefficient, indicating the type of relation between μI and P; and the relation between vI and P is dependent on the empirical constant β. If the increasing concentration of furfural reduces the specific growth rate linearly, then, α = 1. If α < 1, the specific growth rate declines hyperbolically as the concentration of furfural increases and α > 1, the specific growth rate shows a rapid parabolic decline as the concentration of inhibitor increases [50, 52].
Therefore, the effect of low, moderate and high concentrations of furfural, HMF and acetic acid on specific growth rate and fermentation rate of P. stipitis is represented as μI/μOand vI/vO plotted against P/Pmas shown in Fig. 6a and b. It can be observed that the ratio μI/μo remains almost unchanged (= 1) in case of furfural and HMF when α < 1 and P/Pm, increases from 0 to 0.11 and 0.07, respectively, whereas the inhibitory effect of acetic acid had drastic effect on specific growth rate which showed a slow initial drop in the growth rate followed by a rapid decrease was observed when α > 1 (Fig. 6a).
However, the plot between μI/μO and P did not conform to the straight-line relationship as described by Eq. 2. The resulting curve also did not conform to the exponential model or the hyperbolic equation. Also, when P is not zero, Eqs. 2 and 3 can be rearranged as follows
The above equations are represented in Fig. 7a and b in which ln (1 − μI/μO) or ln (1 − vI/vO) was plotted against lnP ; and the kinetic parameters relating the effect of inhibitors on yeast growth rate and xylitol fermentation were estimated. The model parameters (α, Pm, β and \({\mathrm{P}}_{\mathrm{m}}^{' }\)) depends on the microbial species, the physiological conditions of the microorganism in the presence of inhibitors, ability to detoxify the inhibitors in the fermentation media and the individual and synergistic effect of inhibitors on yeast growth and xylitol fermentation. The kinetic data obtained in this study were well represented by the proposed model to predict the effect of furfural, HMF and acetic acid on growth and fermentation. The best-fit values of a were determined to be 2.028, 1.319 and 1.901 in the presence of furfural, HMF and acetic acid, respectively. The maximum allowable concentration of furfural, HMF and acetic acid above which cells do not grow was predicted to be 884.48 mg/L, 3258.42 mg/L and 2921.93 mg/L. The maximum inhibitor concentration above which xylitol production was inhibited at 1069.55 mg/L of furfural, 3498 mg/L of HMF and 3714.50 mg/L in case of acetic acid. Based on the above-predicted inhibitor concentrations, it can be observed that furfural of 500 mg/L had higher inhibitory effect as compared to acetic acid of 1000 mg/L. Also, the α value for furfural was 2.2028 which was higher than acetic acid (1.901), which represented higher toxicity as reported similarly by Noronha et al. [44]. The higher toxicity by furfural can affect the assimilation pathways for pentose-fermenting yeasts. Also, furfural can be converted to lesser toxic compound, furfuryl alcohol, which require the co-factor NADH. This requirement imbalances the NADH/NAD+ which negatively affected the xylitol production. Similar results were observed in ethanol fermentation by engineered S. cerevisiae where addition of furfural enhanced the ethanol production but reduced xylitol production [53]. Similarly, HMF will be reduced to 2,5-bis-hydroxymethylfuran which requires co-factor NADPH, but there was no significant effect on yeast growth rate and xylitol yield. Furthermore, it was proved by Perna et al [23] that acetic acid was co-consumed as a substrate along with sugars (xylose, arabinose and glucose) by M. guilliermondii when furfural was present as an inhibitor. Therefore, this study demonstrated that furfural has major negative effect on xylitol fermentation by P. stipitis as compared to HMF and acetic acid.
To understand the nature of inhibition on xylitol fermentation by furfural, a representation of 1/V versus 1/S was plotted (Fig. 8). The inhibitory effect of low, moderate and high concentrations of furfural, HMF and acetic acid on xylitol fermentation was compared with xylitol fermentation without inhibitors and was represented in Eqs. 4, 5 and 6. Similarly, high concentration of furfural (500 mg/L) exhibited uncompetitive inhibition, whereas moderate concentration of furfural competitively inhibited xylitol production (Fig. 8a). The reduction in both Vm and Km was observed with 100 and 500 mg/L of furfural during xylitol fermentation, while the competitive inhibition at 250 mg/L showed an increase in Km. The growth of yeast during the xylitol production phase with xylose as the only substrate, in which some of the xylitol formed is converted to xylulose by NAD+-dependent xylitol dehydrogenase. But, in the presence of furfural, some of the NAD+ is used in its metabolism to furoic acid [39], a less toxic metabolite compared to furfural. Therefore, metabolism of furfural competes with the metabolism of xylose for the essential resource of NAD+ competitively. As HMF did not affect the P. stipitis growth and xylitol fermentation, hence, there was uncompetitive nature of inhibition which lowered both Km and Vmax (Fig. 8b). This suggested that HMF concentration required to have substantial effect on xylitol fermentation would be much higher and, therefore, can be favourable for xylitol fermentation from the pretreated hydrolysate. Acetic acid generated from the disruption of hemicellulose component showed mixed inhibition of competitive and uncompetitive with 100 and 500 mg/L, respectively, during xylitol fermentation (Fig. 8c). Competitive inhibition exhibited by inhibitors can be avoided by increase in xylose consumption, such that the xylose concentration should be optimum for higher xylitol production. The results of the study showed that improving the xylose utilization capability by metabolic engineering would potentially increase the inhibitor tolerance to HMF and furfural by increasing the supply of ATP and reducing power [54]. Therefore, the increase in xylose consumption can reduce the competitive inhibitory effect by furfural exhibited in this study.
The present investigation, therefore, demonstrated how the different concentration of furfural, acetic and HMF affects the yeast growth and xylitol fermentation, which has not been reported in any other study for xylitol fermentation by P. stipitis. Also, the prediction of inhibitor concentration range essential for the scale up of xylitol production has been evaluated, where the level of toxicity exhibited by the multiple inhibitors was 1.069 mg/L of furfural, 3.498g/L of HMF and 3.714 g/L of acetic acid, completely inhibited xylitol production. The approach described in this work can be used in process optimization, design and control, simulation and optimization of the process which may aid to reduce the development costs of lignocellulosic-based xylitol production.
4 Conclusion
The recent development in the use of various biomass feedstocks and pretreatment methods has led to the interest in the study of the effect of inhibitory compounds associated with the pretreated hydrolysates for production of value-added products. The effect of substrate inhibition on xylitol fermentation using the yeast P. stipitis in the range of 1–8% xylose was studied and 20 g/L of initial xylose was found to be the optimal for the yeast strain used in the present study. Furthermore, the inhibitory effect of furfural, acetic acid and HMF on xylitol fermentation using the model parameters (a, Pm, β and Pm’) for predicting the maximum threshold concentration of select inhibitors was analysed with respect to yeast growth rate and xylitol fermentation rate. Model parameters depends on the microbial species, the physiological conditions of the microorganism in the presence of inhibitors and ability to detoxify the inhibitors in the fermentation media. It was concluded that higher concentration of furfural and acetic acid affected the specific growth rate, and hence, the xylitol production, as compared with HMF. The model used to study the inhibition kinetics can predict the fermentation profiles along with the type of inhibition pattern. The results suggested that inhibitory effect was analysed for a better understanding of the effect of pretreatment process on inhibitor generation that will interfere with the fermentation process of xylitol which can be further optimized for minimal inhibitors generation and enhanced xylose recovery. And finally, the selection of efficient pretreatment process involves the greener methods negating the inhibitor generation, which ultimately influences the sustainability of the bioprocess.
References
Jeyavishnu K, Thulasidharan D, Shereen MF, Arumugam A (2021) Increased revenue with high value-added products from cashew apple (Anacardium occidentale L.)—addressing global challenges. Food Bioproc Tech 1-28. https://doi.org/10.1007/s11947-021-02623-0
Chen X, Jiang ZH, Chen S, Qin W (2010) Microbial and bioconversion production of D-xylitol and its detection and application. Int J Biol Sci 6:834 https://www.ijbs.com/v06p0834.htm
Franceschin G, Sudiro M, Ingram T, Smirnova I, Brunner G, Bertucco A (2011) Conversion of rye straw into fuel and xylitol: a technical and economical assessment based on experimental data. Chem Eng Res Des 89:631–640. https://doi.org/10.1016/j.cherd.2010.11.001
Mussatto SI, Roberto IC (2002) Xylitol: a sweetner with benefits for human health. Revista Brasileira de Ciências Farmacêuticas 38:401–413. https://doi.org/10.1590/S1516-93322002000400003
Ritter AV, Bader JD, Leo MC, Preisser JS, Shugars DA, Vollmer WM, Holland JC (2013) Tooth-surface-specific effects of xylitol: randomized trial results. J Dent Res 92:512–517. https://doi.org/10.1177/0022034513487211
Jain V, Ghosh S (2021) Biotransformation of lignocellulosic biomass to xylitol: an overview. Biomass Convers Biorefinery 1-19. https://doi.org/10.1007/s13399-021-01904-0
de Albuquerque TL, da Silva Jr IJ, de Macedo GR, Rocha MV (2014) Biotechnological production of xylitol from lignocellulosic wastes: a review. Process biochem 49:1779–1789. https://doi.org/10.1016/j.procbio.2014.07.010
Kim Y, Kreke T, Hendrickson R, Parenti J, Ladisch MR (2013) Fractionation of cellulase and fermentation inhibitors from steam pretreated mixed hardwood. Bioresour Technol 135:30–38. https://doi.org/10.1016/j.biortech.2012.10.130
Jönsson LJ, Alriksson B, Nilvebrant NO (2013) Bioconversion of lignocellulose: inhibitors and detoxification. Biotechnol Biofuels 6:1–10. https://doi.org/10.1186/1754-6834-6-16
El-Tayeb TS, Abdelhafez AA, Ali SH, Ramadan EM (2012) Effect of acid hydrolysis and fungal biotreatment on agro-industrial wastes for obtainment of free sugars for bioethanol production. Braz J Microbio. 43:1523–1535. https://doi.org/10.1590/S1517-83822012000400037
Cortez DV, Roberto IC, Barbosa MH, Milagres AM (2014) Evaluation of cellulosic and hemicellulosic hydrolysate fermentability from sugarcane bagasse hybrids with different compositions. Biomass Convers Biorefin 4:351–356. https://doi.org/10.1007/s13399-014-0119-5
Kumar R, Wyman CE (2008) The impact of dilute sulfuric acid on the selectivity of xylooligomer depolymerization to monomers. Carbohydr Res 343:290–300. https://doi.org/10.1016/j.carres.2007.10.022
Panagiotopoulos IA, Bakker RR, De Vrije T, Koukios EG (2011) Effect of pretreatment severity on the conversion of barley straw to fermentable substrates and the release of inhibitory compounds. Bioresour Technol 102:11204–11211. https://doi.org/10.1016/j.biortech.2011.09.090
Sonkar RM, Gade PS, Bokade V, Mudliar SN, Bhatt P (2021) Ozone assisted autohydrolysis of wheat bran enhances xylooligosaccharide production with low generation of inhibitor compounds: a comparative study. Bioresour Technol 338:125559. https://doi.org/10.1016/j.biortech.2021.125559
Fu N, Peiris P (2008) Co-fermentation of a mixture of glucose and xylose to ethanol by Zymomonas mobilis and Pachysolen tannophilus. World J Microbiol Biotechnol 24:1091–1097. https://doi.org/10.1007/s11274-007-9613-2
Agbogbo FK, Wenger KS (2007) Production of ethanol from corn stover hemicellulose hydrolyzate using Pichia stipitis. J Ind Microbiol Biotechnol 34:723–727. https://doi.org/10.1007/s10295-007-0247-z
Modig T, Lidén G, Taherzadeh MJ (2002) Inhibition effects of furfural on alcohol dehydrogenase, aldehyde dehydrogenase and pyruvate dehydrogenase. Biochem J 363:769–776. https://doi.org/10.1042/bj3630769
Liu ZL, Slininger PJ, Dien BS, Berhow MA, Kurtzman CP, Gorsich SW (2004) Adaptive response of yeasts to furfural and 5-hydroxymethylfurfural and new chemical evidence for HMF conversion to 2, 5-bis-hydroxymethylfuran. J Ind Microbiol Biotechnol 31:345–352. https://doi.org/10.1007/s10295-004-0148-3
Palmqvist E, Hahn-Hägerdal B (2000) Fermentation of lignocellulosic hydrolysates. II: inhibitors and mechanisms of inhibition. Bioresour Technol 74:25–33. https://doi.org/10.1016/S0960-8524(99)00161-3
Allen SA, Clark W, McCaffery JM, Cai Z, Lanctot A, Slininger PJ, Liu ZL, Gorsich SW (2010) Furfural induces reactive oxygen species accumulation and cellular damage in Saccharomyces cerevisiae. Biotechnol Biofuels 3:1–10. https://doi.org/10.1186/1754-6834-3-2
Bellido C, Bolado S, Coca M, Lucas S, González-Benito G, García-Cubero MT (2011) Effect of inhibitors formed during wheat straw pretreatment on ethanol fermentation by Pichia stipitis. Bioresour Technol 102:10868–10874. https://doi.org/10.1016/j.biortech.2011.08.128
Almarsdottir AR, Sigurbjornsdottir MA, Orlygsson J (2012) Effect of various factors on ethanol yields from lignocellulosic biomass by Thermoanaerobacterium AK17. Biotechnol Bioeng 109:686–694. https://doi.org/10.1002/bit.24346
Perna MD, Bastos RG, Ceccato-Antonini SR (2018) Single and combined effects of acetic acid, furfural, and sugars on the growth of the pentose-fermenting yeast Meyerozyma guilliermondii. 3 Biotech 8:119. https://doi.org/10.1007/s13205-018-1143-0
Chandel AK, Da Silva SS, Singh OV (2013) Detoxification of lignocellulose hydrolysates: biochemical and metabolic engineering toward white biotechnology. Bioenergy Res 6:388–401. https://doi.org/10.1007/s12155-012-9241-z
Kordala N, Lewandowska M, Bednarski W (2021) Effect of the method for the elimination of inhibitors present in Miscanthus giganteus hydrolysates on ethanol production effectiveness. Biomass Convers Biorefinery 1-9. https://doi.org/10.1007/s13399-020-01255-2
Liu ZL (2006) Genomic adaptation of ethanologenic yeast to biomass conversion inhibitors. Appl Microbiol Biotechnol 73:27–36. https://doi.org/10.1007/s00253-006-0567-3
Wannawilai S, Lee WC, Chisti Y, Sirisansaneeyakul S (2017) Furfural and glucose can enhance conversion of xylose to xylitol by Candida magnoliae TISTR 5663. J Biotechnol 241:147–157. https://doi.org/10.1016/j.jbiotec.2016.11.022
Antal MJ Jr, Leesomboon T, Mok WS, Richards GN (1991) Mechanism of formation of 2-furaldehyde from D-xylose. Carbohydr. Res 217:71–85. https://doi.org/10.1016/0008-6215(91)84118-X
Klinke HB, Ahring BK, Schmidt AS, Thomsen AB (2002) Characterization of degradation products from alkaline wet oxidation of wheat straw. Bioresour Technol 82:15–26. https://doi.org/10.1016/S0960-8524(01)00152-3
Dasgupta D, Sidana A, Ghosh P, Sharma T, Singh J, Prabhune A, Ghosh D (2021) Energy and life cycle impact assessment for xylitol production from corncob. J Clean Prod 278:123217. https://doi.org/10.1016/j.jclepro.2020.123217
Rodrigues RC, Kenealy WR, Jeffries TW (2011) Xylitol production from DEO hydrolysate of corn stover by Pichia stipitis YS-30. J Ind Microbiol Biotechnol 38:1649–1655. https://doi.org/10.1007/s10295-011-0953-4
Banerjee S, Sen R, Pandey RA, Chakrabarti T, Satpute D, Giri BS, Mudliar S (2009) Evaluation of wet air oxidation as a pretreatment strategy for bioethanol production from rice husk and process optimization. Biomass Bioenerg 33:1680–1686. https://doi.org/10.1016/j.biombioe.2009.09.001
Levenspiel O (1980) The Monod equation: a revisit and a generalization to product inhibition situations. Biotechnol Bioeng 22:1671–1687. https://doi.org/10.1002/bit.260220810
Luong JH (1985) Kinetics of ethanol inhibition in alcohol fermentation. Biotechnol Bioeng 27:280–285. https://doi.org/10.1002/bit.260270311
Cer RZ, Mudunuri U, Stephens R, Lebeda FJ (2009) IC50-to-Ki: a web-based tool for converting IC50 to Ki values for inhibitors of enzyme activity and ligand binding. Nucleic Acids Res. https://doi.org/10.1093/nar/gkp253
Nigam JN (2001) Ethanol production from wheat straw hemicellulose hydrolysate by Pichia stipitis. J Biotechnol 87:17–27. https://doi.org/10.1016/S0168-1656(00)00385-0
Cabral JC, Silva JP, Roberto IC (2005) Influence of pH on ethanol production by Pichia stipitis. Latin American Postgraduate Meeting
Farias D, de Andrade RR, Maugeri-Filho F (2014) Kinetic modeling of ethanol production by Scheffersomyces stipitis from xylose. Appl Biochem Biotechnol 172:361–379. https://doi.org/10.1007/s12010-013-0546-y
Horváth IS, Franzén CJ, Taherzadeh MJ, Niklasson C, Lidén G (2003) Effects of furfural on the respiratory metabolism of Saccharomyces cerevisiae in glucose-limited chemostats. Appl Environ Microbiol 69:4076. https://doi.org/10.1128/AEM.69.7.4076-4086.2003
Almeida JR, Modig T, Petersson A, Hähn-Hägerdal B, Lidén G, Gorwa-Grauslund MF (2007) Increased tolerance and conversion of inhibitors in lignocellulosic hydrolysates by Saccharomyces cerevisiae. J Chem Technol Biotechnol 82:340–349. https://doi.org/10.1002/jctb.1676
Vajzovic A, Bura R, Kohlmeier K, Doty SL (2012) Novel endophytic yeast Rhodotorula mucilaginosa strain PTD3 II: production of xylitol and ethanol in the presence of inhibitors. J Ind Microbiol Biotechnol 39:1453–1463. https://doi.org/10.1007/s10295-012-1154-5
Kashid M, Ghosalkar A (2017) Evaluation of fermentation kinetics of acid-treated corn cob hydrolysate for xylose fermentation in the presence of acetic acid by Pichia stipitis. 3 Biotech 7:240. https://doi.org/10.1007/s13205-017-0873-8
Bura R, Vajzovic A, Doty SL (2012) Novel endophytic yeast Rhodotorula mucilaginosa strain PTD3 I: production of xylitol and ethanol. J Ind Microbiol Biotechnol 39:1003–1011. https://doi.org/10.1007/s10295-012-1109-x
Noronha LD, Fonseca CR, Silva CC, Silva MB, Faria LF (2010) Use of different types of aluminum polychlorides for the purification of sugarcane bagasse hydrolyzate through the coagulation and flocculation technique, Chem. New 33 (8). https://doi.org/10.1590/S0100-40422010000800015
Felipe MG, Vieira DC, Vitolo M, Silva SS, Roberto IC, Manchilha IM (1995) Effect of acetic acid on xylose fermentation to xylitol by Candida guilliermondii. J Basic Microbiol 35:171–177. https://doi.org/10.1002/jobm.3620350309
Greetham D, Hart AJ, Tucker GA (2016) Presence of low concentrations of acetic acid improves yeast tolerance to hydroxymethylfurfural (HMF) and furfural. Biomass Bioenergy 85:53–60. https://doi.org/10.1016/j.biombioe.2015.11.026
Zaldivar J, Martinez A, Ingram LO (1999) Effect of selected aldehydes on the growth and fermentation of ethanologenic Escherichia coli. Biotechnol Bioeng 65:24–33. https://doi.org/10.1002/(SICI)1097-0290(19991005)65:1%3C24::AID-BIT4%3E3.0.CO;2-2
Zaldivar J, Martinez A, Ingram LO (2000) Effect of alcohol compounds found in hemicellulose hydrolysate on the growth and fermentation of ethanologenic Escherichia coli. Biotechnol Bioeng 68:524–530. https://doi.org/10.1002/(SICI)1097-0290(20000605)68:5%3C524::AID-BIT6%3E3.0.CO;2-T
Tochampa W, Sirisansaneeyakul S, Vanichsriratana W, Srinophakun P, Bakker HH, Chisti Y (2005) A model of xylitol production by the yeast Candida mogii. Bioproc Biosyst Eng 28:175–183. https://doi.org/10.1007/s00449-005-0025-0
Wannawilai S, Sirisansaneeyakul S, Chisti Y (2015) Benzoate-induced stress enhances xylitol yield in aerobic fed-batch culture of Candida mogii TISTR 5892. J Biotechnol 194:58–66. https://doi.org/10.1016/j.jbiotec.2014.11.037
Slininger PJ, Dien BS, Lomont JM, Bothast RJ, Ladisch MR, Okos MR (2014) Evaluation of a kinetic model for computer simulation of growth and fermentation by Scheffersomyces (Pichia) stipitis fed D-xylose. Biotechnol Bioeng 111:1532–1540. https://doi.org/10.1002/bit.25215
Georgieva TI, Skiadas IV, Ahring BK (2007) Effect of temperature on ethanol tolerance of a thermophilic anaerobic ethanol producer Thermoanaerobacter A10: modeling and simulation. Biotechnol Bioeng 98:1161–1170. https://doi.org/10.1002/bit.21536
Wahlbom CF, Hahn-Hägerdal B (2002) Furfural, 5-hydroxymethyl furfural, and acetoin act as external electron acceptors during anaerobic fermentation of xylose in recombinant Saccharomyces cerevisiae. Biotechnol Bioeng 78:172–178. https://doi.org/10.1002/bit.10188
Ask M, Bettiga M, Duraiswamy VR, Olsson L (2013) Pulsed addition of HMF and furfural to batch-grown xylose-utilizing Saccharomyces cerevisiae results in different physiological responses in glucose and xylose consumption phase. Biotechnol Biofuels 6:1–15. https://doi.org/10.1186/1754-6834-6-181
Thomas KC, Ingledew WM (1992) Production of 21%(v/v) ethanol by fermentation of very high gravity (VHG) wheat mashes. J Ind Microbiol 10:61–68. https://doi.org/10.1007/BF01583635
Acknowledgements
The authors thank the Director, CSIR-CFTRI, Mysore, for providing the infrastructure for carrying out the experiment. The first author gratefully acknowledges the Council and Scientific Industrial Research (CSIR), New Delhi, for providing the financial assistance in the form of Junior Research Fellowship to carry out the present investigation and University of Mysore for successful registration of Ph.D.
Funding
This work was supported by Department of Biotechnology (DBT), India, for financial support through (BT/PR12277/PBD/26/434/2014) project.
Author information
Authors and Affiliations
Contributions
Conceptualization: Bhavana B K, Sandeep N Mudliar, Sukumar Debnath. Methodology: Bhavana B K, Sandeep N Mudliar. Formal analysis and investigation: Bhavana B K. Writing—original draft preparation: Bhavana B K. Writing—review and editing: Bhavana B K, Sandeep N Mudliar, Vijay V Bokade. Funding acquisition: Sandeep N Mudliar, Vijay V Bokade. Supervision: Sandeep N Mudliar, Sukumar Debnath.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
B K, B., Mudliar, S.N., Bokade, V.V. et al. Effect of furfural, acetic acid and 5-hydroxymethylfurfural on yeast growth and xylitol fermentation using Pichia stipitis NCIM 3497. Biomass Conv. Bioref. 14, 4909–4923 (2024). https://doi.org/10.1007/s13399-022-02758-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-022-02758-w